Abstract
High hemoglobin A1c (HbA1c) levels are strongly associated with an increased risk of cardiovascular disease (CVD) in people with and without diabetes. However, information regarding the relationship between low HbA1c levels and the risk of CVD among people without known diabetes is limited. The aim of this large-scale, prospective, population-based cohort study was to clarify the association between HbA1c levels and CVD risk among people without known diabetes.
We followed-up 10,980 men and 18,079 women (46–80 years old and free of CVD and cancer at baseline) in the Japan Public Health Center-based Prospective Study. Using Cox models, we estimated the hazard ratios for CVD risk with adjustments for age, sex, geographic areas, body mass index, smoking status, sports and physical exercise, alcohol intake, systolic blood pressure, non-high-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
During the median follow-up of 9.4 years, 935 CVD events (770 strokes and 165 coronary heart diseases) occurred. We observed a nonlinear association between HbA1c levels and CVD risk in participants without known diabetes. Compared with HbA1c levels of 5.0 to 5.4% (31–36 mmol/mol), the hazard ratios for CVD in participants without known diabetes were 1.50 (95% confidence interval: 1.15–1.95), 1.01 (0.85–1.20), 1.04 (0.82–1.32), and 1.77 (1.32–2.38) for HbA1c levels of <5.0% (<31 mmol/mol), 5.5 to 5.9% (37–41 mmol/mol), 6.0 to 6.4% (42–47 mmol/mol), and ≥6.5% (≥48 mmol/mol), respectively (P value for nonlinear trend: <0.001). In addition, the hazard ratio for CVD was 1.81 (1.43–2.29) in patients with known diabetes compared with participants with HbA1c levels of 5.0 to 5.4% and without known diabetes. This nonlinear relation persisted after excluding people with kidney dysfunction, liver dysfunction, anemia, body mass index <18.5 kg/m2, or early events within 3 years of follow-up (P value for nonlinear trend: <0.01 for all tests).
In conclusion, both low and high levels of HbA1c were associated with a higher risk of CVD in a Japanese general population without known diabetes.
INTRODUCTION
Although substantial efforts have been made to control major cardiovascular disease (CVD) risk factors (eg, hypertension and smoking), CVD remains to be the leading cause of death globally.1–3 Biomarkers, such as hemoglobin A1c (HbA1c), may be useful for identifying people with increased risk of CVD and eventually help reduce the global burden of CVD.4,5
It has been well established that high HbA1c levels are strongly associated with a high risk of CVD in people with6 and without4,7 diabetes. Accordingly, several researchers have suggested that HbA1c measurement may be useful for identifying people with an increased risk of CVD.4,7 However, the association between low HbA1c levels and CVD risk is not well understood. In some studies,8–10 but not all,6 it has been suggested that patients with type 2 diabetes and low HbA1c levels may have a higher CVD risk, which is consistent with the observation that severe hypoglycemia is associated with an increased CVD risk among patients with type 2 diabetes.11 However, the association between low HbA1c levels in people without known diabetes and CVD risk remains unknown. Although a possible association between low HbA1c levels and increased mortality in populations without known diabetes has been previously reported,4,12,13 the biological mechanisms underlying this association are currently unknown.13–15 Investigating the association between low HbA1c levels and CVD risk may improve our understanding of health risks associated with low HbA1c levels. The aim of this large-scale, prospective, population-based cohort study was to address the question whether low HbA1c levels are associated with a higher CVD risk among people without known diabetes using strictly standardized HbA1c levels and detailed measurements of covariates in a general Japanese population free of CVD and cancer at baseline.
METHODS
Study Design and Population
The Japan Public Health Centre-based Prospective Study (JPHC Study) was initiated in 1990 for cohort I and in 1993 to 1994 for cohort II. All subjects were Japanese inhabitants from 11 public health center areas, and aged 40 to 59 years in 1990 (cohort I) and 40 to 69 years in 1993 (cohort II). Details of the study design have been described elsewhere.16 The JPHC Diabetes Study, involving HbA1c measurements and an additional questionnaire concerning diabetes and lifestyle, was conducted among JPHC participants at the time of their health check-ups (the first survey in 1998–2000 and the second survey in 2003–2005).17 Two public health center areas from Tokyo and Osaka were excluded because information regarding the incidence of coronary heart disease and stroke was not available. Therefore, this present study involved subjects from 9 areas (cohort I: 4 areas; cohort II: 5 areas). Individuals who participated in either of the JPHC Diabetes Study surveys were included in the present study. Among the 35,197 participants from the JPHC Diabetes Study, we excluded 1004 and 984 participants with a history of CVD and cancer, respectively, as well as 4150 participants with missing anthropometric or laboratory data. In total, we analyzed data for 29,059 participants. All participants provided written informed consent before participating in this study. The JPHC Diabetes Study was approved by the institutional review board of the National Center for Global Health and Medicine, Japan.
Measurements
Detailed procedures for the HbA1c measurements have been described previously,17 and these were performed using high-performance liquid chromatography or immunochemical assays. The overall intra-assay coefficient of variation for HbA1c ranged from 0.0 to 3.4%, and the maximal inter-assay coefficient of variation among the various laboratories ranged from 2.2 to 2.8%. The HbA1c measurement methods differed according to the public health center areas, and therefore, HbA1c values were strictly standardized to minimize inter-laboratory variation. For the calibration procedure, standard samples (approved by the Japan Diabetes Society) were provided to each public health center area before the surveys. HbA1c values were converted to National Glycohemoglobin Standardization Program values.18 Censoring events (which defined the individual's final data point) were defined as the first CVD event, death, change of residence, loss to follow-up, December 31, 2009 (cohort I), or December 31, 2008 (cohort II). For individuals who participated in both surveys of the JPHC Diabetes Study before the censoring events (35.1% of the study population), the average HbA1c levels were used for analyses to capture their long-term exposure.10 The sensitivity analyses using the time-dependent Cox proportional hazard models to update the HbA1c levels and diabetes status did not materially change the estimates.
Each participant completed a self-administered questionnaire at the 5-year and/or 10-year follow-up of the JPHC Study, which comprised questions regarding previously diagnosed medical conditions, medication, and lifestyle factors, including physical activity, alcohol intake, dietary intake, and smoking.19 In the present study, we used data from the JPHC Study questionnaire at the time of entry into the JPHC Diabetes Study, except for participants from cohort I who only participated in the second JPHC Diabetes Study survey (10% of the study population). These participants completed the JPHC Study questionnaire 5 years before their entry into the JPHC Diabetes Study, and these data were used in the current analysis. Sensitivity analyses for excluding these participants did not materially change study findings. At the time of the 2 JPHC Diabetes Study surveys, blood pressure, weight, height, hemoglobin, serum creatinine, alanine aminotransferase, and lipid levels were measured. Body mass index was calculated as weight (kg) divided by height squared (m2).
We defined CVD as either stroke or coronary heart disease, including myocardial infarction or sudden cardiac death. CVD events were documented based on active patient notifications from the local hospitals, hospital record reviews for participants who reported CVD in the follow-up questionnaires, and a review of death certificates.20 A total of 78 major hospitals capable of treating patients with acute CVD were included in the registry of CVD events within the 9 public health center areas. Overall, 97% of stroke and 92% of myocardial infarction cases in the 9 areas were treated at these registry hospitals. CVD events were included in this study if they occurred between the time of entry into the JPHC Diabetes Study and December 31, 2009 (cohort I) or December 31, 2008 (cohort II). Changes in residential status, including survival status, were identified using the residential registry in each area. During the follow-up period, 1273 (4.4%) participants died, 420 (1.4%) moved out of the study areas, and 28 (0.1%) were lost to follow-up.
Stroke diagnoses were confirmed according to the National Survey of Stroke criteria21 by the presence of sudden or rapid-onset focal neurological deficits that last >24 h or until death. Strokes were classified according to subtype: hemorrhagic or ischemic (lacunar or nonlacunar).20 A diagnosis of definite myocardial infarction was confirmed according to the Monitoring Trends and Determinants of Cardiovascular Disease Project criteria22 on the basis of typical chest pain and evidence from electrocardiograms and/or cardiac enzyme levels. For cases of typical prolonged chest pain (>20 min) that were not confirmed by electrocardiograms or cardiac enzymes (8.5% of the total myocardial infarctions), a diagnosis of possible myocardial infarction was made, and these cases were included in the myocardial infarction cases. Sensitivity analyses for excluding cases with possible myocardial infarction did not materially change the findings. In the absence of myocardial infarction diagnoses, deaths that occurred within 1 h from symptom onset were considered as sudden cardiac deaths. Only the first CVD event during the follow-up was included in the analysis; recurrent events were excluded.
Statistical Methods
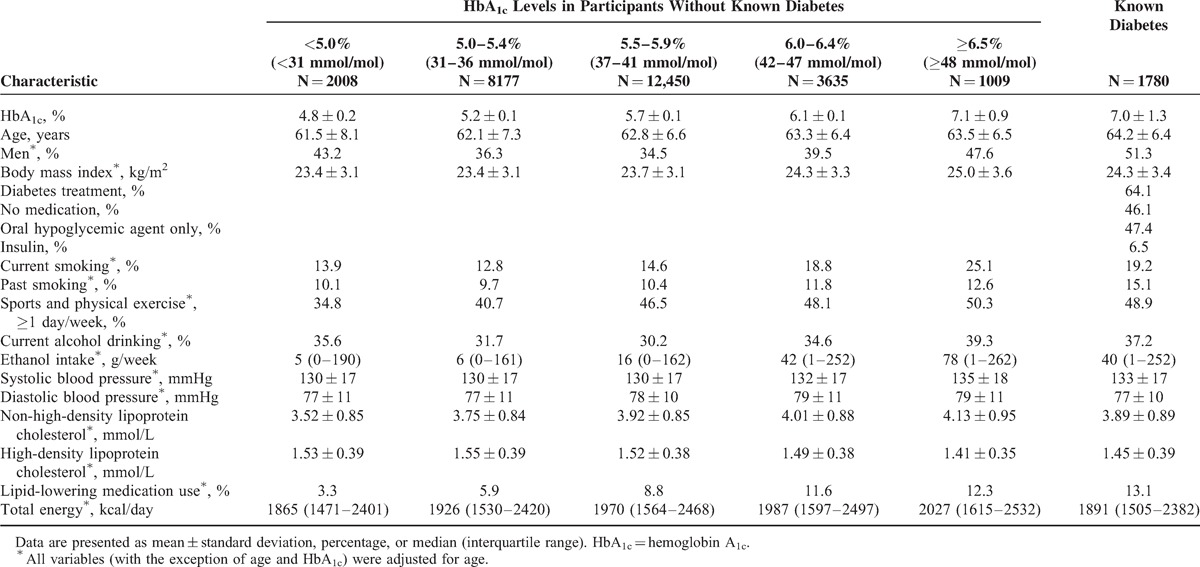
We followed-up 29,059 participants (46–80 years old) and calculated their person-years from the time of entry into the JPHC Diabetes Study until their censoring event. If individuals participated in both JPHC Diabetes Study surveys, the time of entry at the first survey was considered the starting point. We also calculated the baseline characteristics for patients with diabetes and 5 groups of people without known diabetes categorized by their HbA1c levels: <5.0% (<31 mmol/mol), 5.0 to 5.4% (31–36 mmol/mol), 5.5 to 5.9% (37–41 mmol/mol), 6.0 to 6.4% (42–47 mmol/mol), and ≥6.5% (≥48 mmol/mol). We defined participants as having known diabetes if they had self-reported diabetes or were receiving treatment for diabetes. Following conventional practice,4 the HbA1c category of 5.0 to 5.4% (31–36 mmol/mol) was used as the reference category.
To examine the CVD risk in the 6 groups of people, we used Cox proportional hazards models and estimated the hazard ratios and 95% confidence intervals (categorical models). These models were adjusted for age, sex, health center areas, body mass index, smoking status (never smoked, past smoker, or current smoker), alcohol intake (current nondrinker, occasional drinker, or current drinker), sports and physical exercise (≥1 day/week or other), systolic blood pressure (mmHg), high-density lipoprotein cholesterol levels (mmol/L), and non-high-density lipoprotein cholesterol levels (mmol/L). Slightly different physical activity questionnaires were used during the 5-year and 10-year follow-ups of the JPHC Study. Therefore, we first calculated separate estimates for participants who completed the 5-year follow-up questionnaire and for those who completed the 10-year follow-up questionnaire. Because there was no apparent difference in the estimates between these 2 groups, we computed the pooled results using the fixed-effects model with inverse variance weighting.23
Among the participants without known diabetes, we computed 2-sided P values for linear trends by assigning a mean HbA1c value for each category and including the variables as continuous variables in the models. We also computed 2-sided P values for quadratic trends (P value for quadratic trend) by including a quadratic term in each linear trend model. The proportional hazards assumption was assessed using the scaled Schoenfeld residuals24 and found to be appropriate. For sensitivity analysis, we further examined the association between HbA1c levels and CVD after excluding people with kidney dysfunction (estimated glomerular filtration < 60.0 mL·min−1·1.73 m−2),25 liver dysfunction (alanine aminotransferase ≥100 IU/L), anemia (hemoglobin < 100 g/L), or low body mass index (<18.5 kg/m2). Further analyses were also conducted after excluding CVD cases with an early diagnosis (within 3 years of follow-up) from both the numerator and denominator (278 participants). To examine the shape of the association between continuous HbA1c levels and CVD risk among people without known diabetes, we fitted restricted cubic spline models by including transformed variables of HbA1c levels in the Cox models, with adjustment for the same covariates that were used in the categorical models. We fitted the models using 3, 4, and 5 knots at percentiles, and chose the number of knots that produced the smallest Akaike Information Criterion. The level of significance was set at P value < 0.05. Analyses were performed using Stata version 12.1 (StataCorp, College Station, TX).
RESULTS
The baseline characteristics of the study population according to the 6 groups are shown in Table 1. Compared with participants with lower HbA1c levels, participants with higher HbA1c levels or known diabetes tended to be older; current or past smokers; have a higher body mass index, blood pressure, and non-high-density lipoprotein cholesterol levels; have lower high-density lipoprotein cholesterol levels; use lipid-lowering medication(s); be engaged in physical activity; and consume more calories.
TABLE 1.
Baseline Characteristics According to Hemoglobin A1c Levels and Known Diabetes
During the median follow-up of 9.4 years (238,456 person-years), 770 strokes (226 lacunar infarctions, 232 nonlacunar infarctions, 311 hemorrhagic strokes, and 1 stroke of undetermined type) and 165 coronary heart diseases (129 definite myocardial infarctions, 12 possible myocardial infarctions, and 24 sudden cardiac deaths) were documented. Table 2 shows the associations for CVD, coronary heart disease, and stroke risk in the 6 groups. After multivariable adjustment for potential confounding factors, we observed a nonlinear relation between HbA1c levels and CVD risk (model 2; P value for quadratic trend: <0.001). The nonlinear trend was observed even after excluding people with kidney dysfunction, liver dysfunction, anemia, and low body mass index (P value for quadratic trend: <0.05 for all tests). Further adjustment for the use of lipid-lowering medication(s) or total energy intake resulted in similar results (P value for quadratic trend: <0.001 for all tests, data not shown). Similar findings were observed when CVD cases with an early diagnosis (within 3 years of follow-up) were excluded (P value for quadratic trend: 0.002). Spline curves indicated a U-shaped relation, with increased CVD risk observed in participants with low and high levels of HbA1c (Figure 1A). A similar pattern was observed for stroke risk (Table 2 , Figure 1B). Because nonlinear associations were observed, particularly for stroke, we further examined the association between HbA1c levels and stroke subtypes. We observed nonlinear trends for the risks of hemorrhagic and nonlacunar ischemic stroke (model 2; P values for quadratic trend: 0.018 and 0.006, respectively), and a similar pattern was suggested for lacunar stroke (model 2; P value for quadratic trend: 0.066). Participants with high HbA1c levels (≥6.5%, ≥48 mmol/mol) or known diabetes had an increased risk of ischemic stroke (both lacunar and nonlacunar; model 2). A linear relation was suggested for coronary heart disease risk (Table 2 , Figure 1C), although the number of coronary heart disease cases was likely too small to examine its risk in participants with low HbA1c levels. Participants with known diabetes had increased risks of CVD, stroke, and coronary heart disease (model 2).
TABLE 2.
Incidence of Cardiovascular Disease According to Hemoglobin A1c Levels and Known Diabetes
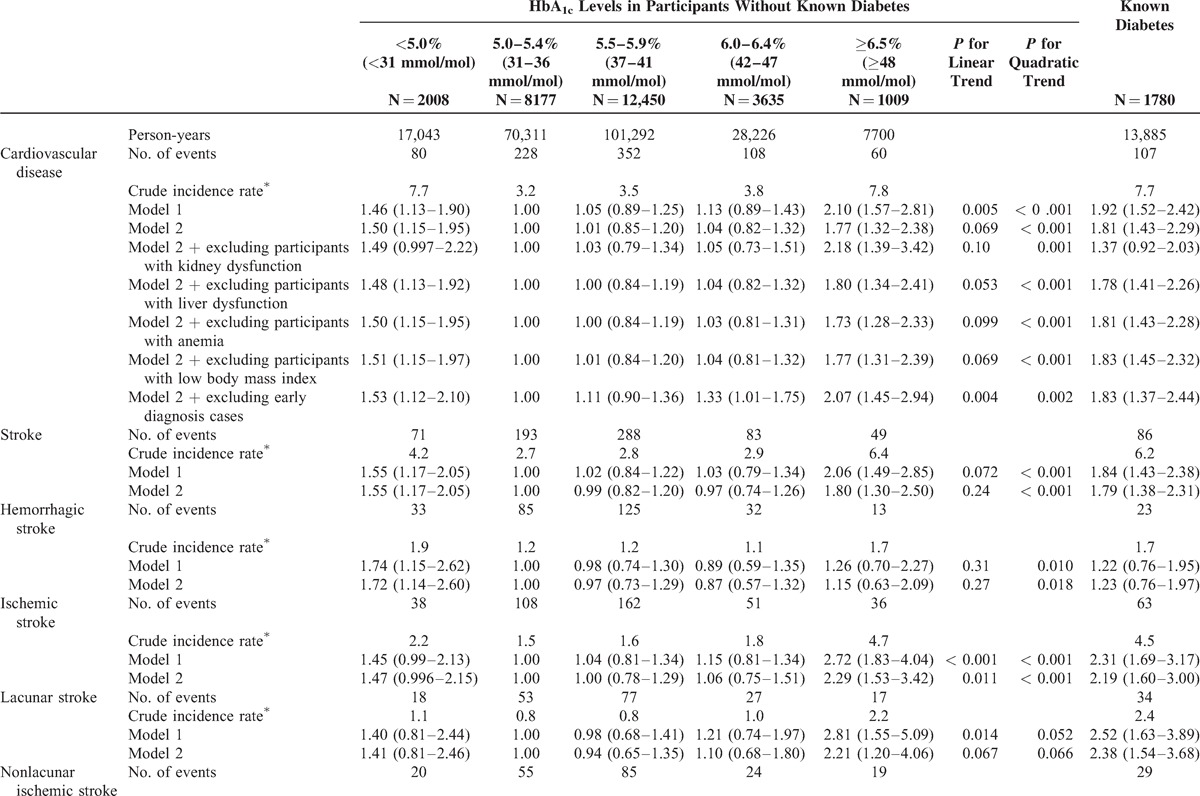
FIGURE 1.
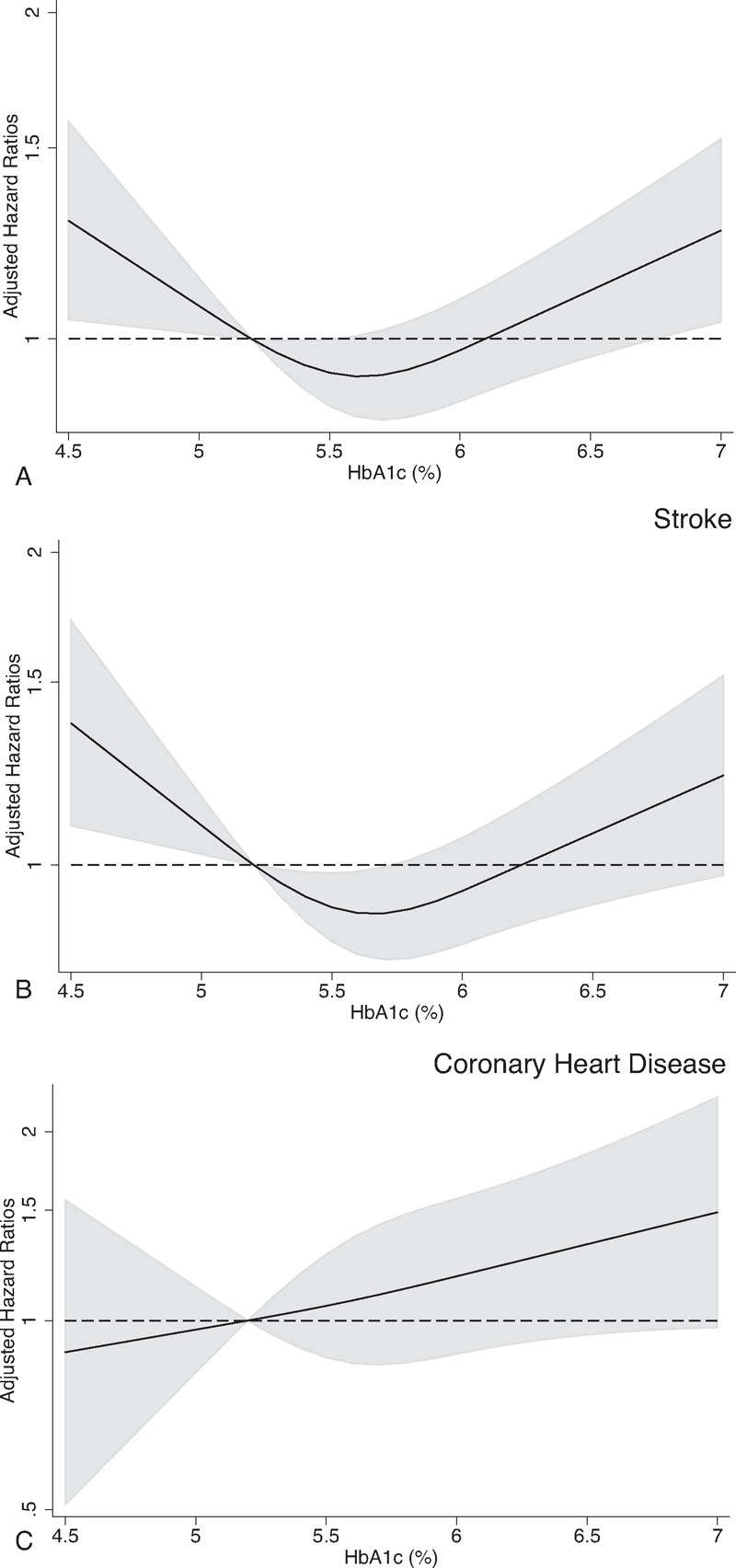
Hazard ratios for cardiovascular events according to continuous hemoglobin A1c (HbA1c) levels among participants without known diabetes. Restricted cubic spline models with the inclusion of transformed variables in the Cox model were used to estimate hazard ratios (solid curve) with point-wise 95% confidence intervals (grey shaded area) for (A) cardiovascular disease, (B) stroke, and (C) coronary heart disease. An HbA1c level of 5.3% (ie, the mean HbA1c level in people with HbA1c levels of 5.0–5.5%) was used to estimate all hazard ratios. We chose the number of knots that produced the smallest Akaike Information Criterion. Hazard ratios were adjusted for age, sex, public health center areas, body mass index, smoking status, alcohol intake, sports and physical exercise, systolic blood pressure, non-high-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
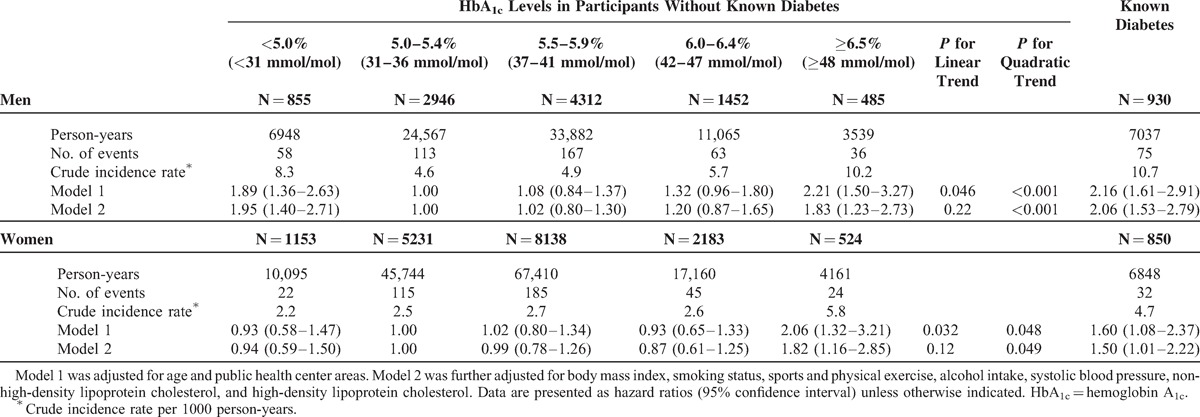
Stratified analysis according to sex suggested an increased CVD risk existed in men with low and high levels of HbA1c (Table 3). Among women, increased CVD risk was apparent in those with HbA1c levels of ≥6.5% (≥48 mmol/mol) or known diabetes.
TABLE 2 (Continued).
Incidence of Cardiovascular Disease According to Hemoglobin A1c Levels and Known Diabetes
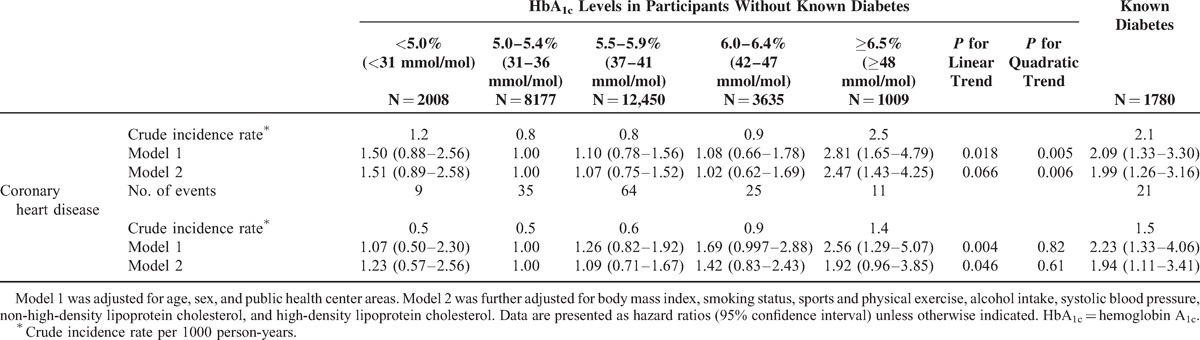
TABLE 3.
Sex-Stratified Incidence of Cardiovascular Disease According to Hemoglobin A1c Levels and Known Diabetes
DISCUSSION
In this large-scale, prospective, cohort study in a general Japanese population, both low and high levels of HbA1c were associated with an increased risk of CVD among participants without known diabetes. The nonlinear association between HbA1c levels and CVD risk persisted even after excluding participants with kidney dysfunction, liver dysfunction, anemia, and low body mass index. Furthermore, the patterns of the association between low HbA1c levels and each CVD subset differed from that for diabetes or high HbA1c levels. Low HbA1c levels were associated with an increased risk of stroke, especially hemorrhagic stroke, while diabetes and high HbA1c levels were associated with increased risks of coronary heart disease and stroke, especially ischemic stroke. The observed CVD risk in individuals with diabetes in this study was also consistent with accumulating evidence of diabetes as a risk factor for CVD.26,27 These findings emphasize a possible increased CVD risk among people without known diabetes and with low HbA1c levels.
The observed CVD risk among people with low HbA1c levels and no known diabetes is particularly important, because this increased risk could not be related to hypoglycemia11 induced by diabetes treatment. Although a possible increased CVD risk or low HbA1c levels among people without known diabetes have been suggested,4,13,28 most studies did not find a statistically significant association.4,13 However, we found a significant association between low HbA1c levels and CVD, particularly stroke, possibly because of a sufficient number of stroke events in this study. Earlier studies were limited by small sample sizes and HbA1c measurements that were obtained from frozen whole blood samples that were stored for >10 years.4,13 A significant nonlinear association between HbA1c levels and CVD risk among people without known diabetes has been reported in a recent pooled analysis; however, the lack of assay standardization and the significant heterogeneity between assay characteristics for HbA1c measurements may have limited the interpretation of the results.28 As previously shown, low HbA1c levels are associated with increased all-cause mortality in people without diabetes.4,12,13 According to our findings, the elevated incidence of CVD may partially explain the increased mortality among people with low HbA1c levels. HbA1c level is increasingly being used to screen for diabetes and therefore, our findings may facilitate the interpretation of low HbA1c levels in the nondiabetic population.
We were also able to confirm that HbA1c levels of ≥6.5% (≥48 mmol/mol) in people without known diabetes were associated with an increased CVD risk, which was consistent with other studies that were conducted in Japan7,29,30 and other countries.4,8 Although a significantly increased CVD risk was not observed for the groups with elevated HbA1c levels in the nondiabetic range, the spline analyses (Figure 1A) appeared to suggest a positive linear association between continuous HbA1c levels and CVD risk in those with HbA1c levels of ≥5.5% (≥37 mmol/mol). Earlier investigators have also documented an increased CVD risk with increasing HbA1c levels within the nondiabetic range.4,7,29,31 Furthermore, individuals with pre-diabetes (defined by glucose levels during oral glucose tolerance tests) may have a 20% increased risk of CVD, compared to those with normal glycemia according to recent meta-analyses.32,33 Therefore, hyperglycemia within the nondiabetic range may be associated with an increased CVD risk in a continuous manner.
The mechanisms responsible for the observed association between low HbA1c levels and increased CVD risk among people without known diabetes remain largely unknown. In addition, it is unknown whether low blood glucose levels not induced by diabetes treatment could have a direct effect on blood vessels. We observed similar results (data not shown) when we adjusted for casual blood glucose levels, which suggested that the association between low HbA1c levels and increased CVD risk may not be explained by blood glucose levels. Abnormal red-cell turnover, which can lead to low HbA1c levels,13 might explain the association. However, the association persisted after we excluded participants with factors that affect red-cell turnover, including kidney dysfunction, liver dysfunction, and anemia. Alternatively, chronic inadequate nutrition may explain the possible increased risk among people with low HbA1c levels. However, the total energy intake did not indicate inadequate nutrition in participants with HbA1c levels of <5.0% (<31 mmol/mol), and adjustment for total energy intake did not change the results. Further, excluding people with a low body mass index at baseline provided similar results. Therefore, confounding by these factors alone may not explain the observed association. Although the biological mechanisms underlying this association remain unresolved, our data support the notion that low HbA1c levels may be a marker for identifying people who are at increased risk of CVD.13 In addition, based on our findings, diabetic patients with low HbA1c levels (eg, <5.0%, <31 mmol/mol) may have an increased risk of CVD, possibly due to glycemic and nonglycemic factors.
This study has several strengths. First, we strictly standardized the HbA1c values using approved standard samples to reduce the possibility of measurement error, leading to less biased estimates. Second, the use of a population-based prospective cohort design with low loss to follow-up and a large sample size should minimize the possibility of selection bias. Third, the systematic surveys of CVD events likely reduced outcome misclassification in our study. Finally, we were able to examine the relation between HbA1c levels and stroke subtypes because of the large number of stroke events.
Despite these strengths, certain limitations of the present study merit consideration. First, HbA1c levels and diabetes status may have changed during the follow-up, but only single measurements of HbA1c were available for most participants (65%). If HbA1c levels during the follow-up had been available for all participants, the association between HbA1c and CVD risk would likely have been stronger. Second, the increased CVD risk observed in individuals with low HbA1c levels may not necessarily indicate a causal association. Third, information regarding socioeconomic status was not available, which might explain the increased CVD risk among people with low HbA1c levels. However, there is no clear evidence that people with low socioeconomic status have low HbA1c levels. Fourth, we could not consider the genetic background of our participants because genetic variants linked to CVD or risk factors (eg, hypertension34) were not measured in our study. However, it is unlikely that the missing information on such variants would bias the association between HbA1c levels and CVD risk because such variants do not tend to affect HbA1c levels. Finally, our results may not be applicable to other populations, especially Western populations, because East Asians tend to have a higher incidence of stroke and lower incidence of coronary heart disease compared with those in Western populations.35 Therefore, the nonlinear relation between HbA1c and stroke might be especially relevant to Asians.
In conclusion, both low and high levels of HbA1c were associated with a higher risk of CVD in a general Japanese population without known diabetes. These data support the notion that very low and high HbA1c levels may be markers for identifying people with an increased health risk.
Acknowledgments
We thank all the staff members in each study area and in the central and cardiovascular offices for their cooperation and technical assistance. We also thank Hadrien Charvat at the Epidemiology and Prevention Group, Research Center for Cancer Prevention and Screening, National Cancer Center for providing statistical advice.
Members of the JPHC Study Group: S. Tsugane (principal investigator), S. Sasazuki, M. Iwasaki, N. Sawada, T. Shimazu, T. Yamaji, and T. Hanaoka, National Cancer Center, Tokyo; J. Ogata, S. Baba, T. Mannami, A. Okayama, and Y. Kokubo, National Cerebral and Cardiovascular Center, Osaka; K. Miyakawa, F. Saito, A. Koizumi, Y. Sano, I. Hashimoto, T. Ikuta, Y. Tanaba, H. Sato and Y. Roppongi, Iwate Prefectural Ninohe Public Health Center, Iwate; Y. Miyajima, N. Suzuki, S. Nagasawa, Y. Furusugi, N. Nagai, Y. Ito and S.Komatsu, Akita Prefectural Yokote Public Health Center, Akita; H. Sanada, Y. Hatayama, F. Kobayashi, H. Uchino, Y. Shirai, T. Kondo, R. Sasaki, Y. Watanabe, Y. Miyagawa, Y. Kobayashi, M. Machida, K. Kobayashi and M. Tsukada, Nagano Prefectural Saku Public Health Center, Nagano; Y. Kishimoto, E. Takara, T. Fukuyama, M. Kinjo, M. Irei, and H. Sakiyama, Okinawa Prefectural Chubu Public Health Center, Okinawa; K. Imoto, H. Yazawa, T. Seo, A. Seiko, F. Ito, F. Shoji and R. Saito, Katsushika Public Health Center, Tokyo; A. Murata, K. Minato, K. Motegi, T. Fujieda and S. Yamato, Ibaraki Prefectural Mito Public Health Center, Ibaraki; K. Matsui, T. Abe, M. Katagiri, M. Suzuki, K. and Matsui, Niigata Prefectural Kashiwazaki and Nagaoka Public Health Center, Niigata; M. Doi, A. Terao, Y. Ishikawa, and T. Tagami, Kochi Prefectural Chuo-higashi Public Health Center, Kochi; H. Sueta, H. Doi, M. Urata, N. Okamoto, and F. Ide and H. Goto, Nagasaki Prefectural Kamigoto Public Health Center, Nagasaki; H. Sakiyama, N. Onga, H. Takaesu, M. Uehara, and T. Nakasone, Okinawa Prefectural Miyako Public Health Center, Okinawa; F. Horii, I. Asano, H. Yamaguchi, K. Aoki, S. Maruyama, M. Ichii, and M. Takano, Osaka Prefectural Suita Public Health Center, Osaka; Y. Tsubono, Tohoku University, Miyagi; K. Suzuki, Research Institute for Brain and Blood Vessels Akita, Akita; Y. Honda, K. Yamagishi, S. Sakurai and N. Tsuchiya, University of Tsukuba, Ibaraki; M. Kabuto, National Institute for Environmental Studies, Ibaraki; M. Yamaguchi, Y. Matsumura, S. Sasaki, and S. Watanabe, National Institute of Health and Nutrition, Tokyo; M. Akabane, Tokyo University of Agriculture, Tokyo; T. Kadowaki and M. Inoue, The University of Tokyo, Tokyo; M. Noda and T. Mizoue, National Center for Global Health and Medicine, Tokyo; Y. Kawaguchi, Tokyo Medical and Dental University, Tokyo; Y. Takashima and Y. Yoshida, Kyorin University, Tokyo; K. Nakamura, Niigata University, Niigata; S. Matsushima and S. Natsukawa, Saku General Hospital, Nagano; H. Shimizu, Sakihae Institute, Gifu; H. Sugimura, Hamamatsu University School of Medicine, Shizuoka; S. Tominaga, Aichi Cancer Center, Aichi; N. Hamajima, Nagoya University, Aichi; H. Iso and T. Sobue, Osaka University, Osaka; M. Iida, W. Ajiki, and A. Ioka, Osaka Medical Center for Cancer and Cardiovascular Disease, Osaka; S. Sato, Chiba Prefectural Institute of Public Health, Chiba; E. Maruyama, Kobe University, Hyogo; M. Konishi, K. Okada, and I. Saito, Ehime University, Ehime; N. Yasuda, Kochi University, Kochi; S. Kono, Kyushu University, Fukuoka; S. Akiba, Kagoshima University, Kagoshima.
FUNDING: This study was supported by Grants-in-Aid for Cancer Research and for the Third Term Comprehensive Control Research for Cancer and Health Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan (Research on Health Services H10–074, Medical Frontier Strategy Research H13–008, Clinical Research for Evidence-based Medicine H14–008 and H15–006, and Comprehensive Research on Life-Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus H16–019, H17–019, H18–028, H19–016, and H25–016). The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to publish the results.
OTHER INFORMATION: CONTRIBUTORS: AG analyzed data, drafted the manuscript, reviewed and edited the manuscript, and contributed to discussion. MN and ST conducted, designed, and supervised the study, and contributed to discussion. YM analyzed data and contributed to discussion. MG analyzed data, reviewed and edited the manuscript, and contributed to discussion. MK, AI, TM, MI, and TK conducted the research. YT, KY, IS, YK, and NS conducted the research and contributed to discussion. KK, SO, and AN contributed to discussion. HY reviewed and edited the manuscript, and contributed to discussion. All authors have read and approved the final manuscript.
Footnotes
Abbreviations: CVD = cardiovascular disease, HbA1c = hemoglobin A1c, JPHC Study = Japan Public Health Center-based Prospective Study.
Conflict of interest: AG has received a lecture fee from Boehringer Ingelheim, outside of the submitted work. MN received grants from Astra Zeneca K.K., Mitsubishi-Tanabe Pharma Corp., Kowa Pharmaceutical Co. Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., and Kyowa Hakko Kirin Co. Ltd. as well as lecture fees from Sanofi K.K., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo company, Eli Lilly Japan K.K., MSD K.K., Novo Nordisk Pharma Ltd., Kissei Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Astellas Pharma Inc., Kowa Pharmaceutical Co. Ltd., Taisho Toyama Pharmaceutical Co. Ltd., Meiji Seika Pharma Co. Ltd., and Kyowa Hakko Kirin Co. Ltd., outside of the submitted work. HY has received lecture fees from Merck/MSD, Takeda Pharmaceutical Company Limited, Boehringer Ingelheim, and Hiroshima Prefectural Medical Association, outside of the submitted work. MI is the beneficiary of a financial contribution from the AXA Research Fund as a chair holder on the AXA Department of Health and Human Security, Graduate School of Medicine, The University of Tokyo. The AXA Research Fund had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to publish the results. All other authors declare that they do not have any potential conflicts of interest to disclose.
REFERENCES
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. J Cardiovasc Dis 2013; 1:1–2. [Google Scholar]
- 3.Santulli G. Coronary heart disease risk factors and mortality. JAMA 2012; 307:1137–1138. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 2007; 297:611–619. [DOI] [PubMed] [Google Scholar]
- 6.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeda F, Doi Y, Ninomiya T, et al. Haemoglobin A1c even within non-diabetic level is a predictor of cardiovascular disease in a general Japanese population: the Hisayama Study. Cardiovasc Diabetol 2013; 12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols GA, Joshua-Gotlib S, Parasuraman S. Glycemic control and risk of cardiovascular disease hospitalization and all-cause mortality. J Am Coll Cardiol 2013; 62:121–127. [DOI] [PubMed] [Google Scholar]
- 9.Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 2012; 55:636–643. [DOI] [PubMed] [Google Scholar]
- 10.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010; 375:481–489. [DOI] [PubMed] [Google Scholar]
- 11.Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013; 347:f4533. [DOI] [PubMed] [Google Scholar]
- 12.Carson AP, Fox CS, McGuire DK, et al. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes 2010; 3:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal V, Schneider AL, Selvin E. Low hemoglobin A1c in nondiabetic adults: an elevated risk state? Diabetes Care 2012; 35:2055–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christman AL, Lazo M, Clark JM, et al. Low glycated hemoglobin and liver disease in the U.S. population. Diabetes Care 2011; 34:2548–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutter MK. Low HbA1c and mortality: causation and confounding. Diabetologia 2012; 55:2307–2311. [DOI] [PubMed] [Google Scholar]
- 16.Tsugane S, Sawada N. The JPHC Study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol 2014; 44:777–782. [DOI] [PubMed] [Google Scholar]
- 17.Noda M, Kato M, Takahashi Y, et al. Fasting plasma glucose and 5-year incidence of diabetes in the JPHC Diabetes Study – suggestion for the threshold for impaired fasting glucose among Japanese. Endocr J 2010; 57:629–637. [DOI] [PubMed] [Google Scholar]
- 18.Kashiwagi A, Kasuga M, Araki E, et al. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3:39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe S, Tsugane S, Sobue T, et al. Study design and organization of the JPHC Study. Japan Public Health Center-based Prospective Study on Cancer and Cardiovascular Diseases. J Epidemiol 2001; 11 (6 Suppl):S3–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagishi K, Iso H, Kokubo Y, et al. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: the JPHC Study. Eur Heart J 2013; 34:1225–1232. [DOI] [PubMed] [Google Scholar]
- 21.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke 1981; 12 (2 Pt 2 Suppl 1):I13–I44. [PubMed] [Google Scholar]
- 22.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, et al. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994; 90:583–612. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S, O’Rourke K. Meta-analysis. Modern Epidemiology. 3rd ed2008; Philadelphia, PA: Lippincott Williams & Wilkins, 652–682. [Google Scholar]
- 24.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81:515–526. [Google Scholar]
- 25.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53:982–992. [DOI] [PubMed] [Google Scholar]
- 26.Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito I, Kokubo Y, Yamagishi K, et al. Diabetes and the risk of coronary heart disease in the general Japanese population: the Japan Public Health Center-based prospective (JPHC) Study. Atherosclerosis 2011; 216:187–191. [DOI] [PubMed] [Google Scholar]
- 28.Emerging Risk Factors Collaboration. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA 2014; 311:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakurai M, Saitoh S, Miura K, et al. HbA1c and the Risks for All-Cause and Cardiovascular Mortality in the General Japanese Population: NIPPON DATA90. Diabetes Care 2013; 36:3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura R, Nakagami T, Sone H, et al. Relationship between hemoglobin A1c and cardiovascular disease in mild-to-moderate hypercholesterolemic Japanese individuals: subanalysis of a large-scale randomized controlled trial. Cardiovasc Diabetol 2011; 10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaw KT, Wareham N, Bingham S, et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol 2010; 55:1310–1317. [DOI] [PubMed] [Google Scholar]
- 33.Lee M, Saver JL, Hong KS, et al. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ 2012; 344:e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 gene deletion induces hypertension. J Am Heart Assoc 2012; 1:e001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation 2011; 124:314–323. [DOI] [PubMed] [Google Scholar]


