Abstract
We evaluate the effects of zolpidem use to develop dementia or Alzheimer disease from the Taiwan National Health Insurance Research Database (NHIRD).
A retrospective population-based nested case–control study. Newly diagnosed dementia patients 65 years and older and controls were sampled. A total of 8406 dementia and 16,812 control subjects were enrolled from Taiwan NHIRD during 2006 to 2010. The relationships between zolpidem use and dementia were measured using odds and adjusted odds ratios. The relationship between the average cumulative doses for zolpidem and dementia was also analyzed.
Zolpidem alone or with other underlying diseases, such as hypertension, diabetes, and stroke, was significantly associated with dementia after controlling for potential confounders, such as age, sex, coronary artery disease, diabetes, anti-hypertension drugs, stroke, anticholesterol statin drugs, depression, anxiety, benzodiazepine, anti-psychotic, and anti-depressant agents’ use (Adjusted OR = 1.33, 95% CI 1.24–1.41). Zolpidem use also has significant dose–response effects for most of the types of dementia. In patient with Alzheimer diseases, the effects of zolpidem among patients with Alzheimer's disease remained obscure. The adjusted OR for patients whose cumulative exposure doses were between 170 and 819 mg/year (adjusted OR: 1.65, 95% CI 1.08–2.51, P = 0.0199) was significant; however, the effects for lower and higher cumulative dose were not significant.
Zolpidem used might be associated with increased risk for dementia in elderly population. Increased accumulative dose might have higher risk to develop dementia, especially in patients with underlying diseases such as hypertension, diabetes, and stroke.
INTRODUCTION
Dementia is a clinical syndrome characterized by “a global deterioration of mental functioning in its cognitive, emotional, and cognitive aspects.”1 Dementia typically involves a long period of progressive decline in memory and other cognitive abilities secondary to brain dysfunction and is a major cause of disability in elderly people.2 Alzheimer disease is the most common dementing disorder, followed by vascular dementia, frontal lobe dementia, and dementia with Lewy bodies. An expert panel estimated that the global prevalence of dementia is 3.9% in people over 60 years of age, and the estimated global annual incidence of dementia is approximately 7.5 per 1000 people.3,4 The incidence of dementia ranges from approximately 1 per 100 person-years in people aged 60 to 64 years to >70 per 1000 person-years in people older than 90 years.4 Because of an increased life expectancy in modern years, the number of people suffering from dementia has rapidly increased, potentially reaching up to 63 million people by 2030.5
The risk factors for dementia include an apolipoprotein E4 genotype, cardiovascular comorbidities, diabetes mellitus, cerebrovascular diseases, alcohol consumption, and a lower education level.6 Benzodiazepine (BZD) and other non-selective γ-aminobutyric acid (GABA) agonists with hypnotic effects similar to those of zolpidem have been shown to disrupt the memory in both human participants and animal subjects.7 The results of previous studies have suggested that BZDs are associated with an increased risk of dementia in the elderly population, and these risks decreased when BZD use is discontinued.8–12 However, the possibility that zolpidem used independently of benzodiazepine derivatives, increases the risk for dementia has not been proposed.
Zolpidem and its derivatives (the Z drugs) are non-BZD hypnotic agent belonging to the imidazopyridine family. Zolpidem acts as an agonist of the benzodiazepine ω1 receptor component of the GABAA receptor complex and is commonly used in patients with insomnia, including elderly patients.13 Zolpidem is well known for having a rapid onset (usually several minutes), short duration of action (the peak time is 2 hours, half time is 1.5–5.5 hours), low tolerance, and a low incidence of adverse effects in treating insomnia.13,14 The most frequent adverse effects associated with zolpidem are nausea, headache, dizziness, drowsiness, hallucination, and short-term memory loss. A 3-week clinical trial revealed psychomotor retardation in 2% of patients receiving zolpidem and in 0% of patients in the placebo group.15 However, there are limited clinical data concerning the effects of long-term zolpidem use on psychomotor or cognitive functions. Thus, the relationship between the use of zolpidem and the potential risk of developing dementia remains unknown. In the present study, we used a national population data bank to explore the associations between zolpidem and all dementia, non-Alzheimer disease dementia, and Alzheimer disease.
MATERIALS AND METHODS
Data Source
A case–control study was conducted using data from the Taiwan National Health Insurance Research Database (NHIRD). The Taiwan National Health Insurance Research Database (NHIRD) contains reimbursement claims data from the Taiwan National Health Insurance (NHI) system, which was established in 1996 and has provided coverage for approximately 99% of the population since 1998. The National Health Research Institute (NHRI) manages the annual claims data in the NHIRD, and the Longitudinal Health Insurance Database (LHID) was established for use in medical research. The demographic data, medications, treatments (including operations), and disease diagnoses (based on the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]) of patients are recorded in the NHIRD. The health facilities enrolled in the Taiwan NHI include local clinics, community hospitals, regional hospitals, and medical centers. With the exception of some local clinics, the Taiwanese NHI includes almost all the primary, secondary, tertiary, and quaternary health care facilities in Taiwan. The LHID comprises historical claims data for 1 million patients randomly selected from the NHIRD. The NHRI encrypts the patients’ personal information for privacy protection and provides researchers with anonymous identification numbers associated with the relevant claim information, which includes the patient's sex, date of birth, registry of medical services, and medication prescriptions. The patient consent is not required for accessing the NHIRD or LHID. This study was approved by the Institutional Review Board of China Medical University in central Taiwan (CMU-REC-101–012).
Study Design
A population-based case–control study was performed. Patients 65 years and older diagnosed with dementia (ICD-9-CM 290, 294) and Alzheimer disease (331.0) in 2006 to 2010 were defined as a case group. The patients with the diagnosis with Alzheimer disease were diagnosed by the board-certified neurologists and meet the following criteria: did not have history of severe trauma, stroke, or other neurology diseases that might interfere with the cognitive functions; have decreased score in Mini-Mental State Examination and Cognitive Ability Screening Instrument, complete blood sampling studies, and imaging studies. Patients with dementia diagnosis before 2006 were excluded. The flow chart in Figure 1 demonstrates the selection process used in this study. The initial date of dementia diagnosis was set as the index date. The control group was selected from the people without dementia diagnosis in LHID observation and was 2-fold frequency matched according to sex, age (per 5 years), and index year. The major risk factor observed was zolpidem exposure. If the patients ever used zolpidem before index date, they were grouped into zolpidem used group. However, patients without zolpidem used before index date classified into non-zolpidem used group. The average zolpidem exposure dose was calculated by dividing the total zolpidem exposure (milligrams) according to the period between the first exposure and the index date (years). The average zolpidem exposure dose was separated into 3 groups according to the tertile.
FIGURE 1.

The flow chart demonstrates the selection process used in this study.
Other dementia-associated comorbidities were also considered potential confounding factors to determine associations between dementia and zolpidem. Data for the underlying comorbidities, including hypertension (ICD-9-CM 401–405), diabetes (ICD-9-CM 250), coronary artery disease (CAD) (ICD-9-CM 410–414), stroke (ICD-9-CM 430–438), hyperlipidemia (ICD-9-CM 272), depression (ICD-9-CM 296.2–296.3 and 300.4), and anxiety (ICD-9-CM 300.0, 300.2, 300.3, 308.3, and 309.81), were collected before the index date.
Drugs potentially associated with the development of dementia, including anti-hypertension agents such as calcium channel blockers (Anatomical Therapeutic Chemical [ATC] code: C08), beta-blockers (ATC code: C07), alpha-blockers (ATC code: C02), diuretics (ATC code: C03), angiotensin-converting enzyme antagonists (ACEI), angiotensin receptor blockers (ARB) (ATC code: C09), and anticholesterol statin drugs (ATC code: C10) benzodiazepine (ATC code: N05), and similar derivative drugs that were available such as zopiclone (ATC code: N05) were analyzed. We also considered effect of anti-psychotic drug used (first generation antipsychotics and second generation antipsychotics) and anti-depression drug used (including selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, heterocyclic antidepressants, and others [bupropion, venlafaxine, mirtazapine, and duloxetine]). All of these drug definitions were considered the drug use group as the patients at least once use before the index date.
Statistical Analysis
The distribution of the study population, which was based on the demographic characteristics and the disease history data, was analyzed. The χ2 test was used for categorical variables, and Student t test was used for continuous variables to evaluate the differences among the study groups. The odds ratio (OR) and 95% confidence interval (95% CI) were measured for each comparison to estimate the associations between zolpidem use and dementia using logistic regression. Adjusted odds ratios (AORs) were also determined after adjusting for potential confounders. To evaluate the dose response of the association between the average zolpidem dose and dementia, logistic regression was used, and the average zolpidem dose was treated as a continuous variable across a range of average doses to evaluate trends in dementia diagnosis. We tested the multiplicative interaction of zolpidem use and each comorbidities (or drugs) by logistic regression and presented the effect of zolpidem for dementia under the different level of each comorbidities (or drugs). All data management and statistical analyses were performed using the SAS 9.1.3 software (SAS Institute, Cary, NC). All statistical tests were 2-sided, and P values <0.05 were considered statistically significant.
RESULTS
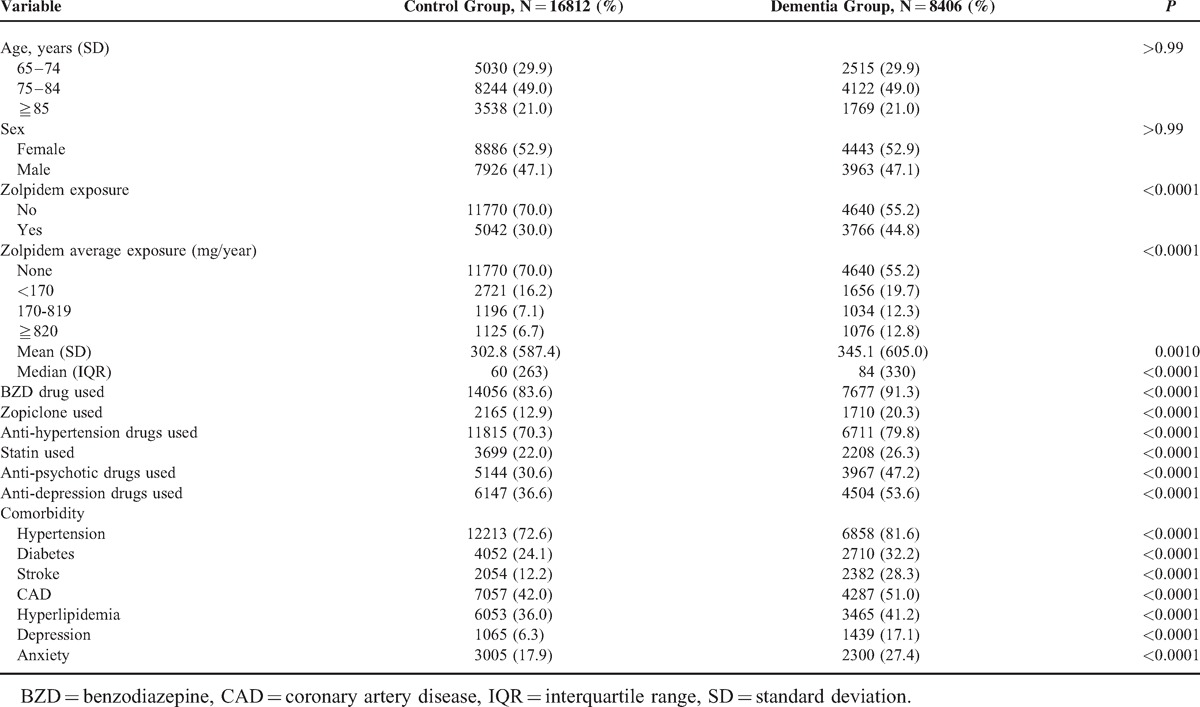
A total of 8406 dementia patient files were identified, and the control group consisted of 16,812 patients. The demographic characteristics of the study population are presented in Table 1. Comparing with the control group, the dementia patients have longer zolpidem exposure time (302.8 days vs 345.1 days, P = 0.001) and higher exposure dose, were more likely to have been exposed to zolpidem (44.8% vs 30%, P < 0.0001), zopiclone (20.3% vs 12.9%, P < 0.0001), and BZDs (91.3% vs 83.6%, P < 0.0001), to have received simultaneous treatments with anti-hypertension agents (79.8% vs 70.3%, P < 0.0001) and anti-cholesterol statin agents (26.3% vs 22%, P < 0.0001), and to have had underlying diseases, such as hypertension (81.6% vs 72.6%, P < 0.0001), diabetes (32.2% vs 24.1%, P < 0.0001), stroke (28.3% vs 12.2%, P < 0.0001), CAD (51% vs 42%, P < 0.0001), hyperlipidemia (41.2% vs 36%, P < 0.0001), depression (17.1% vs 6.3%, P < 0.0001), and anxiety disorder (27.4% vs 17.9%, P < 0.0001).
TABLE 1.
Demographic Status and Comorbidity Compared Between Control Group and Dementia Group
The logistic regression models were adjusted for potential confounders, such as age, sex, CAD, diabetes, stroke, hyperlipidemia, depression, anxiety, and the use of antihypertension agents, anticholesterol statin agents zopiclone and BZD drug uses, to evaluate the relationship between zolpidem use and dementia (Table 2). Zolpidem use and dementia remained significantly associated (dementia: AOR = 1.33, 95% CI 1.24–1.41; P < 0.0001; non-Alzheimer disease dementia: AOR = 1.33, 95% CI 1.25–1.42; P < 0.0001), although the relationship effects between the zolpidem use and Alzheimer disease were not significant (Alzheimer disease: AOR = 1.17, 95% CI 0.90–1.54, P = 0.2410).
TABLE 2.
Effects of Zolpidem Exposure on Dementia in Individuals in Different Average Cumulative Exposure Doses
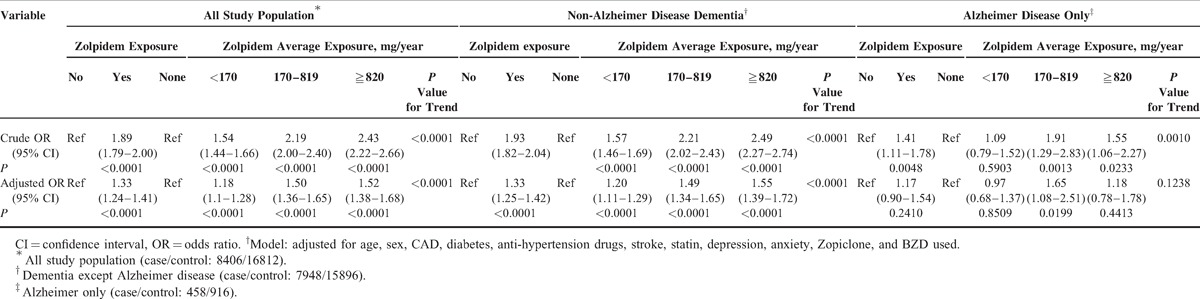
The average cumulative zolpidem doses were analyzed to identify dose effects. Zolpidem use still has significant dose–response effects for most of types of dementia except Alzheimer disease (dementia: <170 mg/y [AOR = 1.18, 95% CI 1.11–1.28], 170–819 mg/y [AOR = 1.50, 95% CI 1.36–1.65], ≧820 mg/y [AOR = 1.52, 95% CI 1.38–1.68], P value for trend <0.001; non-Alzheimer disease dementia: <170 mg/y [AOR = 1.20, 95% CI 1.11–1.29], 170–819 mg/y [AOR = 1.49, 95% CI 1.34–1.65], ≧820 mg/y [AOR = 1.55, 95% CI 1.39–1.72], P value for trend <0.001; and Alzheimer disease: <170 mg/y [AOR = 0.97, 95% CI 0.68–1.37], 170–819 mg/y [AOR = 1.65, 95% CI 1.08–2.51], ≧820 mg/y [AOR = 1.15, 95% CI 0.78–1.78], P value for trend = 0.4413).
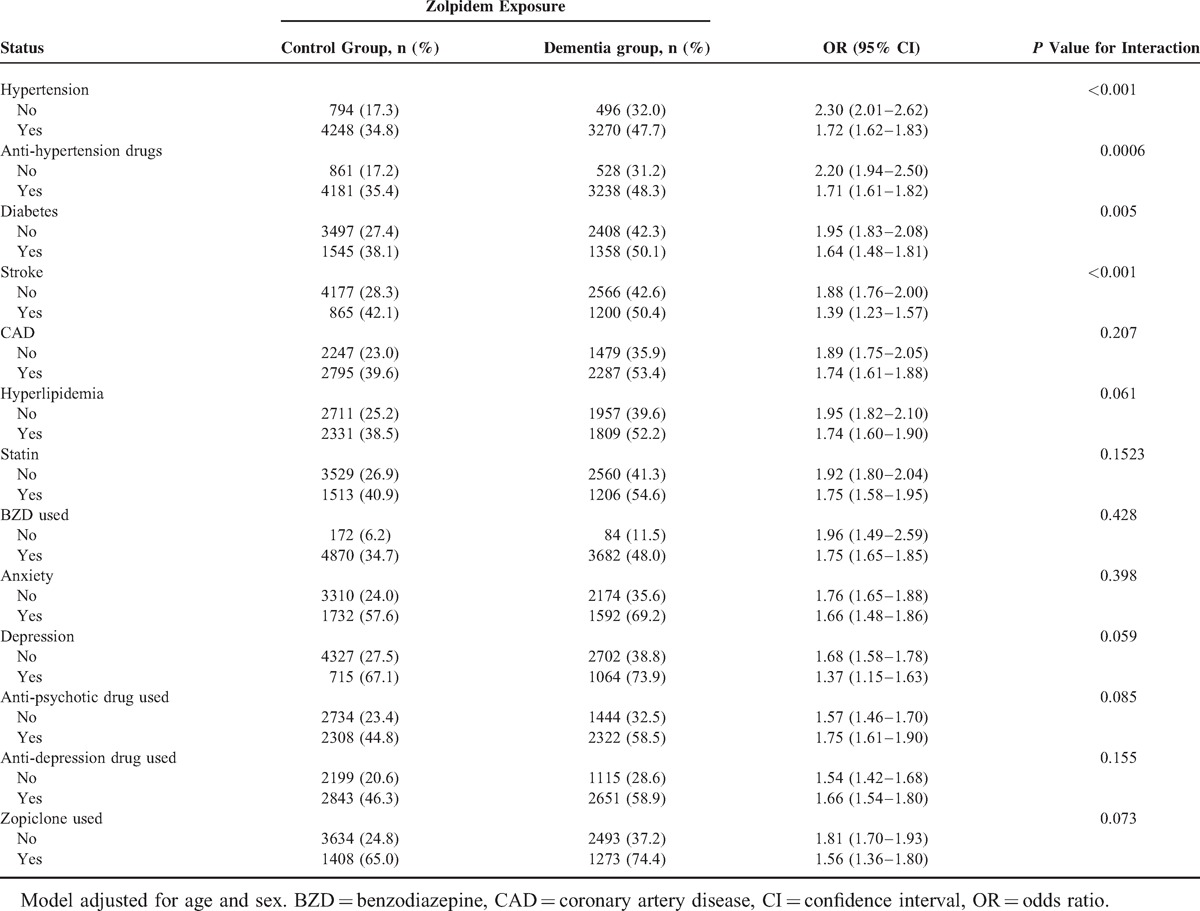
Potential modifying effects for comorbidities that might interfere with the association between dementia and zolpidem were analyzed (Table 3). Of the patients with zolpidem exposure, although effect modifiers such as hypertension, diabetes, stroke, CAD, hyperlipidemia, and anxiety, depression, anti-psychotic agent, and anti-depressant use had positive effects for dementia (hypertension: AOR = 1.72, 95% CI 1.62–1.83; diabetes: AOR = 1.64, 95% CI 1.48–1.81; stroke: AOR = 1.39, 95% CI 1.23–1.57; CAD: AOR = 1.74, 95% CI 1.61–1.88; hyperlipidemia: AOR = 1.74, 95% CI 1.60–1.90; anxiety: AOR = 1.66, 95% CI 1.48–1.86; depression: AOR = 1.37, 95% CI 1.15–1.63), zolpidem exposure alone also has more positive effects on dementia in most circumstances (non-hypertension: AOR = 2.30, 95% CI 2.01–2.62; non-diabetes: AOR = 1.95, 95% CI 1.83–2.08; non-stroke: AOR = 1.88, 95% CI 1.76–2.00; non-CAD: AOR = 1.88, 95% CI 1.76–2.00; non-hyperlipidemia: AOR = 1.95, 95% CI 1.82–2.10; non-statin: AOR = 1.92, 95% CI 1.80–2.04; non-BZD: AOR = 1.96, 95% CI 1.49–2.59; non-anxiety: AOR = 1.76, 95% CI 1.65–1.88; non-depression: AOR = 1.68, 95% CI 1.58–1.78). Patients receiving zolpidem with anti-psychotic or anti-depressant agents at the same time had more positive effects on dementia risk (anti-psychotic agents vs non-antipsychotic agents: AOR = 1.75 [95% CI 1.61–1.90] vs. 1.57 [95% CI 1.46–1.70]; anti-depressant vs non-antidepressant agents: AOR = 1.66 [95% CI 1.54–1.80] vs 1.54 [95% CI 1.42–1.68]). Although large numbers of patients were co-prescribed zolpidem and BZD derivatives, the effects of interactions between zolpidem and BZD derivatives were not significant.
TABLE 3.
Interactions Between Zolpidem Exposure and Comorbidities That Were Associated With Dementia Risk
DISCUSSION
The present population-based case–control study suggests that zolpidem’ use might increase the risk of developing dementia in the elderly population. The accumulative dose of zolpidem, alone, or with other underlying diseases, such as hypertension, diabetes, and stroke, was significantly associated with dementia after controlling for potential confounders, such as age, sex, CAD, diabetes, antihypertension drugs, stroke, anticholesterol statin drugs, depression, anxiety, and BZD use; however, the effects of zolpidem on patients with Alzheimer disease remained obscure. The adjusted odds ratio for patients whose cumulative exposure doses were between 170 and 819 mg/year (adjusted OR: 1.65, 95% CI 1.08–2.51, P = 0.0199) was significant; however, the effects for lower and higher cumulative dose were not significant.
The etiology for dementia is complex.16,17 Alzheimer disease is the leading subtype of dementia and has been identified as a protein degenerative disease that is primarily caused by the accumulation of abnormally folded amyloid beta and phosphorylated tau proteins in the brain.18,19 Clinical presentations, histories, neurological examinations, images, and biomarkers are required to diagnose Alzheimer disease. Dementias other than Alzheimer disease (non-Alzheimer dementias) comprise heterogeneous diseases, including vascular dementia, mixed dementia, Parkinson disease, dementia with Lewy bodies, Huntington disease, Creutzfeldt–Jakob disease, and frontotemporal dementia/Pick disease.16 Interactions between other factors, such as hypercholesterolemia, hypertension, atherosclerosis, coronary heart disease, smoking, obesity, diabetes, and sleep deprivation or sleep fragmentation, were reported to be risk factors that might contribute to dementia.19–21 However, few studies have addressed the potential long-term psychoneurological effects of drugs on non-Alzheimer dementia or Alzheimer disease.8,9,22–24
Zolpidem is an effective non-BZD drug that is primarily used for treating insomnia in the elderly population.25,26 Previous clinical trials have demonstrated the effects of zolpidem on the central nervous system (eg, confusion, impaired cognitive and motor function, postural sway, and ataxia), and an increased risk of falling has also been observed.27–29 Zolpidem has also been shown to disrupt memory in human patients and animal subjects.7 Comparable dose-related impairment was also observed in healthy participants asked to perform cognitive-related tests, such as picture recall, digit entry and recall, a digit symbol substitution test, repeated acquisition and circular lights tasks, and balance tests.30 The cognition-impairing effects in executive function tasks were characterized in animal models before. Previous study results suggested that zolpidem displays a high affinity for α1 subunit of GABAA receptors; therefore, the drug impairs the human cognitive domain in the prefrontal cortical areas, which are involved in processes such as goal forming, planning, initiation, preservation and alteration of goal-directed behaviors, problem-solving, response inhibition, and cognitive flexibility.31,32 Because the aging brain has increased α1 subunit of GABAA receptor–binding sites and α1 subunit of GABAA receptor mRNA levels, the cognition-impairing effects of zolpidem might reflect the elderly population's increased risk of developing non-Alzheimer dementia.33,34 In contrast, the α1 subunit of GABAA receptor protein and mRNA levels were reduced in the brain cortex regions of Alzheimer disease patients.35,36 The different compositions indicate that increases or decreases in the proportion of receptors containing a particular α subunit may reflect the affinity of the receptor for zolpidem, resulting in altered drug responses between patients of non-Alzheimer dementias and Alzheimer disease in the elderly population.
Whether cognitive effects on zolpidem could extend to other Z drugs still remain obscure. Previous studies suggested zaleplon had a rapid elimination and had fewer residual side effects after taking a single dose at bedtime. By comparison, zolpidem and zopiclone have a more delayed elimination than zaleplon. The differences in potency based on plasma concentrations suggested that there are differences in binding to the GABA receptor complex. For example, zopiclone interacts with both benzodiazepine ω1and ω2 GABAA receptors, and zolpidem mainly acts on benzodiazepine ω1 GABAA receptors.37 Therefore, there may be a prolonged drug effect and result in residual sedation and side effects. The Z drugs undergoes hepatic metabolism by cytochrome P450.14 The delayed clearances of the Z drugs were significantly decreased in patients with preexisting advanced liver diseases. Furthermore, variations in genetic polymorphism of the cytochrome P450 in different population might result in different responses as well. A study has suggested that the CYP3A4 and CYP2C19 genetic polymorphisms are associated with the poor metabolism of zolpidem in the Chinese Han population.38
A limited number of human studies have explored the relationship between neuropsychological drugs and the development of dementia or Alzheimer disease. A meta-analysis setting out to ascertain which domains of cognitive function were influenced among the Z drugs indicated very few studies evaluate the individual cognitive effects of the Z drugs. Most of the studies focused on the next-day residual cognitive effects of the Z drugs following nocturnal administration.39 The Z drugs have been heralded as a new frontier with consistent increase in the prescription in the pharmacological treatments available for insomnia patients due to the adverse and deleterious effects associated with long-term benzodiazepine use. There needs to be more thorough investigations into the possible effects on the daily functioning of individuals who take these medications. The results of the present study indicate the potential associations regarding the cumulative dose and interactions among commonly prescribed tranquilizers, zolpidem, underlying comorbidities, and the development of dementia or Alzheimer disease in the elderly population. Although detailed observations and medical records were not obtained and effect modifiers and confounders were not completely controlled, correlations between zolpidem and dementia in the elderly population were observed.
LIMITATIONS
The limitations of the present study include the limitations of the clinical data collected from the NHIRD, the difficulty associated with controlling for confounding factors in a retrospective study design, and the incomplete verification of the data in the NHIRD. The NHIRD does not provide detailed information regarding smoking habits, alcohol consumption, substance use, body mass index, physical activity, socioeconomic status, family history, and detailed medical records such as sleep quality records, reasons for zolpidem withdrawal, or intermittent used, which are potential confounding factors for this analysis.6 Furthermore, some patients in control group might present prodromal symptoms like nxiety, depression, or insomnia before dementia diagnosis was made and led to misclassification and selection bias. The registries in the NHI claims system were primarily designed for administrative billing, and the registry data are not subjected to the stringent levels of verification appropriate for many types of scientific research. There was also no method for directly contacting the patients to obtain additional information on the use of zolpidem because the participants remained anonymous. However, the data from the NHIRD regarding prescriptions and the diagnosis of major underlying diseases and dementia are highly reliable. Qualified neurologists performed a series of neurological examinations. Because of the limitations of the NHIRD, the prescription records for zolpidem before 1996 were not acquired for analysis; therefore, shorter follow-up examinations and lower cumulative doses for zolpidem were calculated, and the risk associations between zolpidem and dementia might also be underestimated. Future studies, such as population-based unbiased randomized observational trials, are warranted to confirm the causal relationships between zolpidem use and dementia.
In conclusion, zolpidem use might be associated with an increased risk for dementia in the elderly population. An increased accumulative dose might result in a significantly higher risk to develop dementia in patients with underlying diseases, such as hypertension, diabetes, and stroke. The long-term effects of zolpidem on patients with Alzheimer disease might not be significant in the elderly population. Therefore, the careful evaluation of the indications of zolpidem use and close follow-up examinations of the cognitive status of elderly patients receiving zolpidem are essential.
Acknowledgments
None.
Footnotes
Abbreviations: AORs = adjusted odds ratios, ATC = anatomical therapeutic chemical, BZD = benzodiazepine, CI = confidence interval, GABA = γ-aminobutyric acid, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHIRD = National Health Insurance Research Database = OR, odds ratio.
Contributors: conception/design: H-IS, C-HK; provision of study materials: Y-FT, C-MC, H-CH, C-HC; collection and/or assembly of data: C-CL, C-HK; data analysis and interpretation: H-IS, C-HK;
manuscript writing: All authors; final approval of manuscript: all authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212–113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103–2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212–124–002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors report no conflicts of interest.
REFERENCES
- 1.Park J, Ko HJ, Park YN, et al. Dementia among the elderly in a rural Korean community. Br J Psychiatry 1994; 164:796–801. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. 2010 Alzheimer's disease facts and figures. Alzheimers Dement 2010; 6:158–194. [DOI] [PubMed] [Google Scholar]
- 3.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366:2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry 2007; 20:380–385. [DOI] [PubMed] [Google Scholar]
- 5.Wimo A, Winblad B, Aguero-Torres H, et al. The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord 2003; 17:63–67. [DOI] [PubMed] [Google Scholar]
- 6.Povova J, Ambroz P, Bar M, et al. Epidemiological of and risk factors for Alzheimer's disease: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2012; 156:108–114. [DOI] [PubMed] [Google Scholar]
- 7.Beracochea D. Anterograde and retrograde effects of benzodiazepines on memory. Sci World J 2006; 6:1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallacher J, Elwood P, Pickering J, et al. Benzodiazepine use and risk of dementia: evidence from the Caerphilly Prospective Study (CaPS). J Epidemiol Community Health 2012; 66:869–873. [DOI] [PubMed] [Google Scholar]
- 9.Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ 2012; 345:e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagnaoui R, Bégaud B, Moore N, et al. Benzodiazepine use and risk of dementia: a nested case-control study. J Clin Epidemiol 2002; 55:314–318. [DOI] [PubMed] [Google Scholar]
- 11.Wu CS, Ting TT, Wang SC, et al. Effect of benzodiazepine discontinuation on dementia risk. Am J Geriatr Psychiatry 2011; 19:151–159. [DOI] [PubMed] [Google Scholar]
- 12.Wu CS, Wang SC, Chang IS, et al. The association between dementia and long-term use of benzodiazepine in the elderly: nested case-control study using claims data. Am J Geriatr Psychiatry 2009; 17:614–620. [DOI] [PubMed] [Google Scholar]
- 13.Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000; 59:865–889. [DOI] [PubMed] [Google Scholar]
- 14.Barkin RL. Zolpidem extended-release: a single insomnia treatment option for sleep induction and sleep maintenance symptoms. Am J Ther 2007; 14:299–305. [DOI] [PubMed] [Google Scholar]
- 15.LLC s-aUS. AMBIEN CR( Prescribing Information. 2013; http://products.sanofi.us/ambien_cr/ambiencr.html Accessed 10/July, 2013. [Google Scholar]
- 16.Chen JH, Lin KP, Chen YC. Risk factors for dementia. J Formos Med Assoc 2009; 108:754–764. [DOI] [PubMed] [Google Scholar]
- 17.McCullagh CD, CraigF D, McIlroy SP. Risk factors for dementia. Adv Psychiatr Treat 2001; 7:24–31. [Google Scholar]
- 18.Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci 2003; 26:81–104. [DOI] [PubMed] [Google Scholar]
- 19.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet 2006; 368:387–403. [DOI] [PubMed] [Google Scholar]
- 20.Exalto LG, Whitmer RA, Kappele LJ, et al. An update on type 2 diabetes, vascular dementia and Alzheimer's disease. Exp Gerontol 2012; 47:858–864. [DOI] [PubMed] [Google Scholar]
- 21.Lim AS, Kowgier M, Yu L, et al. Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep 2013; 36:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdoux H, Lagnaoui R, Begaud B. Is benzodiazepine use a risk factor for cognitive decline and dementia? A literature review of epidemiological studies. Psychol Med 2005; 35:307–315. [DOI] [PubMed] [Google Scholar]
- 23.Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs aging 1999; 15:15–28. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara M, Yamada M. Drug-induced cognitive impairment. Brain Nerve 2012; 64:1405–1410. [PubMed] [Google Scholar]
- 25.Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ 2012; 345:e8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med 2003; 33:1223–1237. [DOI] [PubMed] [Google Scholar]
- 27.Wang PS, Bohn RL, Glynn RJ, et al. Zolpidem use and hip fractures in older people. J Am Geriatr Soc 2001; 49:1685–1690. [DOI] [PubMed] [Google Scholar]
- 28.Kolla BP, Lovely JK, Mansukhani MP, et al. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med 2013; 8:1–6. [DOI] [PubMed] [Google Scholar]
- 29.Kang DY, Park S, Rhee CW, et al. Zolpidem use and risk of fracture in elderly insomnia patients. J Prev Med Public Health 2012; 45:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rush CR. Behavioral pharmacology of zolpidem relative to benzodiazepines: a review. Pharmacol Biochem Behav 1998; 61:253–269. [DOI] [PubMed] [Google Scholar]
- 31.Makaron L, Moran CA, Namjoshi O, et al. Cognition-impairing effects of benzodiazepine-type drugs: role of GABAA receptor subtypes in an executive function task in rhesus monkeys. Pharmacol Biochem Behav 2013; 104:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010; 9:1200–1213. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez A, Khan ZU, Ruano D, et al. Aging-related subunit expression changes of the GABAA receptor in the rat hippocampus. Neuroscience 1996; 74:341–348. [DOI] [PubMed] [Google Scholar]
- 34.Ruano D, Benavides J, Machado A, et al. Aging-associated changes in the pharmacological properties of the benzodiazepine (omega) receptor isotypes in the rat hippocampus. J Neurochem 1995; 64:867–873. [DOI] [PubMed] [Google Scholar]
- 35.Rissman RA, Bennett DA, Armstrong DM. Subregional analysis of GABA(A) receptor subunit mRNAs in the hippocampus of older persons with and without cognitive impairment. J Chem Neuroanat 2004; 28:17–25. [DOI] [PubMed] [Google Scholar]
- 36.Mizukami K, Ikonomovic MD, Grayson DR, et al. Immunohistochemical study of GABAA receptor alpha1 subunit in the hippocampal formation of aged brains with Alzheimer-related neuropathologic changes. Brain Res 1998; 799:148–155. [DOI] [PubMed] [Google Scholar]
- 37.Drover DR. Comparative pharmacokinetics and pharmacodynamics of short-acting hypnosedatives: zaleplon, zolpidem and zopiclone. Clin Pharmacokinet 2004; 43:227–238. [DOI] [PubMed] [Google Scholar]
- 38.Shen M, Shi Y, Xiang P. CYP3A4 and CYP2C19 genetic polymorphisms and zolpidem metabolism in the Chinese Han population: a pilot study. Forensic Sci Int 2013; 227:77–81. [DOI] [PubMed] [Google Scholar]
- 39.Stranks EK, Crowe SF. The acute cognitive effects of zopiclone, zolpidem, zaleplon, and eszopiclone: a systematic review and meta-analysis. J Clin Exp Neuropsychol 2014; 36:691–700. [DOI] [PubMed] [Google Scholar]


