Abstract
Several studies have indicated that air pollution induces systemic as well as tissue-specific inflammation. Chronic inflammatory diseases such as rheumatoid arthritis and chronic obstructive pulmonary disease reduce bone mineral density (BMD), leading to increased release of immune cells from the bone marrow. However, the association between air pollution and osteoporosis remains poorly defined. Therefore, we conducted this population-based retrospective cohort study to evaluate the risk of osteoporosis in Taiwanese residents exposed to air pollution.
We combined 2 nationwide databases in this study. The National Health Insurance Research Database of Taiwan was available from 2000 to 2010. Detailed daily data on air pollution were collected by Taiwan Environmental Protection Agency (EPA) from 1998 to 2010. We calculated the yearly average concentrations of air pollutants from the study start to the date of osteoporosis occurrence, or withdrawal from the NHI program, or December 31, 2010. The yearly average concentrations of air pollutants were categorized into quartiles, and the risks of osteoporosis were evaluated among 4 stages of air pollutants.
Among Q1, Q2, Q3, and Q4 of pollutants in all subjects, the adjusted hazard ratios (HRs) of osteoporosis in Q2, Q3, and Q4 were compared with Q1. For carbon monoxide (CO), the adjusted HRs were 1.05 (95% confidence interval [CI], 0.97–1.14), 1.78 (95% CI, 1.65–1.92), and 1.84 (95% CI, 1.71–1.98), respectively. For nitrogen dioxide (NO2), the adjusted HRs were 1.35 (95% CI, 1.25–1.45), 1.24 (95% CI, 1.15–1.35), and 1.60 (95% CI, 1.48–1.73), respectively, in all subjects.
The findings of the present study show that CO and NO2 exposure is associated with an increased risk of osteoporosis in the Taiwanese population.
INTRODUCTION
Acute and chronic air pollution exposure is associated with the risk of respiratory and cardiovascular morbidity and mortality.1–4 Several studies have indicated that air pollution also induces systemic as well as tissue-specific inflammation.5,6 Chronic inflammatory diseases such as rheumatoid arthritis and chronic obstructive pulmonary disease reduce bone mineral density (BMD), leading to increased release of immune cells from the bone marrow.7,8 A Mexican study suggested that children exposed to air pollution had higher interleukin 6 (IL-6) concentrations than unexposed children, but exhibited no significant change in BMD.9 The associations between cigarette smoking and BMD or bone mineral content are also well established.10–14 A study conducted in Oslo revealed a significant association between air pollution and BMD in men aged 75 to 76 years.15 Another study on elderly men from Oslo suggested that the reduction in BMD was associated with exposure to particulate matter.16 However, the association between air pollution and osteoporosis remains poorly defined. Therefore, we conducted this population-based retrospective cohort study to evaluate the risk of osteoporosis in Taiwanese residents exposed to air pollution.
MATERIALS AND METHODS
Data Source
This retrospective cohort study was used the Longitudinal Health Insurance Database (LHID) and Taiwan Air Quality Monitoring Database (TAQMD). LHID contained 1 million insurant randomly selected from the original 2000 Registry for beneficiaries joining in the Taiwan National Health Insurance program. This program was set up by Taiwan Bureau of National Health Insurance (TBNHI) in March 1995 and covered over 99% Taiwan residents. LHID included all medical records from the start of 1996 to the end of 2010. The identification of insurant was re-coded before it had been released to researchers because of the Personal Information Protection Act. This study was also approved by the Institutional Review Board of China Medical University, Taiwan. To identify the disease in LHID was according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
TAQMD was set up by Taiwan Environmental Protection Administration Executive Yuan and included daily concentrations of carbon monoxide (CO) and nitrogen dioxide (NO2) in 1998 to 2010 from 74 ambient air quality-monitoring stations, which were distributed over Taiwan. Two databases were linked by the insurant living area and the air quality-monitoring stations location. The living area for the insured persons was defined based on the sought treatment for acute upper respiratory tract infection (AURTI) (ICD-9-CM code 460).
Study Subject, Exposure Measurement, and Comorbidity
We selected people living in areas with the air quality-monitoring stations in this study. Patients with osteoporosis history before the year of 2000 were excluded and they were followed from the start of 2000 to the date for osteoporosis development. People without osteoporosis development were followed to the date for withdrew from the program or the end of 2011.17 The yearly average pollutants’ concentrations for each study subject were calculated from 1998 until the end of observation year. Air pollutant concentrations were grouped into 4 levels based on quartile: CO concentration (Q1: <200.9, Q2: 200.9–248.4, Q3: 248.5–295.9, and Q4: >295.9 ppm) and NO2 concentration (Q1: <6600.8, Q2: 6600.8–8339.2, Q3: 8339.3–9825.1, and Q4: >9825.1 ppb). Comorbidity contained diabetes mellitus (DM, ICD-9-CM code 250), ischemic heart disease (IHD, ICD-9-CM codes 410–414), hypertension (HT, ICD-9-CM codes 401–405), chronic obstructive pulmonary disease (COPD, ICD-9-CM codes 490–496), alcoholism (ICD-9-CM codes 303, 305.0, and V113), hyperlipidemia (ICD-9-CM code 272), and estrogen supplement in women.
Statistical Analysis
To test the distributed difference for sex, insurance fee (<14,400, 14,400–18,300, 18,301–21,000, and >21,000 New Taiwan Dollar), urbanization, and comorbidity among air pollutant concentration levels, χ2 test was used. One-way analysis of variance (ANOVA) test was used to test the different of mean age among different air pollutant concentration levels. The incidence of osteoporosis (per 1000 person-years) was counted in different air pollutant concentration levels. Cox proportional hazard regression was used to estimate the hazard ratios (HRs) and 95% confidence interval (CIs) for osteoporosis in Q2–Q4 level for air pollutant concentration compared the lowest one (Q1). Multivariable model was adjusted for age, sex, insurance fee, urbanization, and comorbidity. Kaplan–Meier analysis was used to plot the osteoporosis-free rate curve and log-rank test was used to test the difference among air pollutant concentration levels. All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC) and the Statistical Package for the Social Science (Version 15.1; SPSS Inc, Chicago, IL). All statistical tests were considered statistically significant when 2-tailed P values were <0.05.
RESULTS
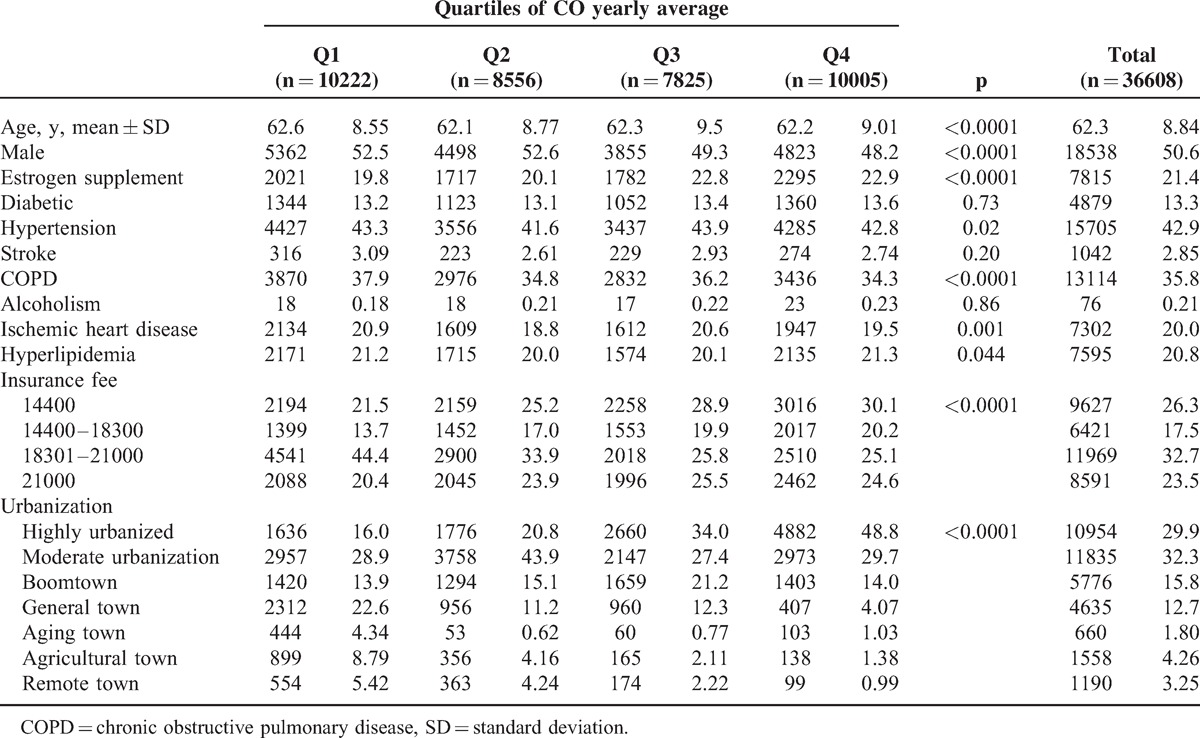
According to the location of the Taiwan air quality monitoring station, we collected the data of 36,608 and 36,561 patients without osteoporosis history under conditions of CO and NO2 exposure, respectively. We categorized the CO and NO2 concentrations into 4 levels based on quartiles, ranging from Q1 (the lowest concentration) to Q4 (the highest concentration). The mean age in CO-exposed patients was 62.3 years (SD = 8.84) (Table 1). The proportion of men and women were similar (50.6% vs 49.4%). Women receiving estrogen supplements were more likely exposed to higher CO level. More patients with hypertension were exposed to Q3 level, more patients with COPD and IHD were exposed to the lowest level, and more hyperlipidemia patients were exposed to the highest level. In the Q4 group, more people had lower incomes and lived areas with higher urbanization.
TABLE 1.
Comparison of Baseline Characteristics Among Quartiles of CO Yearly Average
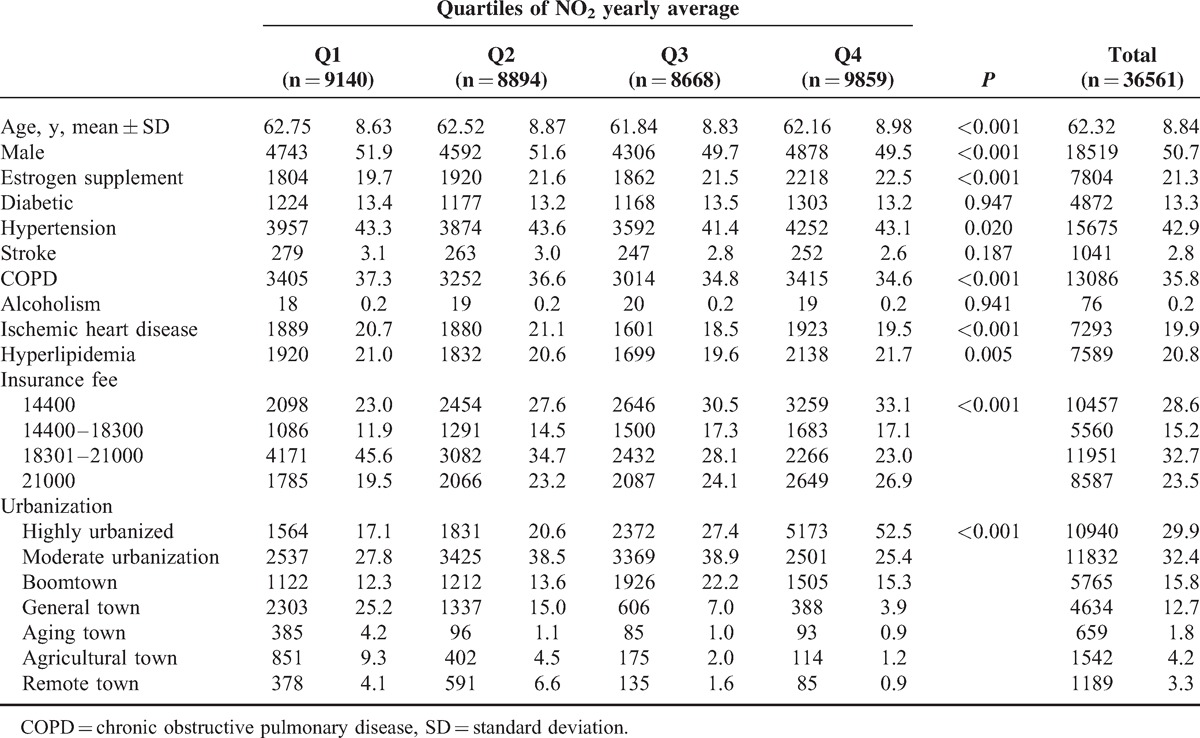
The mean age in the NO2 exposure subjects was 62.3 years’ old (SD = 8.84) (Table 2). Men were more likely exposed to lower NO2 level, but more women undergoing estrogen supplement treatment were exposed to higher NO2 level. More patients with hypertension and ISD were exposed to Q2 level, those with COPD were exposed to lowest level, and those with hyperlipidemia were exposed to highest level. People with highest NO2exposure concentrations were more likely to have lower incomes and live in areas with higher urbanization.
TABLE 2.
Comparison of Baseline Characteristics Among Quartiles of NO2 Yearly Average
The incidence for osteoporosis increased with CO and NO2 exposure concentration, increasing from 13.58 to 22.25 and from 14.33 to 20.37 per 1000 person-years, respectively (Table 3). After 11 follow-up years, the osteoporosis-free rate for people living in areas with lower CO concentration (Q1 and Q2) was approximately 6.5% higher than those living areas with higher CO concentration (Q3 and Q4) (Figure 1). The osteoporosis-free rate among people with the lowest NO2 exposure concentration was 5% higher than those with highest NO2 exposure concentration. In the multivariable Cox proportional hazard regression, the risk for osteoporosis increased with the CO and NO2 exposure concentrations from 1.05 to 1.84 and from 1.35 to 1.60, respectively, compared with those exposed to the corresponding concentrations in Q1 level (Table 3). Regardless of sex, people with highest-level exposure to CO or NO2 exhibited the highest risk for osteoporosis compared with those with lowest level.
TABLE 3.
Comparisons of Difference Osteoporosis Incidences and Associated Hazard Ratios Among 4 Levels of Air Pollutants by Sex Stratification
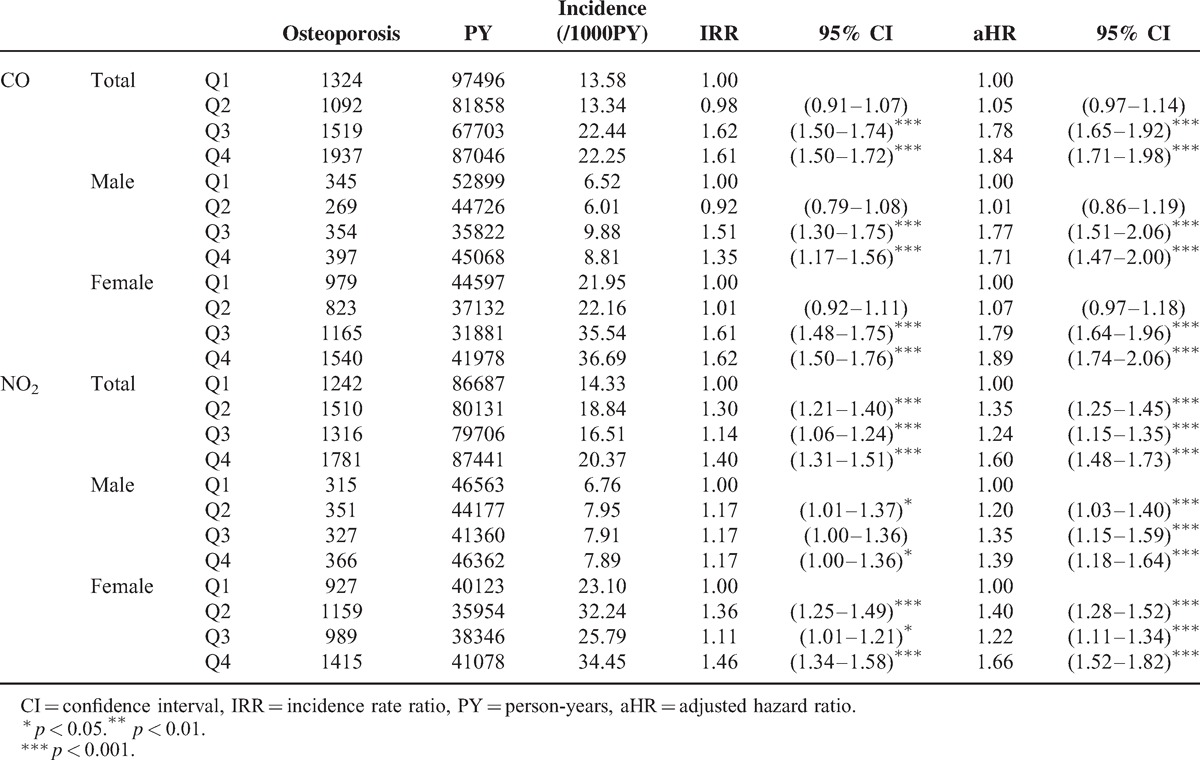
FIGURE 1.
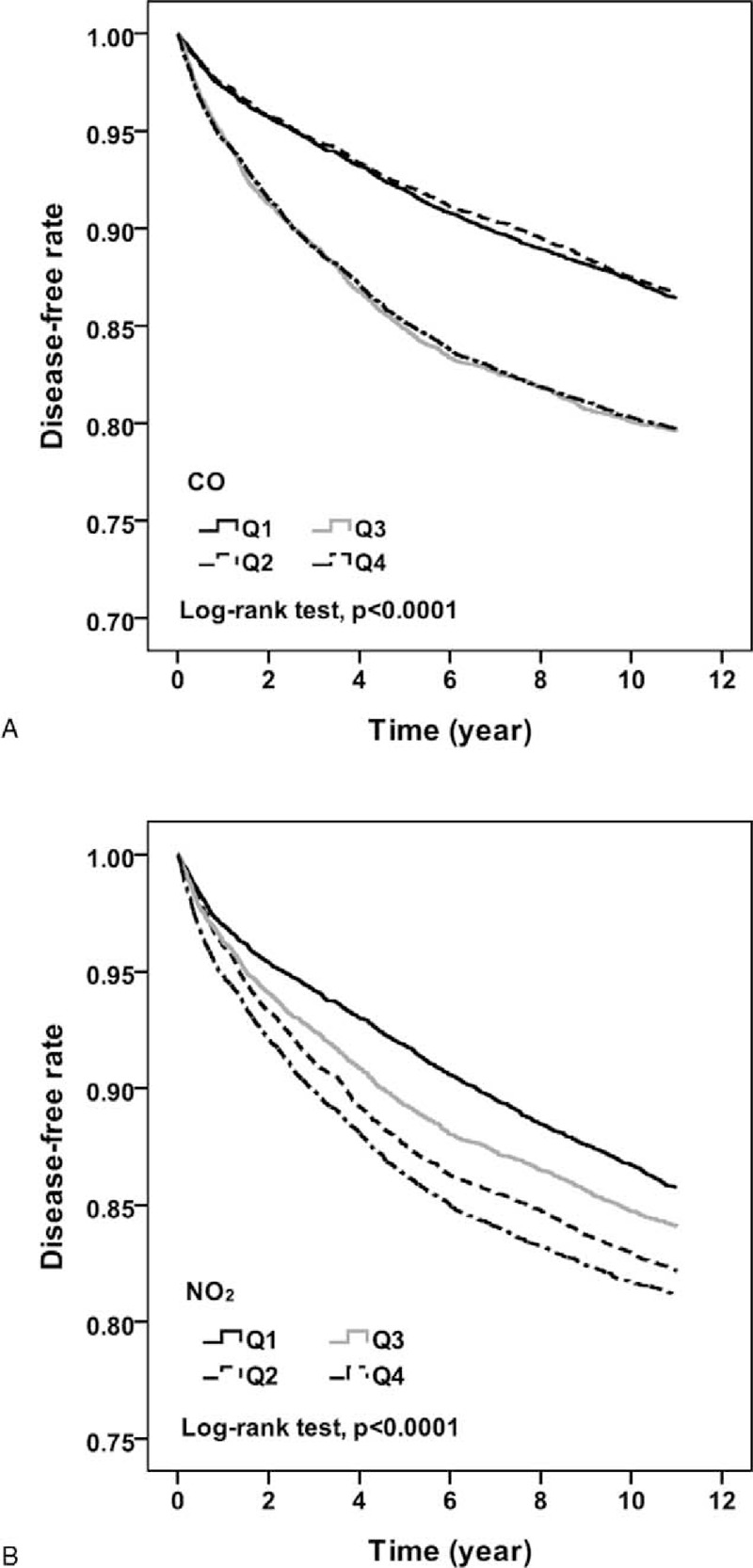
The Kaplan–Meier curves of freedom for osteoporosis are separated by different pollutant concentrations.
DISCUSSION
The major findings of this study were the positive associations between risk of osteoporosis and concentrations of air pollutants in both men and women. Several previous studies have indicated that exposure to air pollutants such as NO2 might induce systemic inflammation.18,19 In bone metabolism, systemic inflammation regulates immune responses and has osteoclastic effects through increased bone-resorption by IL-6.9,20 A previous showed that a low concentration of CO (250 ppm)exposure would inhibit osteoclast genesis and decrease osteoclast-mediated bone erosion.21 Similar results are shown in Table 2, although the CO concentration was a cumulative dose. Therefore, further study is warranted.
In other air studied related to air pollution, the investigators defined the active area of subjects based on geographic information system or insurance area.22,23 In this study, we defined the active areas of the subjects according to the location of the clinics for most frequently sought treatment for AURTI. This definition method was used in our previous study.17
In addition to aging, estrogen deficiency is a critical factor for osteoporosis in women. However, osteoporosis etiology in elderly men was relatively unclear.24 Therefore, we performed a statistical analysis stratified by sex, and considered the effect of estrogen supplements in women.
There were several reasons for COPD adjustment. First, osteoporosis has been one of the most common comorbidities in COPD.25–27 Second, cigarette smoke is the most important risk factor of COPD, and it also induces the osteoporosis.28–32 In addition, alcohol consumption has consistently been recognized as a critical factor of osteoporosis.33,34 Because of the lack of information on healthy behaviors in NHIRD, we considered COPD and alcoholism instead of cigarette smoke and alcohol consumption in the Cox proportional hazard regression. Furthermore, we used urbanization as a covariate in multivariate analysis model in accordance with the suggestion about the bone mineral density of residents was significant difference between rural andurban.35
We observed conflicting results regarding the subjects in the Q4 group who did not have the highest prevalence of comorbidity in Tables 1 and 3. It was more likely consistent with the age distribution. The highest prevalence of comorbidities appeared in areas with the highest air pollution level and oldest subjects.
This retrospective population-based cohort study combined 2 nationwide databases. Although the difference in urbanization level among towns throughout Taiwan was considered, potential bias may have resulted from defining the active area according to the location of medical institutions where residents sought AURTI treatment. Although we rejected residents with no medical record related to AURTI between 2000 and 2010, healthy residents are more likely to be exposed to the lowest level of air pollution. This might lead to the underestimation of osteoporosis risk. We have adjusted many covariates, such as age, sex, urbanization, hyperlipidemia, estrogen supplement usage in women, cerebrovascular disease, and cardiovascular disease, However, because of the various causes of osteoporosis and the limitation of the NHIRD, it was not feasible to consider all of the confounders, such as diet, exercise, related medication, endocrine disease, and gastrointestinal disease, that are likely to be associated with osteoporosis occurrence.
In summary, we observed an increasing trend in the relationship between air pollutant stage and the risk of osteoporosis in both men and women. Exposure to the highest level of air pollutants might increase 39% to 89% risk of osteoporosis. Despite the research limitations, the findings were considered reliable and worthy for conducting further studies.
Footnotes
Abbreviations: BMD = bone mineral density, CIs = confidence intervals, CO = carbon monoxide, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, HRs = hazard ratios, HT = hypertension, ICD-9-CM = International Classification of Diseases, IHD = ischemic heart disease, IRR = incidence rate ratio, NHIRD = National Health Insurance Research Database, Ninth Revision = Clinical Modification, NO2 = nitrogen dioxide.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212–113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103–2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212–124–002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Author contributions: All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript: Study concept and design: K-HC, C-HK; acquisition of data: all authors; analysis and interpretation of data: K-HC, C-HM, C-HK; drafting of the manuscript: all authors; critical revision of the manuscript for important intellectual content: K-HC, C-HM, C-HK; statistical analysis: C-HM; obtained funding: C-HK; administrative, technical, or material support: all authors; study supervision: C-HK.
All of the authors declare no conflicts of interest.
REFERENCES
- 1.Oftedal B, Brunekreef B, Nystad W, et al. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology 2008; 19:129–137. [DOI] [PubMed] [Google Scholar]
- 2.Lisabeth LD, Escobar JD, Dvonch JT, et al. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol 2008; 64:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turin TC, Kita Y, Rumana N, et al. Ambient air pollutants and acute case-fatality of cerebro-cardiovascular events: Takashima Stroke and AMI Registry, Japan (1988∗–∗2004). Cerebrovasc Dis 2012; 34:130–139. [DOI] [PubMed] [Google Scholar]
- 4.Andersen ZJ, Kristiansen LC, Andersen KK, et al. Stroke and long-term exposure to outdoor air pollution from nitrogen dioxide: a cohort study. Stroke 2012; 43:320–325. [DOI] [PubMed] [Google Scholar]
- 5.Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 2009; 32:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunzli N, Jerrett M, Garcia-Esteban R, et al. Ambient air pollution and the progression of atherosclerosis in adults. PLoS One 2010; 5:e9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SH, Kim TS, Choi Y, et al. Osteoimmunology: cytokines and the skeletal system. BMB Rep 2008; 41:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev 2008; 29:403–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderón-Garcidueñas L, Mora-Tiscareño A, Francolira M, et al. Exposure to urban air pollution and bone health in clinically healthy six-year-old children. Arh Hig Rada Toksikol 2013; 64:23–34. [DOI] [PubMed] [Google Scholar]
- 10.Kim KH, Lee CM, Park SM, et al. Secondhand smoke exposure and osteoporosis in never-smoking postmenopausal women: the Fourth Korea National Health and Nutrition Examination Survey. Osteoporos Int 2013; 24:523–532. [DOI] [PubMed] [Google Scholar]
- 11.Holmberg T, Bech M, Curtis T, et al. Association between passive smoking in adulthood and phalangeal bone mineral density: results from the KRAM study-the Danish Health Examination Survey 2007–2008. Osteoporos Int 2011; 22:2989–2999. [DOI] [PubMed] [Google Scholar]
- 12.Benson BW, Shulman JD. Inclusion of tobacco exposure as a predictive factor for decreased bone mineral content. Nicotine Tob Res 2005; 7:719–724. [DOI] [PubMed] [Google Scholar]
- 13.César-Neto JB, Benatti BB, Manzi FR, et al. The influence of cigarette smoke inhalation on bone density. A radiographic study in rats. Braz Oral Res 2005; 19:47–51. [DOI] [PubMed] [Google Scholar]
- 14.Akhter MP, Lund AD, Gairola CG. Bone biomechanical property deterioration due to tobacco smoke exposure. Calcif Tissue Int 2005; 77:319–326. [DOI] [PubMed] [Google Scholar]
- 15.Alver K, Meyer HE, Falch JA, et al. Outdoor air pollution, bone density and self-reported forearm fracture: the Oslo Health Study. Osteoporos Int 2010; 21:1751–1760. [DOI] [PubMed] [Google Scholar]
- 16.Alvaer K, Meyer HE, Falch JA, et al. Outdoor air pollution and bone mineral density in elderly men - the Oslo Health Study. Osteoporos Int 2007; 18:1669–1674. [DOI] [PubMed] [Google Scholar]
- 17.Chang KH, Chang MY, Muo CH, et al. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PLoS One 2014; 9:e103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rückerl R, Greven S, Ljungman P, et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect 2007; 115:1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blomberg A, Krishna MT, Helleday R, et al. Persistent airway inflammation but accommodated antioxidant and lung function responses after repeated daily exposure to nitrogen dioxide. Am J Respir Crit Care Med 1999; 159:536–543. [DOI] [PubMed] [Google Scholar]
- 20.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol 2006; 2:619–626. [DOI] [PubMed] [Google Scholar]
- 21.Tseng FJ, Chia WT, Shyu JF, et al. Interactomics profiling of the negative regulatory function of carbon monoxide on RANKL-treated RAW 264.7 cells during osteoclastogenesis. BMC Syst Biol 2014; 8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatto NM, Henderson VW, Hodis HN, et al. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology 2014; 40:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung CR, Lin YT, Hwang BF. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One 2013; 8:e75510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosla S, Melton LJ, 3rd, Riggs BL. Osteoporosis: gender differences and similarities. Lupus 1999; 8:393–396. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe R, Tanaka T, Aita K, et al. Osteoporosis is highly prevalent in Japanese males with chronic obstructive pulmonary disease and is associated with deteriorated pulmonary function. J Bone Miner Metab 2014; [Epub ahead of print.]. [DOI] [PubMed] [Google Scholar]
- 26.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012; 379:1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences-clinical impact, mechanisms, and potential for early intervention. COPD 2008; 5:235–256. [DOI] [PubMed] [Google Scholar]
- 28.Wright JL, Churg A. Animal models of cigarette smoke-induced COPD. Chest 2002; 122:301S–306S. [DOI] [PubMed] [Google Scholar]
- 29.Rajendrasozhan S, Yang SR, Edirisinghe I, et al. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal 2008; 10:799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SJ, Foreman MG, Demeo DL, et al. Menthol cigarette smoking in the COPDGene cohort: Relationship with COPD, comorbidities and CT metrics. Respirology 2015; 20:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniell HW. Osteoporosis of the slender smoker. Vertebral compression fractures and loss of metacarpal cortex in relation to postmenopausal cigarette smoking and lack of obesity. Arch Intern Med 1976; 136:298–304. [DOI] [PubMed] [Google Scholar]
- 32.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ 1997; 315:841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 2008; 121:406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masoni A, Morosano M, Tomat MF, et al. Association between hip fractures and risk factors for osteoporosis. Multivariate analysis. Medicina (B Aires) 2007; 67:423–428. [PubMed] [Google Scholar]
- 35.Pongchaiyakul C, Nguyen TV, Kosulwat V, et al. Effect of urbanization on bone mineral density: a Thai epidemiological study. BMC Musculoskelet Disord 2005; 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


