Abstract
The purpose of this article is to evaluate the evolution of microbial translocation (MT) and its role in CD4+ and CD8+ T cells immune activation (IA) in HIV-1-infected patients on ritonavir-boosted darunavir monotherapy (mtDRV/rtv).
Prospective study of consecutive HIV-1-infected patients switched to mtDRV/rtv as a simplification regimen. Subjects were classified according to the virological behavior during a 24-month follow-up as continuous undetectable viral load, blips, intermittent viremia, and virological failure (VF). MT was evaluated by plasma LPS and 16S genomic rDNA (16S rDNA) levels, whereas IA was assessed by the coexpression of HLA-DR and CD38 in CD4+ and CD8+ T cells, and plasma sCD14 levels.
Seventy-one patients were included in this substudy of the MonDar cohort (ClinicalTrials.gov: NCT01505722). At baseline, CD4+ (ρ = −0.352, P = 0.01) and CD8+ T-cell activation (ρ = −0.468, P < 0.001) were correlated with time with viral suppression, but not with MT markers. A significant decrease in plasma LPS levels was found only in patients without VF (baseline, 77.8 vs month 24, 60.4 pg/mL; P < 0.001]. Both plasma 16S rDNA and sCD14 levels were unchanged irrespective of the viral behavior. The only variable independently associated with a decrease in CD4+ and CD8+ T cells activation was an undetectable HIV-1 viremia (β = 4.78, P < 0.001 and β = 2.93, P = 0.005, respectively).
MT does not have a pivotal role in T-cell activation, at least in patients with long-term viral suppression. The viremic episodes and VF are the main factors related to CD4+ and CD8+ T-cells IA, even during mtDRV/rtv.
INTRODUCTION
Several studies have highlighted the importance of microbial translocation (MT) as a pivotal mechanism for persistent immune activation (IA) during HIV-1 infection.1–4 This anomalous MT arises as a direct consequence of severe CD4+ T-cell depletion in the gastrointestinal tract and damage to the integrity of the intestinal mucosa.5–7 MT can be measured in plasma by direct quantification of bacterial products, including lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria, as well as conserved sequences of 16S genomic rDNA (16S rDNA) common to most bacteria or other microbe-specific compounds. Both of them were positively correlated with CD8+ T-cell activation in untreated HIV infection and several longitudinal studies have shown important decreases in plasma LPS, 16S rDNA, and sCD14 levels after the initiation of ART, though without reaching the values of healthy individuals.1,8–14
In the past few years, ART simplification strategies, such as ritonavir-boosted protease inhibitor-based monotherapy (mtPI/rtv), have been explored in patients with long-standing virological suppression. Data from several clinical trials of lopinavir/ritonavir and ritonavir-boosted darunavir monotherapy (mtDRV/rtv) have shown that mtPI/rtv has a similar or slightly less efficacy than triple therapy to maintain viral load suppression.15–19 Even so, there is significant controversy regarding the use of this therapeutic strategy,20–25 and information about the impact of this therapeutic option on issues other than virological efficacy and safety is still scarce. Herein, we have assessed the evolution of MT and its role in CD4+ and CD8+ T cells IA profile in HIV-1-infected patients on mtDRV/rtv over a 24-month period.
MATERIAL AND METHODS
As previously described in detail,26,27 the MonDar cohort (Clinical-Trials.gov identifier: NCT01606722) prospectively enrolled 150 HIV-1-infected patients with virological suppression for ≥6 months who were switched to mtDRV/rtv (800/100 mg once daily) as a simplification strategy from January 2010 to April 2011. The study was conducted after obtaining informed consent according to the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee in Biomedical Research of Andalucía and the Spanish Agency for Medicines.
Patients, Follow-Up, and Samples
Clinical assessments and sampling were performed just before switching to mtDRV/rtv and quarterly thereafter, including biochemical and hematological profiles, CD4+ T cells counts, and plasma HIV-1 viremia (COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0; Roche, Branchburg, NJ). In this substudy, only patients with available samples just before and at month 6, 12, 18, and 24 after switching to mtDRV/rtv were included. Patients who presented virological failure (VF) on mtDRV/rtv were also followed up for 24 months after reachieving virological control by triple therapy reintroduction or by encouraging adherence.
Patients were classified according to their virological behavior into 4 groups: continuous undetectable viremia (CUV) (<20 copies/mL); blips or transitory episodes of HIV-RNA >20 copies/mL, preceded and followed by undetectable viremia without changes in ART; VF, defined as 2 consecutive HIV-RNA >200 copies/mL; and intermittent viremia (IV) defined as episodes of detectable HIV-RNA during the follow-up yet without meeting the blip or VF criteria.
Laboratory Procedures
All samples were tested in duplicate. Plasma LPS and sCD14 were measured by the QCL-1000 Limulus Amebocyte Lysate kit (Lonza, Basel, Switzerland) and the Human sCD14 Quantikine ELISA kit (R&D Systems, Abingdon, Oxfordshire, UK), respectively, following manufacturer's instructions. Plasma 16S rDNA was measured by quantitative polymerase chain reaction by using degenerate forward and reverse primers (8F: 5′-AGAGTTTGATYMTGGCTCAG-3′; 361R: 5′-CGYCCATTGBGBAADATTCC-3′) and a TaqMan probe (338P: 5′-FAM-TACGGGAGGCAGCAGT-BHQ1-3′).10 The IA profile was measured as the coexpression of HLA-DR and CD38 in both CD4+ and CD8+ T cells as previously described.28
Statistical Analysis
Results are expressed as median and interquartile range for continuous variables and as number of cases and percentages for categorical variables. The Kruskal–Wallis and analysis of variance tests were performed to compare continuous variables, whereas the χ2 and Fisher exact tests were run to compare categorical variables. The relationships between continuous variables were assessed by Spearman rank correlation coefficients (ρ test). The variables that were statistically significant in the univariate analysis were included in a multiple linear regression model. The differences were considered statistically significant for P values <0.05. The statistical analyses were performed using SPSS software (v. 19.0, Chicago, IL).
RESULTS
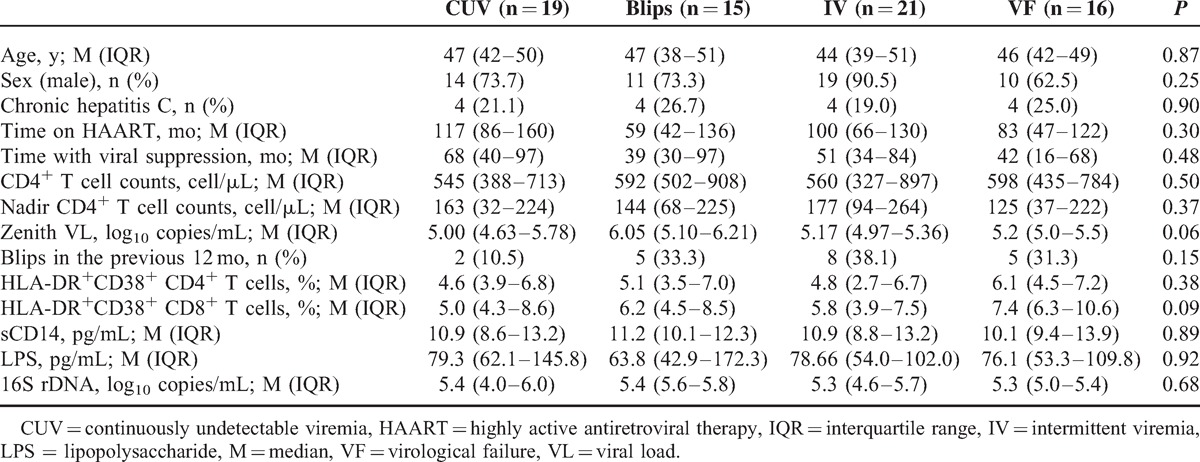
A total of 71 patients were included in this study and classified as CUV: 19 (26.8%); blips: 15 (21.1%); IV: 21 (29.6%); and VF: 16 (22.5%). Patients’ baseline characteristics are shown in Table 1. At baseline, a weak correlation was found between time with viral suppression and plasma levels of LPS (ρ = −0.311, P = 0.03), but not with 16S rDNA (ρ = 0.051, P = 0.67) or sCD14 (ρ = −0.075, P = 0.53). Moreover, time with viral suppression was inversely correlated with the percentages of HLA-DR+CD38+ CD4+ (ρ = −0.352, P = 0.01) and HLA-DR+CD38+ CD8+ T cells (ρ = −0.468, P = 0.001). The IA of CD4+ and CD8+ T cells were correlated with the sCD14 levels (ρ = 0.277, P = 0.05 and ρ = 0.412, P = 0.003), but not with LPS or 16S rDNA levels. Other epidemiological factors, such as age or hepatitis C virus coinfection, did not correlate with any of the MT or IA markers.
TABLE 1.
Patients’ Baseline Characteristics
MT Evolution Over 24 Months on mtDRV/rtv
A significant decrease in plasma LPS levels was found in patients without VF (CUV, blips, and IV groups) (month 0, 77.8 [51.6–108.96] vs month 24, 60.4 pg/mL [51.8–74.4], P = 0.006; Figure 1A). By contrast, in those patients who experienced VF, the LPS levels remained unchanged in comparison with baseline values both at VF time and at month 24, despite of reachieving virological control (month 0, 83.6 [54.3–111.8] vs at virological failure, 75.7 [49.1–148.9] vs month 24, 72.1 pg/mL [50.8–134.6], P = 0.76; Figure 1B).
FIGURE 1.
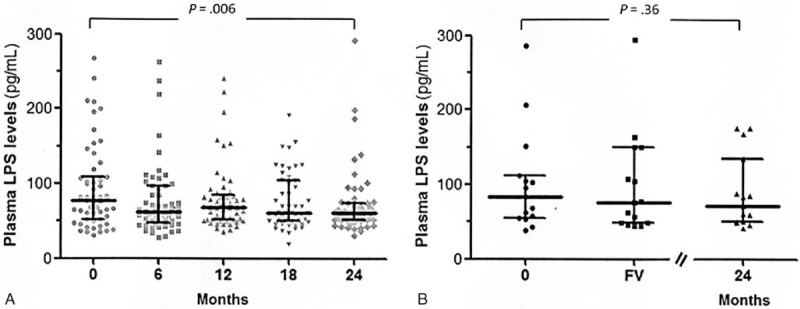
Evolution of plasma LPS levels during the 24 months of mtDRV/rtv in patients (A) without and (B) with VF. VF occurred at a median of 12 mo (IQR, 6–18; range, 6–21). IQR = interquartile range, LPS = lipopolysaccharide, mtDRV/rtv = ritonavir-boosted darunavir monotherapy, VF = virological failure.
Both plasma 16S rDNA (month 0, 5.35 [4.97–5.79] vs month 24, 5.44 log10 copies/mL [4.93–6.05]; P = 0.62) and sCD14 levels (month 0, 10.9 [9.4–13.1] vs month 24, 10.4 pg/mL [9.1–12.8]; P = 0.18) were unchanged during the 24 months on mtDRV/rtv irrespective of the viral outcome. No correlations between plasma LPS and 16S rDNA or sCD14 levels were found either at baseline (ρ = −0.185, P = 0.123; ρ = −0.112, P = 0.176) or throughout the follow-up (ρ = −0.151, P = 0.20; ρ = −0.146, P = 0.11), although there were strong correlations between the baseline values of each of them and the geometric means of their values during the follow-up (ρ = 0.749 for LPS, ρ = 0.671 for 16S rDNA and ρ = 0.485 for sCD14 levels; P < 0.001).
Relationship Between IA, MT, and HIV-1 Viremia
The IA profiles of CD4+ and CD8+ T cells were related with the virological behavior after switching to mtDRV/rtv. During the 24 months, IA decreased in CUV patients (baseline HLA-DR+CD38+ CD4+ T cells, 4.6% vs month 24, 4.0%, P = 0.01; baseline HLA-DR+ CD38+ CD8+ T cells, 5.3% vs month 24: 4.4%, P = 0.001), and remained stable in blips and in IV patients (baseline HLA-DR+CD38+ CD4+ T cells, 5.0% vs month 24, 4.0%, P = 0.13; baseline HLA-DR+CD38+ CD8+ T cells, 5.9% vs month 24, 4.8%, P = 0.75). By contrast, IA increased in patients with VF (baseline HLA-DR+CD38+ CD4+ T cells, 6.1% vs month 24, 7.1%, P = 0.02; baseline HLA-DR+CD38+ CD8+ T cells, 7.4% vs month 24, 9.4%, P = 0.06).
Throughout the follow-up, the changes in HLA-DR+CD38+ CD4+ and CD8+ T cells were not associated to LPS or sCD14 levels, but weak correlations were found only between plasma 16S rDNA levels and HLA-DR+CD38+ CD4+ (ρ = 0.286, P = 0.07) and CD8+ T cells (ρ = 0.331, P = 0.04).
On the other hand, there were no correlations between HIV-RNA and LPS levels (ρ = −0.085, P = 0.13) or 16S rDNA levels (ρ = 0.013, P = 0.82) or sCD14 levels (ρ = 0.013, P = 0.31) during the 24 months on mtDRV/rtv. In contrast, positive correlations were found between HIV-RNA and the percentages of HLA-DR+CD38+ CD4+ (ρ = 0.102, P = 0.05) and CD8+ T cells (ρ = 0.162, P = 0.01). Finally, a multiple linear regression model showed that HIV-RNA was the only variable associated with changes in HLA-DR+CD38+ CD4+ and CD8+ throughout the follow-up (β = 4.78, P < 0.001 and β = 2.93, P = 0.005, respectively).
DISCUSSION
We have assessed MT by 2 different markers (LPS and 16S rDNA) and a surrogate marker (sCD14). As in the study by Abad-Fernández et al,14 no correlations of bacterial 16S rDNA with either with LPS or sCD14 were found. In addition, we also failed to find a relationship between LPS and sCD14 levels perhaps because sCD14 is an activation marker for monocytes, but not a direct or specific marker of MT,29 and stimuli other than MT could have a major influence on innate immunity activation in patients with a long time of viral suppression and immune reconstitution.30,31
Our results show that although plasma 16S rDNA and sCD14 levels remained stable throughout the 24 months on mtDRV/rtv irrespective of patients’ viral outcomes, LPS levels decreased, but only in patients without VF. This decline was more pronounced among patients with CUV, which might suggest a continuous recovery of the mucosal immune dysfunction and/or LPS clearance mechanisms, including the clearance by Kupffer cells.32,33 On the contrary, neither at baseline nor during the follow-up was CD4+ and CD8+ T cells IA related to MT markers. The only variable independently associated with a decrease in CD4+ and CD8+ T cells activation was an undetectable viremia throughout all the follow-up. In a completely different clinical setting, Srinivasula et al34 also found that HIV was the primary driving force behind the activation and proliferation of most subsets of both CD4 and CD8 T lymphocytes in HIV-infected patients, whereas MT had not an important role in this proliferation.
Nevertheless, this observational study have several limitations among which are the small sample size in each group of patients and that it was a single-arm trial, without a comparator group on triple therapy. Thus, these results need to be confirmed with larger prospective clinical studies including both types of treatment. Likewise, these results could be different in earlier treatment phases or in patients with higher immunological impairment. However, to date, there are few data on factors influencing the CD4+ and CD8+ T cells IA in HIV-1-infected patients on ritonavir-boosted protease inhibitor monotherapy. In this context, our results might help to clarify one of the several concerns about this maintenance strategy in patients with long-term viral suppression.
In summary, our results suggest that MT does not have a pivotal role in T-cell activation in patients with long-term viral suppression and relatively high CD4+ T-cell counts. The viremia and VF are the main factors related to CD4+ and CD8+ T cells IA, even during mtDRV/rtv.
Acknowledgments
The authors are indebted to patients for their involvement in this study, and to M. Rodríguez, F. Cano, and R. Martin for their help with specimen processing.
Footnotes
Abbreviations: 16S rDNA = 16S genomic rDNA, CUV = continuous undetectable viremia, IA = immune activation, IV = intermittent viremia, LPS = lipopolysaccharide, MT = microbial translocation, mtDRV/rtv = ritonavir-boosted darunavir monotherapy, mtPI/rtv = ritonavir-boosted protease inhibitor-based monotherapy, VF = virological failure.
This study was supported by a grant from Dirección Gerencia del Servicio Andaluz de Salud, Consejería de Salud, Junta de Andalucía (exp. SAS 111237).
LFL-C and PV have received unrestricted research funding, consultancy fees, and lecture fees from and have served on the advisory boards of Abbott, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, Roche España, and ViiV Healthcare. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–1371. [DOI] [PubMed] [Google Scholar]
- 2.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS 2008; 22:2035–2038. [DOI] [PubMed] [Google Scholar]
- 3.Karlsson AC, Younger SR, Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS 2004; 18:981–989. [DOI] [PubMed] [Google Scholar]
- 4.Richman DD, Margolis DM, Delaney M, et al. The challenge of finding a cure for HIV infection. Science 2009; 323:1304–1307. [DOI] [PubMed] [Google Scholar]
- 5.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004; 200:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattapallil JJ, Douek DC, Hill B, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005; 434:1093–1097. [DOI] [PubMed] [Google Scholar]
- 7.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 2004; 200:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallet MA, Rodriguez CA, Yin L, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS 2010; 24:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajasuriar R, Booth D, Solomon A, et al. Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor alpha and microbial translocation. J Infect Dis 2010; 202:1254–1264. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Lederman M, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009; 199:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reus S, Portilla J, Sánchez-Payá J, et al. Low-level HIV viremia is associated with microbial translocation and inflammation. J Acquir Immune Defic Syndr 2013; 62:129–134. [DOI] [PubMed] [Google Scholar]
- 12.Pilakka-Kanthikeel S, Kris A, Selvaraj A, et al. Immune activation is associated with increased gut microbial translocation in treatment-naive, HIV-infected children in a resource-limited setting. J Acquir Immune Defic Syndr 2014; 66:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.d’Ettorre G, Baroncelli S, Micci L, et al. Reconstitution of intestinal CD4 and Th17 T cells in antiretroviral therapy suppressed HIV-infected subjects: implication for residual immune activation from the results of a clinical trial. PLoS One 2014; 9:e109791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abad-Fernandez M, Vallejo A, Hernandez-Novoa B, et al. Correlation between different methods to measure microbial translocation and its association with immune activation in long-term suppressed HIV-1-infected individuals. J Acquir Immune Defic Syndr 2013; 64:149–153. [DOI] [PubMed] [Google Scholar]
- 15.Pulido F, Delgado R, Pérez-Valero I, et al. Long-term (4 years) efficacy of lopinavir/ritonavir monotherapy for maintenance of HIV suppression. J Antimicrob Chemother 2008; 61:1359–1361. [DOI] [PubMed] [Google Scholar]
- 16.Arribas JR, Delgado R, Arranz A, et al. Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and 2 nucleosides for maintenance therapy of HIV: 96-week analysis. J Acquir Immune Defic Syndr 2009; 51:147–152. [DOI] [PubMed] [Google Scholar]
- 17.Meynard JL, Bouteloup V, Landman R, et al. Lopinavir/ritonavir monotherapy versus current treatment continuation for maintenance therapy of HIV-1 infection: the KALESOLO trial. J Antimicrob Chemother 2010; 65:2436–2444. [DOI] [PubMed] [Google Scholar]
- 18.Valantin MA, Lambert-Niclot S, Flandre P, et al. Long-term efficacy of darunavir/ritonavir monotherapy in patients with HIV-1 viral suppression: week 96 results from the MONOI ANRS 136 study. J Antimicrob Chemother 2012; 67:691–695. [DOI] [PubMed] [Google Scholar]
- 19.Clumeck N, Rieger A, Banhegyi D, et al. 96 week results from the MONET trial: a randomized comparison of darunavir/ritonavir with versus without nucleoside analogues, for patients with HIV RNA < 50 copies/mL at baseline. J Antimicrob Chemother 2011; 66:1878–1885. [DOI] [PubMed] [Google Scholar]
- 20.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. 2014. http://aidsinfo.nih.gov/guidelines/ Accessed date: January 7, 2015. [Google Scholar]
- 21.Günthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. J Am Med Assoc 2014; 312:410–425. [DOI] [PubMed] [Google Scholar]
- 22.European AIDS Clinical Society. European Guidelines for Treatment of HIV Infected Adults in Europe. 2014. Version 7.02 http://eacsociety.org/Guidelines.aspx Accessed date: January 7, 2015. [Google Scholar]
- 23.Panel de expertos de GeSIDA y Plan Nacional sobre el Sida. Documento de consenso de GeSIDA/Plan Nacionalsobre el Sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana (Actualización enero 2014). Enferm Infecc Microbiol Clin. 2014;32:446.e1-e42. [DOI] [PubMed] [Google Scholar]
- 24.Williams I, Churchill D, Anderson J, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (2013 update). HIV Med 2014; 15 suppl 1:1–85. [DOI] [PubMed] [Google Scholar]
- 25.Hoen B, Bonnet F, Delaugerre C, et al. French 2013 guidelines for antiretroviral therapy of HIV-1 infection in adults. J Int AIDS Soc 2014; 17:19034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BenMarzouk-Hidalgo OJ, Torres-Cornejo A, Gutierrez-Valencia A, et al. Immune activation throughout a boosted darunavir monotherapy simplification strategy. Clin Microbiol Infect 2014; 20:1297–1303. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez-Valencia A, Torres-Cornejo A, Benmarzouk-Hidalgo OJ, et al. Darunavir Cmin and ritonavir-boosted darunavir monotherapy outcome in HIV-infected patients. Antivir Ther 2014; 19:443–447. [DOI] [PubMed] [Google Scholar]
- 28.Wittkop L, Bitard J, Lazaro E, et al. Effect of cytomegalovirus-induced immune response, self antigen-induced immune response, and microbial translocation on chronic immune activation in successfully treated HIV type 1-infected patients: the ANRS CO3 Aquitaine Cohort. J Infect Dis 2012; 207:622–627. [DOI] [PubMed] [Google Scholar]
- 29.Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol 2000; 12:13–19. [DOI] [PubMed] [Google Scholar]
- 30.Marchetti G, Cozzi-Lepri A, Merlini E, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS 2011; 25:1385–1394. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti G, Nasta P, Bai F, et al. Circulating sCD14 is associated with virological response to pegylated-interferon-alpha/ribavirin treatment in HIV/HCV co-infected patients. PLoS One 2012; 7:e32028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003; 77:11708–11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balagopal A, Ray SC, De Oca RM, et al. Kupffer cells are depleted with HIV immunodeficiency and partially recovered with antiretroviral immune reconstitution. AIDS 2009; 23:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivasula S, Lempicki RA, Adelsberger JW, et al. Differential effects of HIV viral load and CD4 count on proliferation of naive and memory CD4 and CD8 T lymphocytes. Blood 2011; 118:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]


