Abstract
Renal dysfunction is a prevalent comorbidity in acute ischemic stroke patients requiring thrombolytic therapy. However, the effect of renal dysfunction on the clinical outcome of this population remains controversial.
This study aimed to evaluate the safety and effectiveness of thrombolytic therapy in acute stroke patients with renal dysfunction using a meta-analysis.
We systematically searched PubMed and EMBASE for studies that evaluated the relationship between renal dysfunction and intravenous tissue plasminogen activator (tPA) in patients with acute ischemic stroke. Poor outcome (modified Rankin Scale ≥2), mortality, and symptomatic intracranial hemorrhage (ICH) and any ICH were analyzed.
Fourteen studies were included (N = 53,553 patients). The mean age ranged from 66 to 75 years. The proportion of male participants was 49% to 74%. The proportion of renal dysfunction varied from 21.9% to 83% according to different definitions. Based on 9 studies with a total of 7796 patients, the meta-analysis did not identify a significant difference in the odds of poor outcome (odds ratio [OR] = 1.06; 95% confidence interval [CI]: 0.96–1.16; I2 = 44.5) between patients with renal dysfunction and those without renal dysfunction. Patients with renal dysfunction were more likely to die after intravenous thrombolysis (OR = 1.13; 95% CI: 1.05–1.21; I2 = 70.3). No association was observed between symptomatic ICH (OR = 1.02; 95% CI: 0.94–1.10; I2 = 0) and any ICH (OR = 1.07; 95% CI: 0.96–1.18; I2 = 25.8).
Renal dysfunction does not increase the risk of poor outcome and ICH after stroke thrombolysis. Renal dysfunction should not be a contraindication for administration of intravenous thrombolysis to eligible patients.
INTRODUCTION
Thrombolytic therapy with intravenous tissue plasminogen activator (tPA) is an effective treatment of acute ischemic stroke in patients presenting within 3 or 4.5 hours of onset of symptoms.1,2 More than one third of acute stroke patients have comorbidity of chronic kidney disease (CKD), defined as the presence of reduced estimated glomerular filtration rate (eGFR) or kidney injury.3 Stroke patients with renal dysfunction are more likely to have a poor outcome in the natural course.3–5 Although current guidelines do not include renal dysfunction as a contraindication to tPA therapy, some clinicians hesitate to administer tPA because of a tendency of bleeding in these patients.6,7 The real risk and benefit of thrombolytic therapy in this high-risk population are unknown. Reports on the relationship between renal dysfunction and the risk of poor outcome and symptomatic intracerebral hemorrhage (sICH) are contradictory.8–11 The effectiveness and safety of thrombolysis in patients with renal dysfunction has not been clearly determined.
Therefore, we aimed to perform a systematic review to evaluate the evidence of the safety and effectiveness of thrombolytic therapy in acute stroke patients with renal dysfunction.
METHODS
Search Strategy and Eligibility Studies
We systematically searched PubMed and EMBASE (from its earliest date to August 2014) for studies that evaluated the relationship between renal dysfunction and intravenous tPA in patients with acute ischemic stroke. The terms “renal dysfunction,” “kidney dysfunction,” “renal impairment,” “eGFR,” “creatinine”, “urea,” “estimated glomerular filtration rate,” “thrombolysis,” “recombinant tissue plasminogen activator,” and “rt-PA” (recombinant tissue plasminogen activator) were combined using “and” or “or” for searching relevant studies. The bibliographies of relevant articles were screened. Only studies that met the following criteria were included: (1) they evaluated the association between renal dysfunction and the outcome of intravenous thrombolysis; (2) at least one of following outcomes was reported: modified Rankin Scale (mRS), mortality, or intracerebral hemorrhage (ICH); and (3) results were reported in a manner that allowed calculation of the odds ratio (OR) for outcomes. Articles were excluded if they were case reports. In case of multiple publications from the same study population, only the report with the most complete data was included. Our study was a systematic review of published studies. Therefore ethical approval was not required.
Selection of Studies and Extraction of Data
One reviewer independently screened the titles and abstracts of every record. The full articles were obtained when the information provided in the title or abstracts conformed to the selection criteria outlined above. Two reviewers independently performed extraction of data and compared the results. The following data were extracted: (1) general characteristics of the studies and participants, (2) sample size, (3) the diagnostic criteria for renal dysfunction, and (4) outcome measurements (eg, mRS, mortality, and ICH). Articles that met all of the inclusion criteria, but specific data extraction was not possible, were defined as “NG” (not given). Discrepancies were resolved by consensus.
Quality Assessment and Statistical Methods
We performed quality assessment using the Newcastle–Ottawa Scale (NOS) for cohort studies.12 The NOS uses a “star” rating system to judge quality based on 3 aspects of the study: selection of participants, comparability of study groups, and outcome of interest. The maximum number of stars that a study may receive in each of these 3 categories is 4, 2, and 3, respectively. The highest-quality study receives 9 stars. We extracted adjusted ORs from logistic regression models reporting the association between renal dysfunction and outcomes after intravenous thrombolysis treatment or calculated the ORs for outcomes. We evaluated heterogeneity among included studies using the I2 test. We considered a value greater than 50% to indicate substantial heterogeneity. Regardless of the size of heterogeneity, the random effects model was used for statistical analysis. We conducted the meta-analysis using STATA 11.0.
RESULTS
Identified Studies
The selection of studies is shown in Figure 1. The initial literature search identified 1328 relevant articles. After reading the titles and abstracts, we retained 26 studies for further assessment. Of these, we excluded 13 studies.13–25 A recently published study was included.26 Ultimately, 14 studies, containing 53,553 patients, were included in this systematic review.8–11,27–35
FIGURE 1.
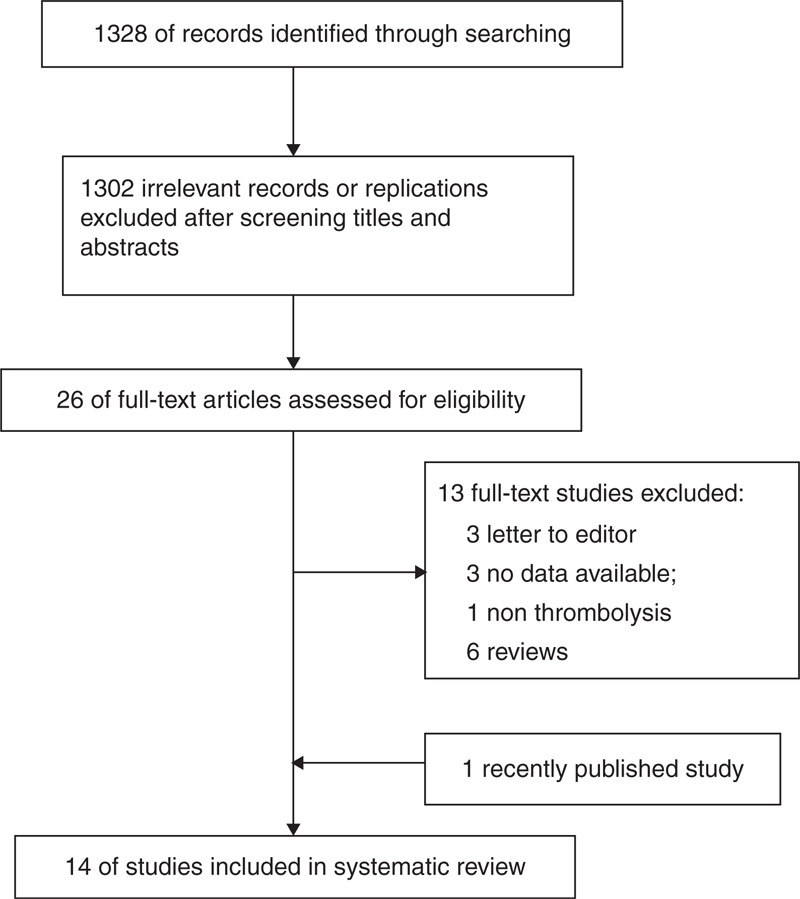
Flow chart of literature screening and selection process.
Characteristics of the Included Studies
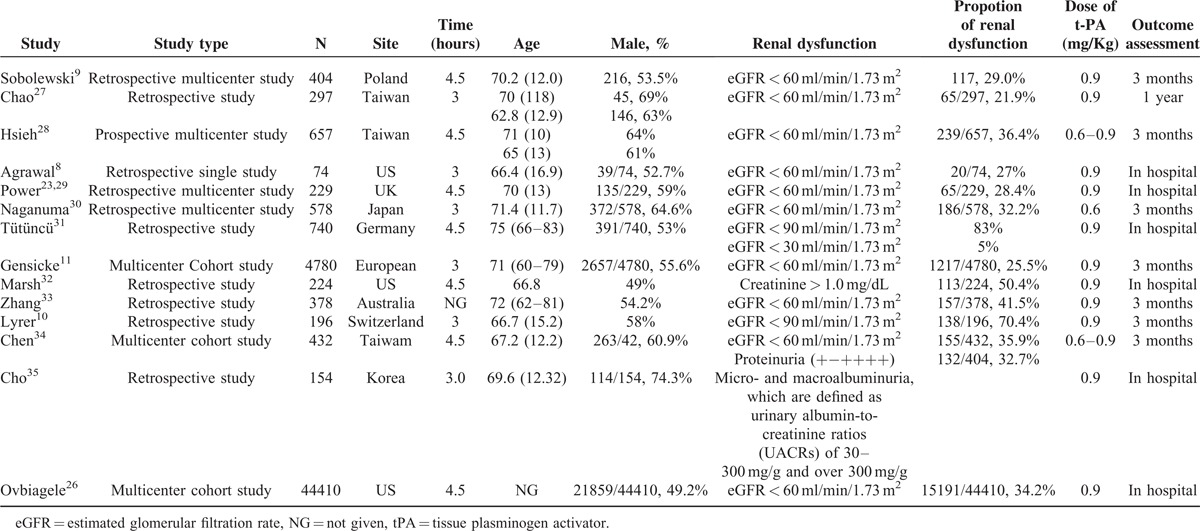
Five studies were performed in Europe, 5 in Asia, 3 in the United States, and 1 in Australia. The number of participants ranged from 74 to 44410. The mean age of participants ranged from 66 to 75 years. The proportion of male participants was 49% to –74% among these trials. The proportion of renal dysfunction varied from 21.9% to 83% according to different definitions, including eGFR, creatinine, proteinuria, and micro- and macro-albuminuria. Further details are summarized in Table 1. The number of stars of studies as assessed by the NOS was more than 7.
TABLE 1.
Characteristics of Studies on Intravenous tPA-Treated Patients With Renal Dysfunction
Poor Outcome at the End of the Follow-Up Period
Nine studies reported a poor outcome at the end of the follow-up period (Table 2). The cut-off to define a poor outcome was an mRS ≥2 (3 studies), 3 (4 studies), and 4 (2 studies). Based on 9 studies with a total of 7796 patients, the meta-analysis did not identify a significant difference in the odds of a poor outcome (OR = 1.06; 95% confidence interval [CI]: 0.96–1.16; I2 = 44.5) between patients with renal dysfunction and those without renal dysfunction (Figure 2).
TABLE 2.
Outcomes of Studies on Intravenous tPA-Treated Patients With Renal Dysfunction
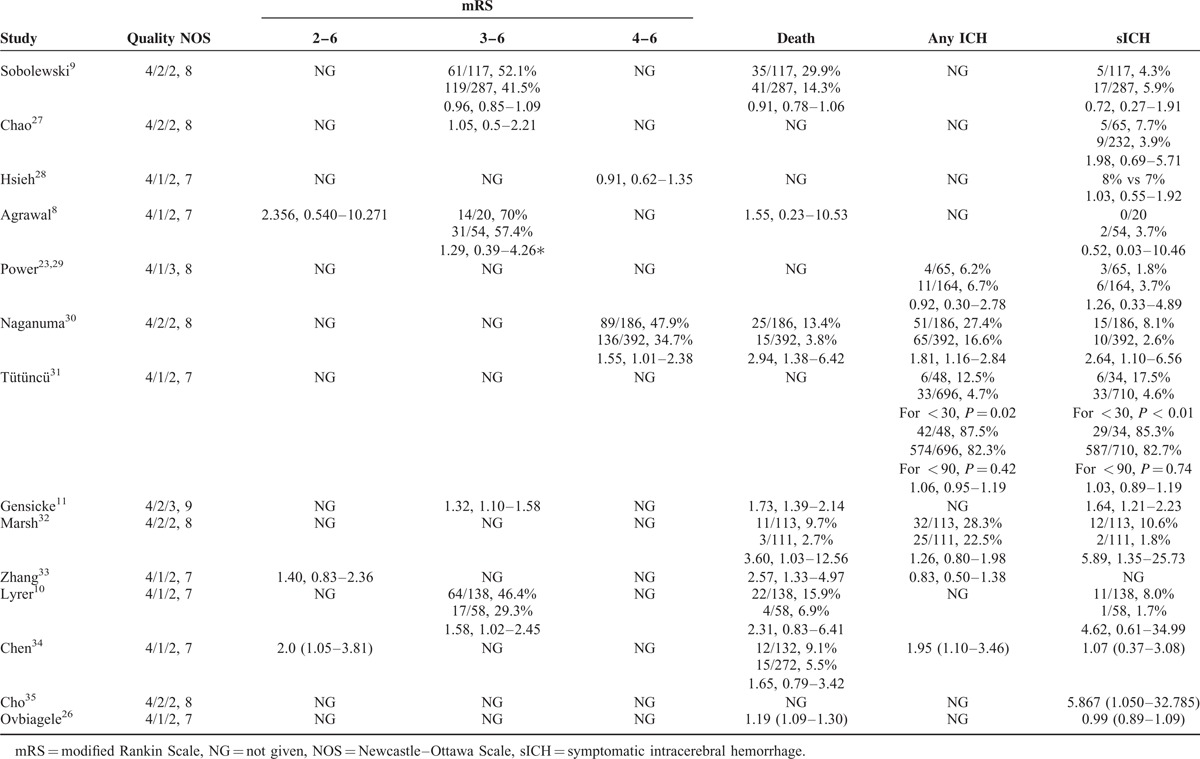
FIGURE 2.
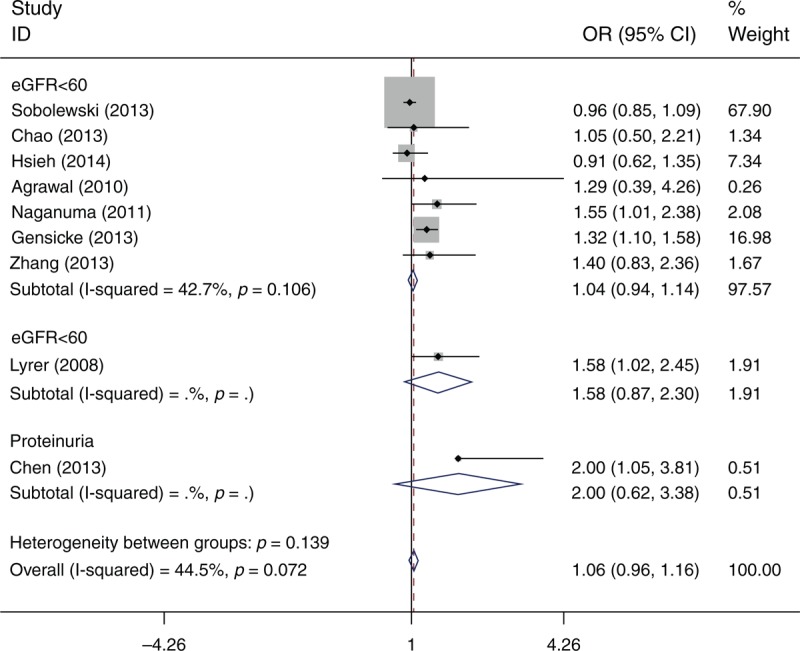
Odds ratio for poor outcome of intravenous rtPA-treated patients with renal function compared with those without renal dysfunction.
Mortality
Nine studies (N = 51476 patients) reported the mortality in hospital or at the end of the follow-up period (Table 2). Meta-analysis showed that patients with renal dysfunction were more likely to die after intravenous thrombolysis (OR = 1.13; 95% CI: 1.05–1.210; I2 = 70.3) (Figure 3).
FIGURE 3.
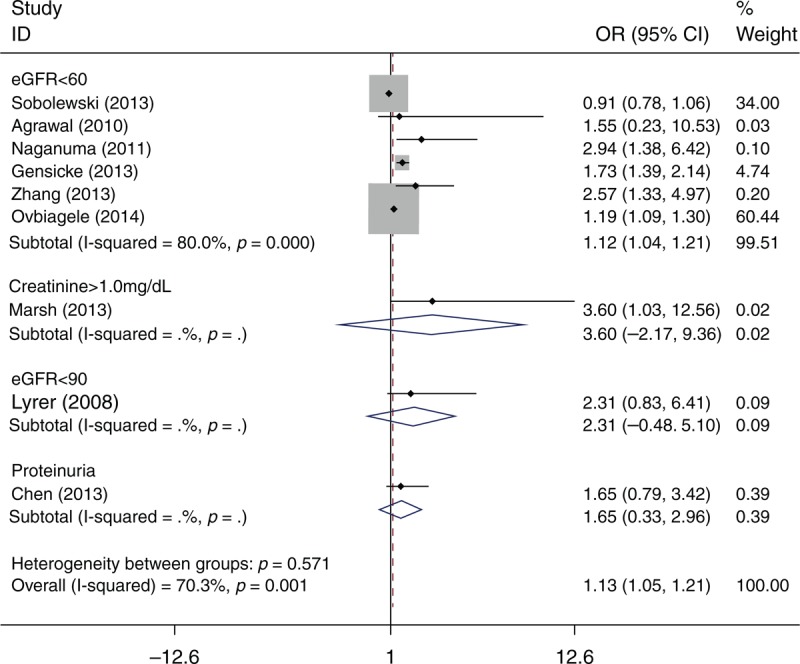
Odds ratio for mortality of intravenous rtPA-treated patients with renal function compared with those without renal dysfunction.
Occurrence of sICH and any ICH
Twelve studies (N = 53175 patients) reported the occurrence of sICH (Table 2). The definition of sICH varied among the different studies (Table 3). Meta-analysis showed that there was no association of sICH (OR = 1.02; 95% CI: 0.94–1.10; I2 = 0) between patients with renal dysfunction and those without renal dysfunction (Figure 4). There was also no association of any ICH (OR = 1.07; 95% CI: 0.96–1.18; I2 = 25.8) between patients with renal dysfunction and those without renal dysfunction.
TABLE 3.
The Definition of sICH in Included Studies

FIGURE 4.
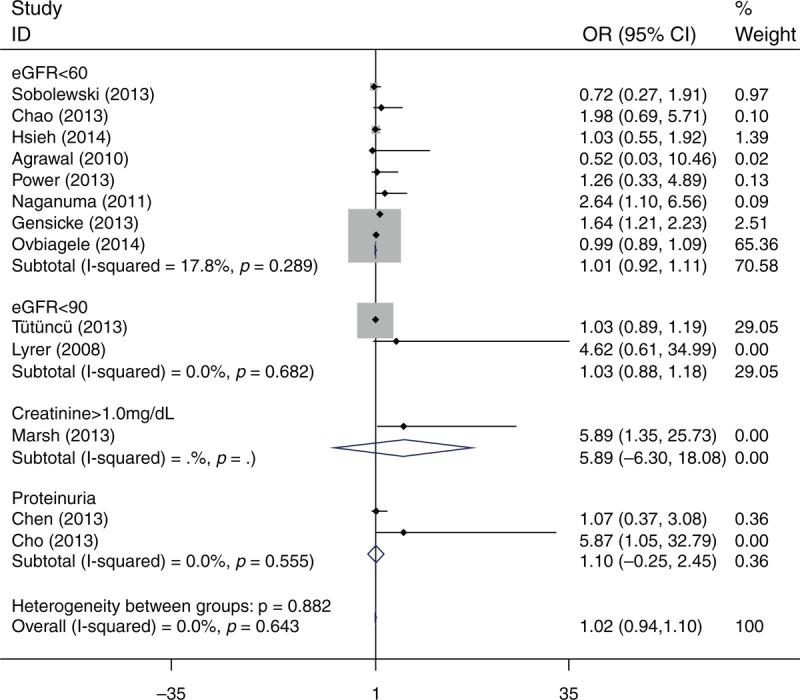
Odds ratio for sICH of intravenous rtPA-treated patients with renal function compared with those without renal dysfunction.
DISCUSSION
This systematic review showed that the prevalence of renal dysfunction in acute ischemic stroke varied from 21.9% to 83% according to different definitions. Meta-analysis showed that renal dysfunction did not increase the risk of a poor outcome and ICH after stroke thrombolysis. However, patients with renal dysfunction were more likely to die after intravenous thrombolysis.
The quality of reporting in general was good. The main problem was that most of the studies did not represent the population well. The statistical heterogeneity in outcome measurements was probably related to different baseline characteristics of the participants, different methods of evaluating renal function, and different classifications of renal dysfunction.
The prevalence of CKD is increasing. Studies have shown that the incidence of CKD in people with cardiovascular disease is higher than that in healthy people, and it is an independent risk factor for recurrence of cardiovascular disease and death.36 Several studies have recently found that renal dysfunction increases the risk of stroke.37,38 Renal dysfunction can also predict short-term and long-term case fatality rates in patients with stroke.4 Impairment in small vessel vasculature, atherosclerotic changes in large vessels, and coagulation abnormalities in CKD probably underlie the specific characteristics of stroke in these patients.10,16 Therefore, we speculate that CKD patients probably have worse bleeding complications compared with those without CKD, thus increasing poor outcomes. Lyrer et al10 found that impaired renal function before thrombolysis is associated with an increased odds for a poor outcome and there is a trend for more sICH compared with stroke patients with normal renal function. A retrospective, multicenter, observational study that was conducted in Japan also showed that reduced eGFR was associated with early ICH and a 3-month unfavorable outcome in stroke patients receiving intravenous tPA.30 In contrast, another study showed that the presence of an eGFR <60 ml/min/1.73 m2 was not associated with increased ICH, poor functional outcome, or death.8 Recently, Ovbiagele et al26 showed that there was no independent relationship between the presence of CKD and occurrence of sICH in patients with intravenous rt-PA. However, they found that patients with CKD were more likely to die in the hospital because of the presence of other harmful conditions, such as anemia, oxidative stress, electrolyte imbalances, and chronic inflammation.26 Our results on studies of the association between renal dysfunction and outcomes in patients with acute ischemic stroke receiving intravenous tPA showed that the presence of renal dysfunction was not associated with increased ICH or a poor functional outcome. Patients with renal dysfunction were more likely to die after intravenous thrombolysis. Notably, more than 50% of the data from our analysis including 44,410 participants and with the greater proportion of patients had CKD stages 3 to 4 versus stage 5.
The main limitation of the study is that most of the included studies were retrospective and causality cannot be proved. Second, renal function, represented by eGFR, was estimated using the Modification of Diet in Renal Disease (MDRD) formula instead of direct laboratory measurements. This difference between true GFR and eGFR may have led to misclassification of some of the subjects. However, eGFR was easily obtained before thrombolysis and served as a practical reference of renal function. Third, there was lack of adjustment for baseline differences. Fourth, there was no consensus definition of renal dysfunction and sICH. Finally, there was a lack of individual patient data and a limited number of trials. Therefore, we were not able to assess whether there were significant differences in treatment effects in important subgroups, such as patients treated within 3 hours compared with those treated later.
CONCLUSIONS
Renal dysfunction does not increase the risk of poor outcome and ICH after stroke thrombolysis. Renal dysfunction should not be a contraindication for administration of intravenous thrombolysis to eligible patients.
Footnotes
Abbreviations: CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, MDRD = Modification of Diet in Renal Disease, mRS = modified Rankin Scale, NG = not given, NOS = Newcastle-Ottawa Scale, OR = Odds Ratio, sICH = symptomatic intracerebral hemorrhage, tPA = tissue plasminogen activator.
Zilong Hao and Chunsong Yang contributed equally to this study.
This research was supported by the Science and Technology Infrastructure Projects of Sichuan Province (2012JCPT008).
ZH and ML designed the study. ZH and CY performed the literature searches and data extraction. ZH wrote the first draft of the manuscript. All authors helped to organize the information, and wrote and edited subsequent drafts of the manuscript.
The authors have no competing interests to declare.
REFERENCES
- 1.Tissue plasminogen activator for acute ischemic, stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med 1995; 333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 3.Rowat A, Graham C, Dennis M. Renal dysfunction in stroke patients: a hospital-based cohort study and systematic review. Int J Stroke 2014; 9:633–639. [DOI] [PubMed] [Google Scholar]
- 4.Hojs Fabjan T, Hojs R, Tetickovic E, et al. Ischaemic stroke–impact of renal dysfunction on in-hospital mortality. Eur J Neurol. 2007; 14:1351–1356. [DOI] [PubMed] [Google Scholar]
- 5.Yahalom G, Schwartz R, Schwammenthal Y, et al. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke 2009; 40:1296–1303. [DOI] [PubMed] [Google Scholar]
- 6.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2013; 44:870–947. [DOI] [PubMed] [Google Scholar]
- 7.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack. Cerebrovasc Dis 2008; 25:457–507. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal V, Rai B, Fellows J, et al. In-hospital outcomes with thrombolytic therapy in patients with renal dysfunction presenting with acute ischaemic stroke. Nephrol Dial Transplant 2010; 25:1150–1157. [DOI] [PubMed] [Google Scholar]
- 9.Sobolewski P, Kozera G, Kazmierski R, et al. Intravenous rt-pa in patients with ischaemic stroke, renal dysfunction. Clin Neurol Neurosurg 2013; 115:1770–1774. [DOI] [PubMed] [Google Scholar]
- 10.Lyrer PA, Fluri F, Gisler D, et al. Renal function and outcome among stroke patients treated with iv thrombolysis. Neurology 2008; 71:1548–1550. [DOI] [PubMed] [Google Scholar]
- 11.Gensicke H, Zinkstok SM, Roos YB, et al. Iv thrombolysis and renal function. Neurology 2013; 81:1780–1788. [DOI] [PubMed] [Google Scholar]
- 12.Wells GA SB OCD, Peterson J, Welch V, et al. The newcastle–ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses. 2009. http://wwwohrica/programs/clinical_epidemiology/oxfordhtm. [Google Scholar]
- 13.Tütüncü S, Ziegler AM, Nolte CH. Response to letter regarding article, “Severe renal impairment is associated with symptomatic intracerebral hemorrhage after thrombolysis for ischemic stroke”. Stroke 2014; 45:e29. [DOI] [PubMed] [Google Scholar]
- 14.Power A. Letter by power regarding article, “Severe renal impairment is associated with symptomatic intracerebral hemorrhage after thrombolysis for ischemic stroke”. Stroke 2014; 45:e28. [DOI] [PubMed] [Google Scholar]
- 15.Hirano T. Thrombolysis, hyperacute reperfusion therapy for stroke in renal patients. Contrib Nephrol 2013; 179:110–118. [DOI] [PubMed] [Google Scholar]
- 16.Kamouchi M. Stroke features, management in patients with chronic kidney disease. Contrib Nephrol 2013; 179:92–99. [DOI] [PubMed] [Google Scholar]
- 17.Lee JG, Lee KB, Jang IM, et al. Low glomerular filtration rate increases hemorrhagic transformation in acute ischemic stroke. Cerebrovasc Dis 2013; 35:53–59. [DOI] [PubMed] [Google Scholar]
- 18.Whiteley WN, Slot KB, Fernandes P, et al. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review, meta-analysis of 55 studies. Stroke 2012; 43:2904–2909. [DOI] [PubMed] [Google Scholar]
- 19.Palacio S, Gonzales NR, Sangha NS, et al. Thrombolysis for acute stroke in hemodialysis: International survey of expert opinion. Clin J Am Soc Nephrol 2011; 6:1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu K, Mars WM, et al. Novel actions of tissue-type plasminogen activator in chronic kidney disease. Front Biosci 2008; 13:5174–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opatrny K, Jr, Zemanova P, Opatrna S, et al. Fibrinolysis in chronic renal failure, dialysis and renal transplantation. Ann Transplant 2002; 7:34–43. [PubMed] [Google Scholar]
- 22.Khalid MI. Bleeding complications after thrombolysis. BMJ 1993; 307:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power A. Stroke in dialysis and chronic kidney disease. Blood Purif 2013; 36:179–183. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi T, Yamada T, Hirota T, et al. Prognostic factors for successful short term outcome (the modified rankin score <2; mrs) after thrombolysis,. J Neurol 2014; 261:S416. [Google Scholar]
- 25.Rakusa M, Dzordzevic M, Menih M. Reducing frequency of symptomatic intracranial hemorrhage in patients with acute ischemic stroke treated by recombinant tissue-plasminogen activator; interim result of a prospective observational cohort study. Stroke 2013; 44: (2 Meeting Abstract). [Google Scholar]
- 26.Ovbiagele B, Smith EE, Schwamm LH, et al. Chronic kidney disease and bleeding complications after intravenous thrombolytic therapy for acute ischemic stroke. Circ Cardiovasc Qual Outcomes 2014; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Chao TH, Lin TC, Shieh Y, et al. Intracerebral hemorrhage after thrombolytic therapy in acute ischemic stroke patients with renal dysfunction. Eur Neurol 2013; 70:316–321. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh CY, Lin HJ, Sung SF, et al. Is renal dysfunction associated with adverse stroke outcome after thrombolytic therapy? Cerebrovasc Dis 2014; 37:51–56. [DOI] [PubMed] [Google Scholar]
- 29.Power A, Epstein D, Cohen D, et al. Renal impairment reduces the efficacy of thrombolytic therapy in acute ischemic stroke. Cerebrovasc Dis 2013; 35:45–52. [DOI] [PubMed] [Google Scholar]
- 30.Naganuma M, Koga M, Shiokawa Y, et al. Reduced estimated glomerular filtration rate is associated with stroke outcome after intravenous rt-pa: the stroke acute management with urgent risk-factor assessment and improvement (samurai) rt-pa registry. Cerebrovasc Dis 2011; 31:123–129. [DOI] [PubMed] [Google Scholar]
- 31.Tütüncü S, Ziegler AM, Scheitz JF, et al. Severe renal impairment is associated with symptomatic intracerebral hemorrhage after thrombolysis for ischemic stroke. Stroke 2013; 44:3217–3219. [DOI] [PubMed] [Google Scholar]
- 32.Marsh EB, Gottesman RF, Hillis AE, et al. Serum creatinine may indicate risk of symptomatic intracranial hemorrhage after intravenous tissue plasminogen activator (iv tpa). Medicine (Baltimore) 2013; 92:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Churilov L, Meretoja A, et al. Elevated urea level is associated with poor clinical outcome and increased mortality post intravenous tissue plasminogen activator in stroke patients. J Neurol Sci 2013; 332:110–115. [DOI] [PubMed] [Google Scholar]
- 34.Chen CH, Tang SC, Tsai LK, et al. Proteinuria independently predicts unfavorable outcome of ischemic stroke patients receiving intravenous thrombolysis. PLoS ONE 2013; 8:e80527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho BH, Kim JT, Chang J, et al. Prediction of hemorrhagic transformation in acute ischaemic stroke by micro- and macroalbuminuria after intravenous thrombolysis. Eur J Neurol 2013; 20:1145–1152. [DOI] [PubMed] [Google Scholar]
- 36.Ovbiagele B. Impairment in glomerular filtration rate or glomerular filtration barrier and occurrence of stroke. Arch Neurol 2008; 65:934–938. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama M, Metoki H, Terawaki H, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general japanese population-the ohasama study. Nephrol Dial Transplant 2007; 22:1910–1915. [DOI] [PubMed] [Google Scholar]
- 38.Nickolas TL, Khatri M, Boden-Albala B, et al. The association between kidney disease and cardiovascular risk in a multiethnic cohort: findings from the northern manhattan study (nomas). Stroke 2008; 39:2876–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]


