Abstract
Epidemiologic studies have reported increased incidence, prevalence and acuity of periodontitis in adults with diabetes and some have also suggested that treating periodontal disease may improve glycemic control in diabetic patients.
This meta-analysis was conducted to evaluate the effects of different periodontal therapies on metabolic control in patients with type 2 diabetes mellitus (T2DM) and periodontal disease.
We searched the Medline, EMBASE and Cochrane Library (Central) databases up to January 2014 for relevant studies pertaining to periodontal treatments and glycemic control in adults with T2DM. The search terms were periodontal treatment/periodontal therapy, diabetes/diabetes mellitus, periodontitis/periodontal and glycemic control. The primary outcome measure taken from the included studies was glycated hemoglobin (HbA1c).
We compared differences in patients’ pre- and post-intervention HbA1c results between a treatment group receiving scaling and root planing (SRP) combined with administration of oral doxycycline (n = 71) and controls receiving SRP alone or SRP plus placebo (n = 72). Meta-analysis was performed using Comprehensive Meta Analysis software.
Nineteen randomized controlled trials (RCTs) were identified. Four trials involving a total of 143 patients with T2DM and periodontal disease were determined to be eligible for analysis. Data of 1 study were not retained for meta-analysis because HbA1c results were recorded as median with IQR. Meta-analysis of the included 3 studies revealed no significant differences in HbA1c results between the periodontal treatment group (n = 71) and control group (n = 72) (HbA1c SMD = −0.238, 95% CI = −0.616 to 0.140; P = 0.217).
Systemic doxycycline added to SRP does not significantly improve metabolic control in patients with T2DM and chronic periodontitis. Current evidence is insufficient to support a significant association between periodontal therapy and metabolic control in this patient population. However, evidence suggests that periodontal therapy itself improves metabolic control and reinforces that T2DM is a risk factor for periodontitis.
INTRODUCTION
Periodontal disease (PD) is a chronic inflammatory disease that destroys the gingiva or tooth-supporting tissues. It is one of the most prevalent chronic infections in adults between ages 30 and 90 years in the United States and the most prevalent dental disease in people with diabetes, affecting up to 22% of diabetic patients.1 PD is reported to affect about 90% of people globally.2 The 2 major forms of PD, gingivitis and periodontitis, are the result of bacterial plaque, which ultimately destroys gingival tissue and periodontal attachment apparatus.3 Although tooth-adherent microorganisms initiate PD, the individual inflammatory response along with concomitant chronic disease such as cardiovascular disease, chronic pulmonary disorders and diabetes, is responsible for the chronic nature of the disease and eventual breakdown of tissue. These conditions suggest that type 2 diabetes mellitus (T2DM) is a risk factor for periodontitis.1 Epidemiologic studies over the years have consistently reported that increased incidence, prevalence and acuity of periodontitis is found among adults with T2DM.4 Incidence of periodontitis increases as diabetic patients age and is both more frequent and more severe in T2DM patients with advanced systemic complications.5
Studies of diabetes patients with PD have examined different treatments for gingivitis and periodontitis that are targeted toward reducing oral bacteria and associated calculus. Patients with gingivitis without concomitant disease that influences oral health, may respond well to simply improving personal plaque control using a mix of mechanical and hygienic processes.6 However, self-treatment alone is inconsistent and seldom results in maintaining plaque-free status, so professional reinforcement is usually advised.6 Treatment of periodontitis is directed toward removing pathogenic bacteria and preventing recolonization; correcting modifiable risk factors such as smoking, alcohol abuse, medications or stress; and controlling systemic conditions such as autoimmune disease, diabetes mellitus, cardiovascular disease, lung disease, and osteoporosis.7 Standard nonsurgical treatment for PD includes scaling and root planing (SRP), thorough removal of calculus and debris from tooth surfaces and gingiva, and adjunctive therapy such as laser treatment.8 Adjunctive treatment also may include local or systemic antimicrobial administration,9 and improving inflammatory host response via non-steroidal anti-inflammatory agents or biophosphonates.7
Although many studies have demonstrated associations between T2DM and PD, fewer studies have addressed whether effective treatment of periodontal disease effectively improves glycemic control. Some studies have shown that treating periodontal conditions results in improved metabolic control.10–12 The results of previously published meta-analyses13 and randomized controlled trials (RCTs)14,15 also suggest that periodontal treatment can improve glycemic control in T2DM patients. Nevertheless, although nonsurgical treatment of periodontitis may improve periodontal and inflammatory status in diabetic patients, strong evidence is lacking to support improved glycemic control.16 Current evidence is conflicting and appears to be insufficient to make clinical recommendations with confidence. Therefore, the purpose of the present meta-analysis was to evaluate the reported effects of periodontal therapies on metabolic control in T2DM patients with PD.
METHODS
Literature Search
The Medline, EMBASE and Cochrane Library (CENTRAL) databases were searched up to January 2014 using the following search terms: periodontal treatment/periodontal therapy, diabetes/diabetes mellitus, periodontitis/periodontal, and glycemic control. Reference lists of the relevant studies were searched manually. Titles and abstracts were screened for all studies and full-text was then obtained for those that met the inclusion criteria (below).
Study Selection
Studies that met the following criteria were included: an original investigation excluding review articles and meta-analyses; published RCT only; study subjects with T2DM and PD; comparison of different periodontal therapies; and studies providing comparable numerical results for glycated hemoglobin (HbA1c) measurement. Case reports, letters to the editor, systematic reviews and commentaries were excluded.
We initially identified RCTs that had investigated periodontal treatments and glycemic control in adults with T2DM. The primary outcome measure in the included studies was HbA1c, and differences between pre- and post-intervention HbA1c were compared in a treatment group and controls in all studies. Two author-reviewers independently determined the eligibility of all retrieved studies and uncertainties were resolved through discussion or by consultation with a third author-reviewer. The internal review board of National Yang Ming University reviewed and approved the study protocol for this meta-analysis.
Data Extraction and Quality Assessment of Included Studies
Data were extracted by two author-reviewers, including first author's name, year of publication, study design, treatments/interventions, number of participants in each group; and participants’ ages and gender, periodontal disease index, follow-up period and HbA1c as primary outcome. The methodological quality of each study was assessed using the risk-of-bias assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0.).17 Two author-reviewers subjectively reviewed all studies and assigned a value of “low risk,” “high risk,” or “unclear” to the following aspects: (a) random sequence generation; (b) allocation concealment; (c) blinding function (patients, personnel, and assessors); (d) adequate assessment of each outcome; (e) avoidance of selective outcome reporting; and (f) whether or not intention-to-treat analysis was included.
Primary Outcome Measure
The main outcome measure is the differences in patients’ HbA1c values between pre- (baseline) and post-treatment/intervention (at final visit) in the included studies.
Statistical Analysis
Differences in patients’ HbA1c values from baseline to final visit were compared between participants receiving treatment (treatment group) vs those receiving standard control therapies (control group). An χ2-based test of homogeneity was performed and the Q and inconsistency index (I2) statistics were determined. If I2 was >50% or >75%, the trials were considered to be heterogeneous or highly heterogeneous, respectively. If I2 was <25%, the studies were considered to be homogeneous. If the I2 statistic (>50%) indicated that heterogeneity existed between studies, a random-effects model (DerSimonian–Laird method) was performed. Otherwise, fixed-effects models (Mantel–Haenszel method) were performed. For each outcome measure, standardized mean difference (SMD) with corresponding 95% confidence intervals (CIs: lower and upper limits) were calculated for each individual study and for the studies combined. A two-sided P-value <0.05 was established as statistical significance when comparing groups. Sensitivity analysis of the outcomes was performed using the leave-one-out approach. Due to the small number of selected studies, assessing for publication bias using funnel plot was considered inappropriate.18 All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
RESULTS
Literature Search
Detailed search procedures are summarized in Figure 1. The database search identified 19 studies, of which 4 met the inclusion criteria and were potentially eligible for analysis.19–22 Fifteen studies were excluded10,12,14–16,23–32 based on lack of treatment intervention,7 an untreated control group,5 and no numerical results data.1 The full text of the 4 potentially eligible studies met all inclusion criteria and these studies were determined to be eligible for inclusion in the meta-analysis (Figure 1).
FIGURE 1.

Flow chart of literature search and study selection.
Baseline Characteristics of Selected Studies
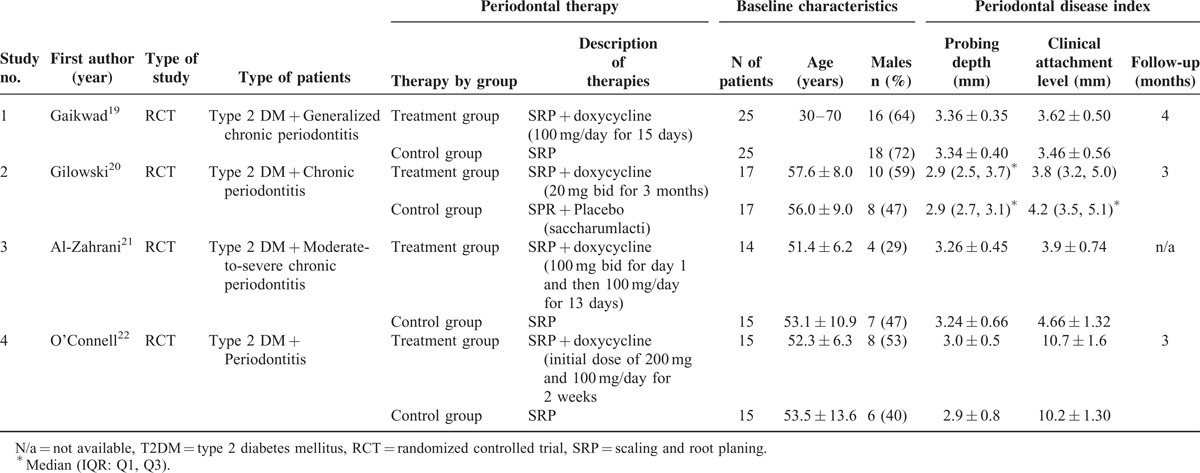
Characteristics of the 4 included studies are summarized in Table 1. Enrolled subjects in the 4 RCTs included a total of 143 patients with T2DM and PD; periodontitis was described as “chronic” or “severe chronic” in the included studies. Participants’ ages were similar among all studies and between therapies; gender distribution varied between studies and between treatment and control groups in individual studies. Overall, 71 patients received SRP combined with administration of oral doxycycline (treatment group), while 72 participants received SRP alone or SRP plus placebo (control group) (Table 1).
TABLE 1.
Baseline Characteristics of the Included Studies
Quality Assessment of Included Studies
The risk of bias in the included studies is summarized in Figure 2A and an overall assessment of risk of bias is presented in Figure 2B. Assessment of the studies by independent reviewers revealed the following: (a) random sequence generation was appropriate in all 4 studies; (b) allocation concealment was appropriate in 3 studies;19–21 (c) only 1 study reported personnel, participants and assessor blinded to treatment,20 (d) all studies were judged to be at low risk of attrition bias; (e) 2 studies did not have reporting bias,20,22 and (f) intention-to-treat analysis was not included in any of the 4 studies.
FIGURE 2.
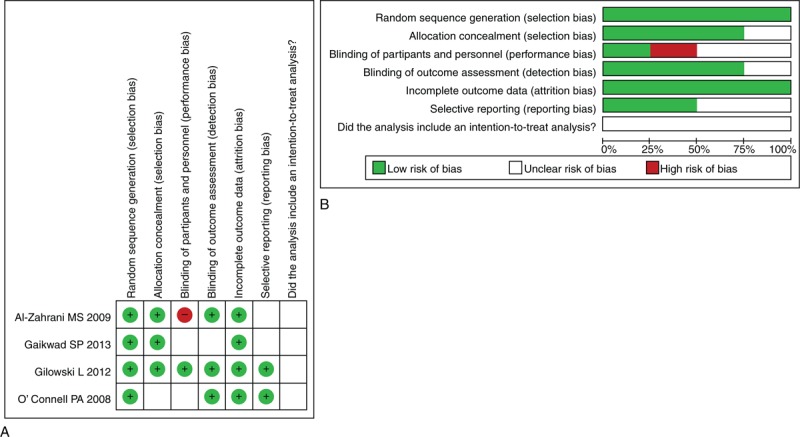
Assessment of risk of bias. (A) Summary of risk assessment of bias (B) Overall risk assessment of bias.
Study Outcomes
The primary outcomes are the HbA1c results of patients in the included studies, which are summarized in Table 2. Data of 1 study20 were not retained for meta-analysis because HbA1c results were recorded as median with IQR.
TABLE 2.
Differences in Subjects’ HbA1c Results at Baseline and Final Visit by Study

HbA1c SMD Forest Plot
Figure 3 shows the Forest plot for the SMD of the primary outcome HbA1c between patients in the treatment group and those in the control group. A fixed-effect analysis was applied because there was no evidence of heterogeneity among the studies (Q-statistic = 1.093, I2 = 0%, P = 0.579). Meta-analysis of the included 3 studies revealed no significant differences in HbA1c results between all patients in the periodontal treatment group (n = 71) and control group (n = 72) (HbA1c SMD = −0.238, 95% CI = −0.616 to 0.140; P = 0.217) (Figure 3).
FIGURE 3.

Forrest plot comparing before/after HbA1c in treated subjects vs control subjects by study. CI = confidence interval, Lower limit = lower bound of 95% CI, SMD = standardized mean difference, Upper limit = upper bound of 95% CI, 1st AU = first author.
Sensitivity Analysis
Figure 4 shows the Forrest plot for the SMD of HbA1c with individual studies evaluated separately among the treatment group and the control group. The direction and magnitude did not change with the exclusion of individual studies, indicating good reliability of the meta-analysis (Figure 4).
FIGURE 4.
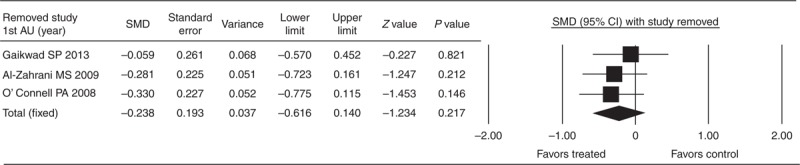
Sensitivity analysis of the influence of each study on pooled estimates of HbA1c. The leave-one-out approach was used. CI = confidence interval, Lower limit = lower bound of 95% CI, Upper limit = upper bound of 95% CI, SMD = standardized mean difference, 1st AU = first author.
DISCUSSION
In the present meta-analysis, among the 19 RCTs identified initially, only 4 with a total of 143 patients with T2DM and PD were determined to be eligible for analysis. Data of 15 studies were excluded and data of 1 study were excluded from meta-analysis because HbA1c results were shown as median with IQR. Meta-analysis of the remaining 3 studies revealed no significant differences in HbA1c results between the periodontal treatment group and control group, which does not appear to support an association between periodontal therapy with doxycycline and metabolic control in T2DM patients with periodontal disease even though HbA1c levels decreased somewhat in both groups.
A study conducted in China demonstrated that non-surgical therapy for periodontitis was beneficial in both maintaining periodontal health and reducing blood glucose levels in patients with T2DM and chronic PD.10 The treatment group in that study received doxycycline and SRP as in the present study, resulting in significantly reduced plaque index and bleeding on probing scores and improved HbA1c results compared to controls. Stewart et al12 also found that periodontal treatment for 9 months had a 17.1% improvement in glucose levels compared to those of controls. Another study found that inflammatory cytokine levels decreased and adiponectin levels increased in patients with moderately or poorly controlled T2DM.32 Our study focused on the differences in pre- and post-intervention HbA1c results as a measure of glycemic control, while other studies relied on outcomes of plaque index, probing scores, glucose levels and other glycemic parameters.
It must be remembered when comparing studies that HbA1c results monitor overall glycemic control achieved within the previous 2 to 3 months and the intervals between tests must be at least 2 months to be able to note relevant differences or changes between pre- and post-intervention.16 This was considered in each of the 4 studies selected for analysis and all authors had included appropriate intervals in follow-up times. However, in the interpretation of HbA1c results, SMDs are especially important as a uniform scale applicable in meta-analysis when methodological differences in HbA1c measurement are noted between studies. For example, although 1 study was excluded from meta-analysis in the present study because HbA1c results were shown as median with IQR, the HbA1c SMD of the included studies was −0.238 (95% CI = −0.616 to 0.140; P = 0.217). One meta-analysis by Teeuw et al33 demonstrated a weighted mean difference in HbA1c for pre- and post-intervention of 0.40 (95% CI 0.77 to −0.04%, P = 0.03), which favored periodontal intervention in patients with T2DM. However, this improvement in HbA1c must be interpreted carefully due to limited robustness as evidenced by heterogeneity among studies selected for meta-analysis. Darré et al34 reported an SMD of 0.46 (95% CI 0.11 to 0.82), indicating a significant reduction in HbA1c post-treatment. The meta-analysis by Janket et al13 reported a weighted average decrease of 0.38% in actual HbA1c levels of all included studies, 0.66% when restricted to patients with T2DM and 0.71% when patients also received antibiotic treatment. However, differences in pre- and post-intervention HbA1c results were not significant.
A previous meta-analysis of intervention studies that focused on the effects of periodontal treatment on glycemic control in T2DM patients suggested that successful management of periodontal infection leads to both reductions in local symptoms of the disease and improved control of glucose metabolism. However, in that meta-analysis, post-intervention results were not significantly different from baseline results.13 In the RCT conducted by Singh et al,14 2 treatment groups were employed, 1 receiving systemic doxycycline plus SRP and 1 receiving SRP alone, and effects of periodontal treatment on metabolic control in diabetes patients were evaluated. Those investigators found a statistically significant improvement in glycemic control in the group receiving periodontal treatment combined with the antibacterial effects of doxycycline, which was demonstrated by improved periodontal results and reduced HbA1c results; however, the decreases in fasting blood glucose and 2-hour post-prandial blood glucose levels were not significant. Systemic doxycycline inhibits metalloproteinase activity and also has antimicrobial effects beneficial to controlling periodontal inflammation. With these special advantages of docycycline, it is not surprising that the combination therapy in the treatment group in our study had measureable clinical effects compared to the control group, and had noticeable but non-significant effects in other studies.10,12 Among our included studies, anti-infective and systemic doxycycline periodontal therapy was shown to influence patients’ systemic conditions favorably even though statistically significant differences were not observed. However, 1 study suggested that the noted improvements in glycemic control and reduced inflammatory markers could just as easily have been the result of diet,22 and yet diet was not controlled in that study nor evaluated in any of the included studies. Previous systematic reviews and meta-analyses have suggested that periodontal therapy has a positive effect on glycemic control in patients with T2DM.13,17,33,34 Results of a recent meta-analysis by Sgolastra et al35 indicated that SRP significantly reduced HbA1c in the diabetic dental patients of 5 RCTs, which supports the effectiveness of SRP alone in improving glycemic control in T2DM patients with chronic PD. In the present study, it appears that even in the absence of significant differences between groups with and without doxycycline therapy, the measurable clinical effects are definitely signs of improved metabolic control, and the lack of significant between-group differences primarily suggests that periodontal therapy itself is able to improve metabolic control.
Limitations
Results of this study are limited by the small number of studies analyzed and the related small number of subjects. In this meta-analysis, the lack of significant differences in HbA1c results between all patients in the periodontal treatment group (n = 71) and control group (n = 72) suggests that evidence in the small number of included studies may be insufficient to demonstrate significant differences between the SRP + doxycycline treatment in the treatment group vs that of SRP and placebo in the controls. Typically, a meta-analysis is conducted because of the advantage of having greater statistical power and its value as an evidence-based resource able to extrapolate confirmatory data analysis across the affected population. However, the lack of sufficient evidence in the present meta-analysis may not contribute to making clinical recommendations with confidence. More RCTs with larger cohorts and longer-term treatment are still needed to evaluate variables of periodontal disease and glycemic control and to investigate associations between periodontal therapy and metabolic control in T2DM patients with PD.
CONCLUSIONS
In conclusion, adding doxycycline to periodontal therapy with SRP does not significantly improve metabolic control in patients with T2DM and chronic periodontitis. Currently, available evidence is insufficient to support a significant association between periodontal therapy and metabolic control in T2DM patients with PD, however, evidence suggests that periodontal therapy itself improves metabolic control. Results also reinforce that diabetes is a risk factor for periodontitis. More and larger RCTs are needed before findings of metabolic control in T2DM patients with PD can be generalized to other patients with diabetes.
Footnotes
Abbreviations: HbA1c = glycated hemoglobin, PD = periodontal disease, RCT = randomized controlled trial, SMD = standardized mean difference, SRP = scaling and root planing, T2DM = type 2 diabetes mellitus.
No funding was received for this study.
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States 1988–1994. J Periodontol 1999; 70:13–29. [DOI] [PubMed] [Google Scholar]
- 2.Cullinan MP, Ford PJ, Seymour GJ. Periodontal disease and systemic health: current status. Aust Dent J 2009; 54:1S62–69. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Periodontology The pathogenesis of periodontal diseases. Periodontol 1999; 70:457–470. [DOI] [PubMed] [Google Scholar]
- 4.Löe H. Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care 1993; 16:329–334. [PubMed] [Google Scholar]
- 5.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol 2006; 77:1289–1303. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Periodontology Research Therapy Committee Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. Pediatr Dent 2004; 35:13–14. [Google Scholar]
- 7.Serio FG, Duncan TB. The pathogenesis and treatment of periodontal disease. 2010. http://www.ineedce.com Accessed March 7, 2014. [Google Scholar]
- 8.Kamma JJ, Vasdekis VG, Romanos GE. The effect of diode laser (980 nm) treatment on aggressive periodontitis: evaluation of microbial and clinical parameters. Photomed Laser Surg 2009; 27:11–19. [DOI] [PubMed] [Google Scholar]
- 9.Mobelli A, Samaranayake LP. Topical and systemic antibiotics in the management of periodontal diseases. Int Dent J 2004; 54:3–14. [DOI] [PubMed] [Google Scholar]
- 10.Yun D, Firkova EI, Lin JQ, Xun H. Effect of non-surgical periodontal therapy on patients with type 2 diabetes mellitus. Folia Medica 2007. [PubMed] [Google Scholar]
- 11.Wang TT, Chen TH, Wang PE. A population-based study on the association between type 2 diabetes and periodontal disease in 12,123 middle-aged Taiwanese. J Clin Periodontol 2009; 36:372–379. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol 2001; 28:306–310. [DOI] [PubMed] [Google Scholar]
- 13.Janket SJ, Wightman A, Baired AE, et al. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res 2005; 84:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S, Kumar V, Kumar S, Subbappa A. The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Diabetes in Developing Countries 2008; 28:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koromantzos PA, Makrilakis K, Dereka X, et al. A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol 2011; 38:142–147. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Luo G, Xuan D, et al. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic controlin patients with type 2 diabetes: a randomized study. J Periodontol 2012; 83:435–443. [DOI] [PubMed] [Google Scholar]
- 17.The Cochrane Collaboration; 2011; Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [Google Scholar]
- 18.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000; 320:1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaikwad SP, Gurav AN, Shete AR, Desarda HM. Effect of scaling and root planing combined with systemic doxycycline therapy on glycemic control in diabetes mellitus subjects with chronic generalized periodontitis: a clinical study. J Periodontal Implant Sci 2013; 43:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilowski L, Kondzielnik P, Wiench R, et al. Efficacy of short-term adjunctive subantimicrobial dose doxycycline in diabetic patients—a randomized study. Oral Dis 2012; 18:763–770. [DOI] [PubMed] [Google Scholar]
- 21.Al-Zahrani MS, Bamshmous SO, Alhassani AA, Al-Sherbini MM. Short-term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J Periodontol 2009; 80:1568–1573. [DOI] [PubMed] [Google Scholar]
- 22.O’Connell PA, Taba M, Nomizo A, et al. Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol 2008; 79:774–783. [DOI] [PubMed] [Google Scholar]
- 23.Santos VR, Lima JA, Miranda TS, et al. Full-mouth disinfection as a therapeutic protocol for type-2 diabetic subjects with chronic periodontitis: twelve-month clinical outcomes: a randomized controlled clinical trial. J Clin Periodontol 2013; 40:155–162. [DOI] [PubMed] [Google Scholar]
- 24.Moeintaghavi A, Arab HR, Bozorgnia Y, et al. Non-surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust Dent J 2012; 57:31–37. [DOI] [PubMed] [Google Scholar]
- 25.Khader YS, Al Habashneh R, Al Malalheh M, Bataineh A. The effect of full-mouth tooth extraction on glycemic control among patients with type 2 diabetes requiring extraction of all remaining teeth: a randomized clinical trial. J Periodontal Res 2010; 45:741–747. [DOI] [PubMed] [Google Scholar]
- 26.Santos VR, Lima JA, De Mendonça AC, et al. Effectiveness of full-mouth and partial-mouth scaling and root planing in treating chronic periodontitis in subjects with type 2 diabetes. J Periodontol 2009; 80:1237–1245. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri S, Nitta H, Nagasawa T, et al. Multi-center intervention study on glycohemoglobin (HbA1c) and serum, high-sensitivity CRP (hs-CRP) after local anti-infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res Clin Pract 2009; 83:308–315. [DOI] [PubMed] [Google Scholar]
- 28.Promsudthi A, Pimapansri S, Deerochanawong C, Kanchanavasita W. The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis 2005; 11:293–298. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues DC, Taba MJ, Novaes AB, et al. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol 2003; 74:1361–1367. [DOI] [PubMed] [Google Scholar]
- 30.Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol 1997; 68:713–719. [DOI] [PubMed] [Google Scholar]
- 31.Telgi RL, Tandon V, Tangade PS, et al. Efficacy of nonsurgical periodontal therapy on glycaemic controlin type II diabetic patients: a randomized controlled clinical trial. J Periodontal Implant Sci 2013; 43:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun WL, Chen LL, Zhang SZ, et al. Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med 2011; 50:1569–1574. [DOI] [PubMed] [Google Scholar]
- 33.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care 2010; 33:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darré L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabetes Metab 2008; 34:497–506. [DOI] [PubMed] [Google Scholar]
- 35.Sgolastra F, Severin M, Pietropaoli D, et al. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol 2013; 84:958–973. [DOI] [PubMed] [Google Scholar]


