Abstract
Aupuncture is widely used for functional constipation. Effect of acupuncture might be related to the depth of needling; however, the evidence is limited. This trial aimed to evaluate the effect and safety of deep needling and shallow needling for functional constipation, and to assess if the deep needling and shallow needling are superior to lactulose.
We conducted a prospective, superiority-design, 5-center, 3-arm randomized controlled trial. A total of 475 patients with functional constipation were randomized to the deep needling group (237), shallow needling group (119), and lactulose-controlled group (119) in a ratio of 2:1:1. Sessions lasted 30 minutes each time and took place 5 times a week for 4 weeks in 2 acupuncture groups. Participants in the lactulose group took lactulose orally for 16 continuous weeks. The primary outcome was the change from baseline of mean weekly spontaneous bowel movements (SBMs) during week 1 to 4 (changes from the baselines of the weekly SBMs at week 8 and week 16 in follow-up period were also assessed simultaneously). Secondary outcomes were the weekly SBMs of each assessing week, the mean score change from the baseline of constipation-related symptoms over week 1 to 4, and the time to the first SBM. Emergency drug usage and adverse effects were monitored throughout the study.
SBMs and constipation-related symptoms were all improved in the 3 groups compared with baseline at each time frame (P < 0.01, all). The changes in the mean weekly SBMs over week 1 to 4 were 2 (1.75) in the deep needling group, 2 (1.75) in the shallow needling group, and 2 (2) in the lactulose group (P > 0.05, both compared with the lactulose group). The changes of mean weekly SBMs at week 8 and week 16 in the follow-up period were 2 (2), 2 (2.5) in the deep needling group, 2 (3), 1.5 (2.5) in the shallow needling group, and 1 (2), 1 (2) in the lactulose group (P < 0.05, all compared with the lactulose group). No significant difference was observed among the 3 groups regarding the score changes of straining, incomplete evacuation, abdominal distention during spontaneous defecating, or Cleveland Clinic Scores over week 1 to 4. However, the lactulose group got better effect than other 2 acupuncture groups in improving stool consistency (P < 0.01, both) and shortening the time to the first SBM (P < 0.05, both). The percentage of emergency drugs used in the 2 acupuncture groups were both lower than in the lactulose group at each time frame (P < 0.01, all). No obvious adverse event was observed in the deep or shallow needling group.
Deep and shallow needling at Tianshu (ST25) can improve intestinal function remarkably and safely. Therapeutic effects of deep and shallow needling are not superior to that of lactulose; however, the sustained effects of deep and shallow needling after stopping the acupuncture treatments are superior to the therapeutic effect of lactulose, which might qualify the superiority of deep and shallow needling.
BACKGROUND
Functional constipation, which is not caused by organic problems or medicines, is a functional bowel disorder that presents as persistently difficult, infrequent, or incomplete defecation but does not meet the criteria for irritable bowel syndrome (IBS).1 This condition is so common that it affects nearly 12% of people around the world,2 with a prevalence of approximately 15% in North America,3 8.75% in Europe,4 and 3% to 17% in China.5 Systematic review has indicated that acupuncture might be effective for constipation.6 In the acupoints used for treating constipation, Tianshu (ST25) tops the list, at approximately 60.17%.7 In Western medicine, lactulose is recommended by the American College of Gastroenterology (Grade A recommendation) and has been shown to be effective in producing bowel movements (BMs) and improving stool consistency.3 Therefore, the effects of acupuncture can be better tested by using lactulose as a control treatment.
At present, the evidence supporting acupuncture for functional constipation is limited and lacking in quality,8 trials comparing the effects of deep needling and shallow needling for functional constipation are especially rare. In addition to acupoint selecting, the effect and safety of acupuncture might also be related to the depth of the needling.9 In our previous study on deep needling, using the depth of 30 to 70 mm at ST25 for functional constipation, weekly spontaneous bowel movements (SBMs) increased from 1.79 ± 1.05 times at baseline to 3.90 ± 1.43 times after 4 weeks of treatment (P < 0.01). Increases in the SBMs from the baseline were maintained at 1.67 ± 1.43 and 1.71 ± 1.39 times, respectively, at the 12-week and 6-month follow-ups after the treatment.10 Nonetheless, in Japan and Korea, the depth of needling is usually quite shallow, at approximately 2 to 4 mm.11,12 Hence, our trial was designed to evaluate the effect and safety of deep and shallow needling for functional constipation, and to assess whether the effectiveness of deep and shallow needling at ST25 are superior to lactulose.
METHODS
Trial Design
A prospective, superiority-designed, multicenter, 3-arm randomized controlled trial was conducted between March 2008 and September 2011. Participants were randomized into the deep needling group, shallow needling group, and lactulose-controlled group at a ratio of 2:1:1. We set this proportion in view of the patients’ willingness toward the perception of strong needling and the high acceptability of deep needling in China. According to the Declaration of Helsinki,13 the protocol of this trial was reviewed and approved by the ethics committees of the responsible centers. Participants gave written informed consent. The authors vouch for the completeness and veracity of the data and data analyses. The cycle of our trial was 17 weeks, which contained a 1-week run-in period (week -1), a 4-week treatment period (week 1–4), and a 12-week follow-up period (week 5–16).
Participants
Patients with functional constipation were enrolled from 5 centers in China, including Guang’anmen Hospital, China Academy of Chinese Medical Sciences (Beijing); Third Affiliated Hospital of Najing University of Chinese Medicine (Nanjing); Jiangsu Province Hospital of Traditional Chinese Medicine (Nanjing); West China Hospital, Sichuan University (Chengdu); and Huguosi Hospital of Chinese Medicine, Beijing University of Chinese Medicine (Beijing). All of these hospitals are rated by the Chinese government as first-grade hospitals and located in medium or large cities with rich source of patients. Participants were recruited through newspaper ads, hospital posters, and websites. Prior to the baseline visit, patients were diagnosed by the clinicians of Digestive Department or Anorectal Department based on the patients’ symptoms and the history of constipation according to Rome III criteria,14 and related examinations such as stool routine, colonoscopy, or barium enema. Eligible subjects completed a 1-week screening diary that contained weekly SBMs and constipation-related symptoms. At the baseline visit, subjects returned the screening diaries and were asked to have the form for the Cleveland Constipation Score filled out by gastrointestinal or anorectal specialists. Then, the subjects were randomized into the different groups by acupuncturists. The cost of patients’ treatment and related examination were all exempted. During the follow-up period, we expressed the defecating diaries to patients for free.
Inclusion Criteria
Patients were included if they fulfilled all of the following criteria: met the diagnosis of functional constipation based on Rome III criteria,14 aged from 18 to 75 years, have never used lactulose for constipation in the past, no use of medicine for constipation for approximately 2 weeks before the enrollment, no acupuncture treatment for constipation in the previous 3 months, never joined any other trial in progress in the previous 3 months, volunteered to join this trial and signed the informed consent form.
Exclusion Criteria
Patients possessing any of the following exclusion criteria were omitted from the study groups: IBS or organic constipation or secondary constipation caused by endocrine, metabolic, nervous, or postoperative diseases, or by drugs; constipation with serious cardiovascular, hepatic, or renal diseases, cognitive dysfunction, aphasia, mental disorders, or illness that affects the patient's cooperation for examination and treatment; women in gestation or lactation periods; such events as abdominal aortic aneurysm or hepatosplenomegaly; blood coagulation disorders or regular anticoagulant use, including Warfarin or Heparin (exception: patients under antiplatelet treatment as using asprin or clopidogrel are eligible); or cardiac pacemaker carrier.
Interventions
All of the acupuncturists had a 5-year undergraduate education background, and all of them are registered practitioners of traditional Chinese medicine with no <2 years of clinical experience. Huatuo needles (size 0.30 × 25 mm and 0.35 × 75 mm; Suzhou Medical Appliance, Suzhou, Jiangsu Province, China), Korean EA apparatus (LH202H; Huawei Industrial Development Company, Beijing, China), and lactulose oral solution (15 mL; Solvay Pharmaceuticals B.V., Netherland) were used.
Deep Needling Group
Acupoints of bilateral ST25, which were located according to the World Health Organization Standardized Acupuncture Points Location,15 were used. After sterilizing the skin, needles of the size of 0.35 × 0.75 mm were inserted into ST25 vertically and slowly, without manipulation, for approximately 20 to 60 mm until they pierced into the muscle layer of the abdominal wall for 1 to 2 mm. Paired alligator clips of the EA apparatus were attached transversely to the needle holders of the bilateral ST25. EA stimulation lasted for 30 minutes with a dilatational wave of 2/15 Hz and current intensity of 0.1 to 1 mA. The skin around the acupoints shivering mildly indicated the proper dose.
Patients were treated once a day, 5 times a week for 4 continuous weeks.
Shallow Needling Group
Bilateral ST25, the same acupoints as in the deep needling group, were used. After skin disinfection, needles of the size of 0.30 × 25 mm penetrated the skin upright at approximately 3 to 5 mm into the fat layer without manipulation. The usage of EA apparatus and the treatment course were the same as in the deep needling group.
Lactulose-Controlled Group
Lactulose oral solution was taken orally at the dose of 15 mL once every morning after breakfast for 4 continuous weeks. The dose would increase to 30 mL if the patients have no BMs >3 days. Patients should continue to take lactulose for the next 3 months if no severe adverse effects were found.
Note that patients were asked to refrain from making any major lifestyle changes (eg, starting a new diet or changing their exercise patterns) during the trial. For patients who had no BMs for 3 or more consecutive days, bisacodyl or glycerin enema was used as an emergency drug. Details (such as date, time, and dosage) related to the usage of emergency drugs were recorded at length.
Outcome Measurements
Defecation Diaries
Patients recorded in daily diaries their SBMs, BMs, scores of straining, incomplete evacuation, stool consistency during spontaneous defecating, scores of abdominal distention, and the details of emergency drug usage. SBMs and BMs were recorded every day from 1-week run-in until the end of week 4, as well as during week 8 and week 16. Scores of straining, incomplete evacuation, stool consistency during spontaneous defecating, and abdominal distention were recorded every day from 1-week run-in until the end of week 4. Patients also recorded in their dairies the dates and times of their first treatment (by acupuncture or lactulose), and the dates and times of their first SBM after the first treatment. Furthermore, the date, time and dosage of the intake of study drug or emergency drugs (such as bisacodyl or glycerin enemas) were recorded by patients at length. Defecation within 24 hours after taking an emergency drug was considered a non-SBM, whereas defecation exceeding 24 hours was deemed a SBM.
The response rates of patients’ defecation diaries during the treatment period were 94.74% (216/228), 91.07% (102/112), and 86.09% (99/115) in deep needling, shallow needling, and lactulose groups, respectively. The rates during the follow-up period were 85.96% (196/228), 85.71% (96/112), and 85.22% (98/115) in deep needling, shallow needling, and lactulose groups, respectively.
Primary outcomes included the change from the baseline of mean weekly SBMs over week 1 to 4. Thus, the total number of SBMs was summed and divided by 4. The change in the SBMs/week at week 8 and week 16 were evaluated simultaneously. Secondary outcomes included the following: SBMs/week at each assessing week; change from the baseline of mean scores of straining (0 = absent, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe), incomplete evacuation (0 = absent, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe), stool consistency (0 = loose, 1 = soft and solid, 2 = comfortable and solid, 3 = hard and solid, 4 = hard, pellet-like) during spontaneous defecating, and abdominal distention (0 = absent, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe) over week 1 to 4; scores of straining, incomplete evacuation, and stool consistency of each SBM were summed up and divided by the times of spontaneous defecating; scores of each abdominal distention were summed up and divided by the times of abdominal distention occurrence; change from the baseline of mean weekly Cleveland Clinic Scores (CCS) over week 1 to 4 (The score was summed and divided by 4. CCS was evaluated by gastrointestinal or anorectal specialists after asking the participants about their symptoms. Content for the CCS involved 8 aspects, including spontaneous defecating frequency, straining, incomplete evacuation, abdominal pain, defecating time, assisted defecating measures, unsuccessful defecating frequency [failure to defecate although having the feeling of BMs], and a constipation course. The scores of the CCS ranged from 0 to 30, with a higher score indicating a more severe state of constipation.); time to the first SBM after the first treatment; percentage of emergency drug usage; proportion of adverse effects. The adverse effects of acupuncture include needling, pricking, fatigue, nausea, subcutaneous hemorrhage, needle breaking, local infection, abdominal infection, intestinal infection, or intestinal hemorrhage. Needling pricking was divided into 3 levels as mild, moderate, and severe. Considering the invasiveness of acupuncture, moderate or severe pricking was recorded as an adverse effect of acupuncture. The adverse effects of lactulose included diarrhea, abdominal pain, and abdominal distention.
Randomization
Participants were randomized to the deep needling group, shallow needling group, or lactulose group at a ratio of 2:1:1 by using central randomization. Acupuncturists and participants can be concealed through the method of central randomization. A random sequence was generated by the Central Randomization System of China Academy of Chinese Medical Sciences. Patients’ birthdates and sex were input by acupuncturists through phones or the Internet to obtain the random numbers. Staff members from the Clinical Evaluation Center of the China Academy of Chinese Medical Sciences who took no part in this clinic trial kept the blind codes. The randomizing scheme was not allowed to be checked by anyone except the top system administrator.
Blinding
In consideration of the differing characteristics of acupuncture and lactulose, blinding was not performed on patients or acupuncturists, except for the patients in the 2 acupuncture groups, which means that patients knew that they were treated by acupuncture not medicine but did not know to which acupuncture group they were assigned. In addition to the depth of needling, the 2 acupuncture groups had the same acupoints, parameters of electroacupuncture, and course of treatment. Participants in the deep needling group were treated and visited in the morning, whereas participants in the shallow needling group were treated and visited in the afternoon, so that we can avoid the communication among patients in different acupuncture groups. Manipulators were in charge of the patients’ randomization and clinical interventions. Gastrointestinal or anorectal specialists took charge of the clinical diagnosis and CCS evaluation (knowing nothing about the assignment), and 1 special evaluator from each center who was unaware of the assignment undertook the work of screening, data collecting, and organizing of patients’ diaries. Statistical specialists in the Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, who were also unaware of the assignment, were responsible for the statistical analysis.
Sample Size
A sample estimated formula for the multigroups is given below:
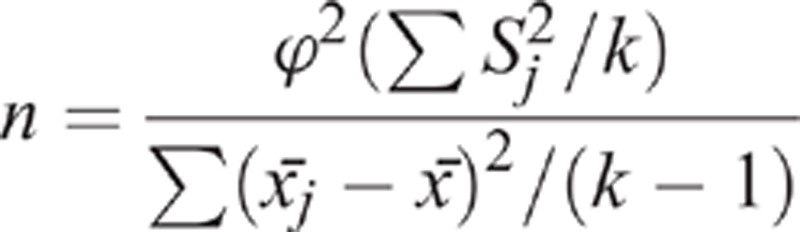 |
We aimed to assess the effects and safety of deep needling and shallow needling, and whether the effects of acupuncture are superior to lactulose. The primary outcome in this trial was the change from baseline of mean weekly SBMs over week 1 to 4. According to our pilot trial, the increase from the baseline of weekly SBMs was 3.20 ± 2.56 in the deep needling group and 2.97 ± 2.67 in the shallow needling group. Considering the enrolled participants searching for treatment in outpatient services of hospitals have more severe states of constipation, the figure using lactulose was estimated as 2.20 ± 1.7.16 With a statistical power of 90%, and a 1-sided type I error rate of 0.05%, we obtained a sample size of 390 and expanded it to 475 in view of a drop-out proportion of 20% and to balance the 3 groups.
Statistical Analysis
SPSS statistical package program (ver. 20.0, International Business Machines Corporation, China) was used. P < 0.05 was considered to be a statistically significant difference. Normally distributed measurement data were expressed with mean ± standard deviation (M ± SD), abnormally distributed measurement data were expressed with Median(Quartile Range) [M(QR)]. Differences from the baseline of normally distributed measurements were tested via t test, and differences from the baseline of abnormally distributed measurements were tested through Wilcoxon test. For testing differences among the 3 groups in the change of mean weekly SBMs over week 1 to 4, in weekly SBMs at week 1, 2, 3, 4, 8, 16, and in the change of incomplete evacuation's score over week 1 to 4, a Kruskal–Wallis test was used; a Student–Newman–Keuls test would be used for further pairwise comparison of each of the 2 groups if the 3 groups showed a significant difference. For the mean scores of straining, stool consistency, and abdominal distention, whose data had a significant difference at baseline, a covariance correction was used for correcting the difference at baseline, and we used a 1-way analysis of variance (ANOVA) to test differentiate in the score change among the 3 groups. Then a least-significant difference (LSD) test would be used to test differences for further pairwise comparison of each of the 2 groups if the 3 groups showed a significant difference. For testing differences among the 3 groups in the change of CCS score and in time to the first SBMs, an 1-way ANOVA was used; a LSD test was used for further pairwise comparison of each of the 2 groups if a significant difference was manifested among the 3 groups. A χ2 test was used for testing differences among the 3 groups in the percentage of emergency drugs used and the proportion of adverse effects. If the 3 groups exhibited significant differences, a χ2 test was also used for further pairwise comparison; however, the α here should be corrected as 0.0167 through the method of Bonferroni (0.05/3 = 0.0167). This trial used a modified intention-to-treat (m-ITT) analysis. Data sets of the m-ITT analysis have been defined as patients who accepted at least 1 treatment and had at least 7 nonmissing diary entry days after the baseline. Missing data were filled out according to the last observation carried forward (LOCF) technique to impute data for missing value. Such imputations were performed through the treatment and follow-up period approximately 14.04% (32/228) in the deep needling group, 14.29% (16/112) in the shallow needling group, and 14.78% (17/115) in the lactulose group. Participants with <7 diary days after baseline were deemed nonresponders.
RESULTS
Participants’ Flow
Of the 475 patients who signed consent forms, 455 were randomly assigned to the 3 groups and received related therapies, whereas 20 of them dropped out from this trial with no treatments (More information is detailed in Figure 1).
FIGURE 1.
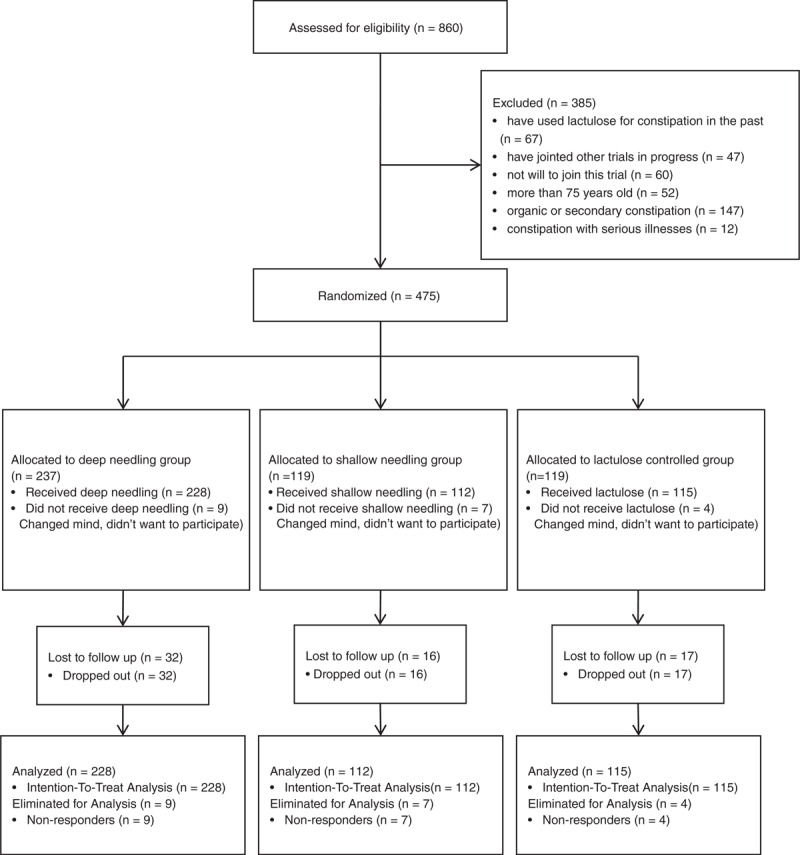
Flow chart of study participants.
Baseline Data
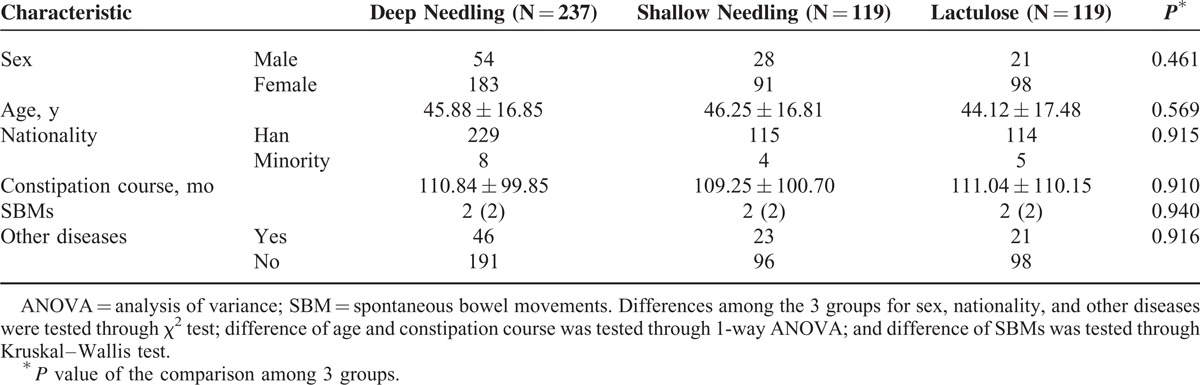
Baseline demographic and clinical characteristics of patients in the 3 groups are detailed in Table 1. There was no significant difference among the 3 groups at baseline (P > 0.05, all).
TABLE 1.
Demographic and Clinical Characteristic of Patients in 3 Groups at Baseline
Outcomes
Primary Outcome
The mean SBMs/week over week 1 to 4 were improved remarkably in the 3 groups compared with baseline (P < 0.01, all). The weekly SBMs at week 8 and week 16 in the 3 groups had also progressed (P < 0.01, all). In increasing mean weekly SBMs of deep needling and shallow needling over week 1 to 4 were not superior to lactulose, whereas at week 8 and week 16, the sustained effect of deep needling and shallow needling were superior to the therapeutic effect of lactulose.
Change of Mean Weekly SBMs/Week During Week 1 to 4
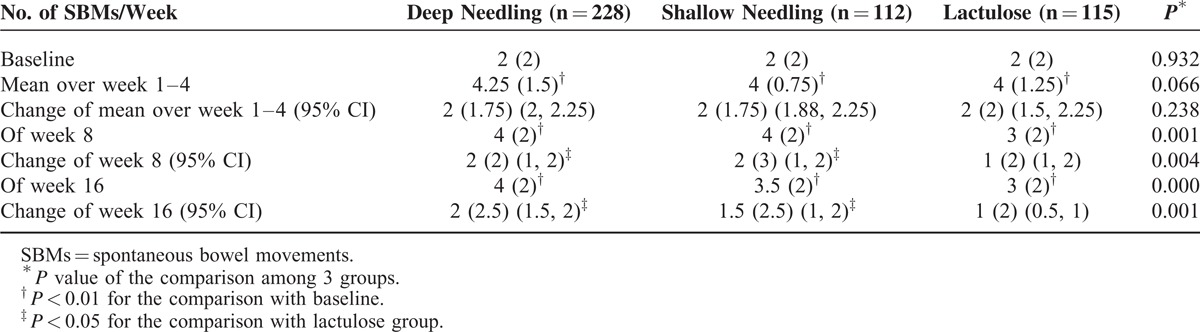
Compared with the baseline in the deep needling group, the mean value of SBMs/week over week 1 to 4 improved from 2 (2) to 4.25 (1.5), with a difference of 2 (1.75) (P < 0.01); the mean value in the shallow needling group increased from 2 (2) to 4 (0.75), with a difference of 2 (1.75) (P < 0.01); and the average number in the lactulose group grew from 2 (2) to 4 (1.25), with a difference of 2 (2) (P < 0.01). No difference was shown in the changes of the mean weekly SBMs among the 3 groups during week 1 to 4 (P = 0.238).
Change in the SBMs/Week at Week 8 and Week 16
At week 8, the SBMs/week remained at 4 (2) in the deep needling group, increasing by 2 (2) from baseline; at 4 (2) in the shallow needling group, raising by 2 (3); and at 3 (2) in the lactulose group, gaining by 1 (2). Both the deep and shallow needling groups exhibited greater improvements compared with the lactulose group (P < 0.05, respectively). At week 16, the mean weekly SBMs were 4 (2) in the deep needling group, increasing by 2 (2.5); 3.5 (2) in the shallow needling group, progressing by 1.5 (2.5); and 3 (2) in the lactulose group, gaining by 1 (2). The improvement in the SBMs/week in both the deep and shallow needling groups were greater than that in the lactulose group (P < 0.05, respectively). More information is detailed in Table 2.
TABLE 2.
Primary Outcomes
Secondary Outcomes
The weekly SBMs at week 1, 2, 3, 4, 8, and 16 in the 3 groups had all progressed compared with the baseline (P < 0.01, all), and all >3 times. More details are shown in Figure 2.
FIGURE 2.
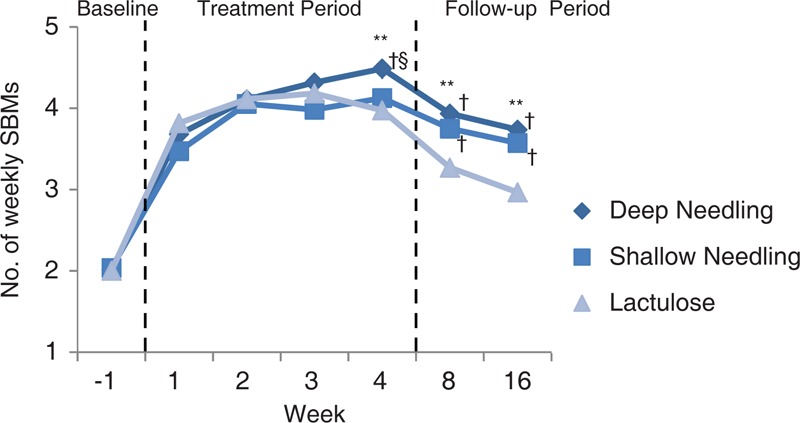
Number of SBMs/week at each assessing week. SBMs = spontaneous bowel movements. ∗∗P < 0.01 for the comparison among the 3 groups. †P < 0.05 compared with lactulose group. §P < 0.05 compared with shallow needling group.
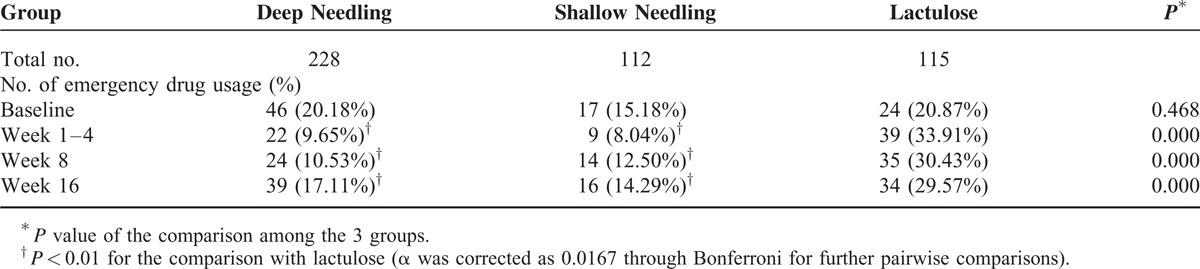
The mean scores for straining, incomplete evacuation, stool consistency during spontaneous defecating, abdominal distention, and CCS were all improved markedly in the 3 groups over week 1 to 4 from the baseline, (P < 0.01, all). There was no statistical difference among the 3 groups in improving straining, incomplete evacuation, abdominal distention, and CCS (P > 0.05, all). However, lactulose exhibit greater effect in progressing the stool consistency (P < 0.01, both compared with other 2 acupuncture groups) and shortening the time to the first SBM (16.52 ± 18.84 hours by lactulose vs 27.08 ± 27.53 hours through deep needling vs 36.68 ± 32.75 hours through shallow needling, P < 0.05, both). Meanwhile, the deep needling group had shorter times to the first SBM than the shallow needling group (P < 0.05). In terms of the percentages of emergency drugs used, the 2 acupuncture groups had lower values than the lactulose group during each evaluated time frame (P < 0.01, all). More details are revealed in Tables 3 and 4.
TABLE 3.
Secondary Outcomes of Constipation-Related Symptoms
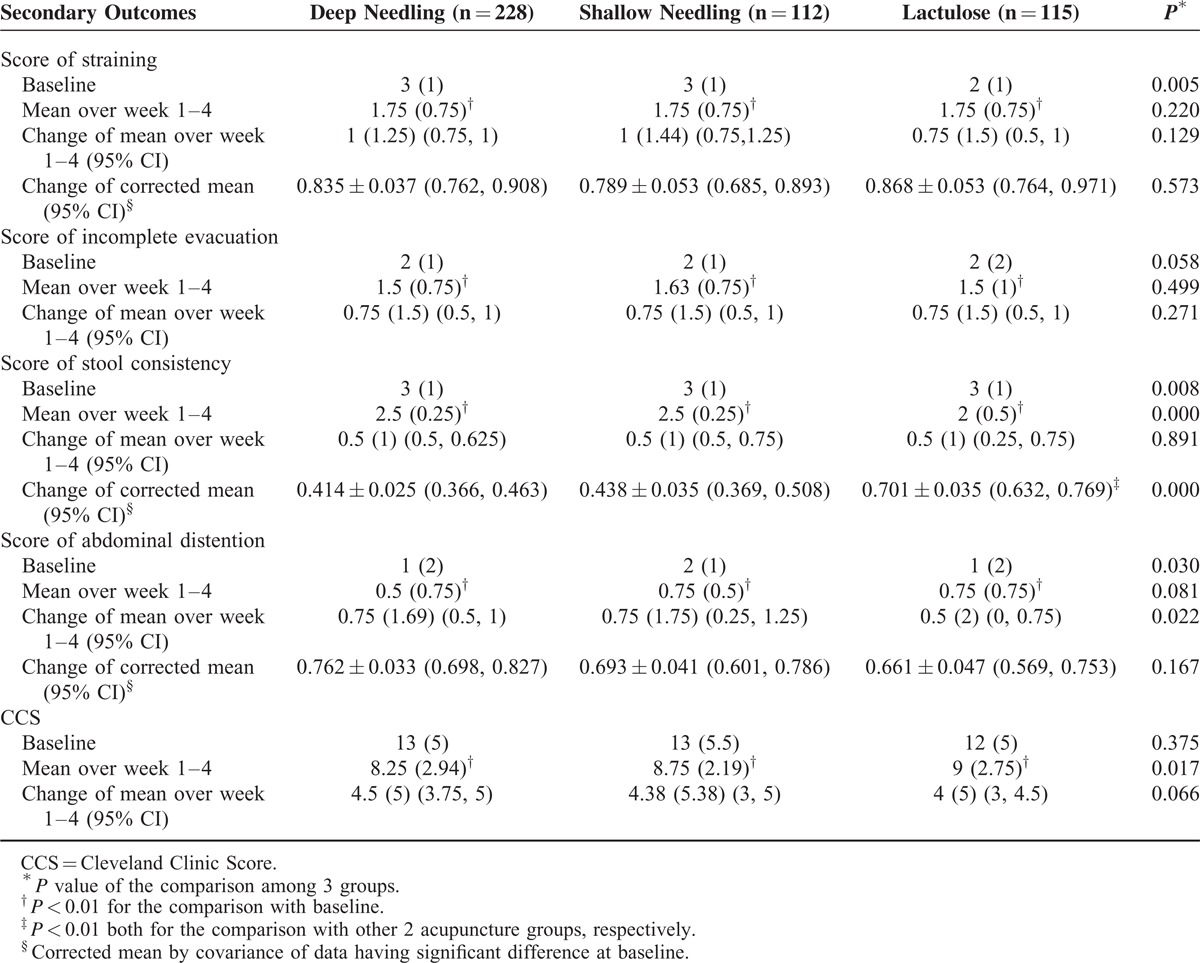
TABLE 4.
Usage of Emergency Drugs
Adverse Effects
The total proportion of adverse effects was 4.62% (21/455) in our trial. No obvious side effects were found in the 3 groups. Overall, 6 patients (2.63%, 6/228) in the deep needling group had mild side effects, including moderate or above pricking, mild fatigue, or subcutaneous hemorrhage; 5 patients (4.46%, 5/112) in the shallow needling group had the same mild side effects as the deep needling group; and 10 patients (8.70%, 10/115) in the lactulose group experienced the side effects of diarrhea and abdominal discomfort. This difference was significant between the deep needling group and lactulose group (P = 0.012, α was corrected as 0.0167 through Bonferroni for further pairwise comparisons).
DISCUSSION
The results of this superiority-design, multicenter, 3-arm, randomized, lactulose-controlled trial show that deep needling and shallow needling are effective and safe for patients with functional constipation. The 4-week therapeutic effects of deep needling and shallow needling are not superior to that of lactulose; however, the sustained effects of 2 kinds of needling after stopping the acupuncture treatments are superior to the therapeutic effect of lactulose.
Although functional constipation is related with various persistent symptoms, infrequent SBMs are generally viewed as the defining and clinically significant symptom of functional constipation.17 For patients with constipation, increased instances of SBMs represent meaningful clinical improvements.18 Thus, a change in the weekly mean SBMs as a primary outcome based on patients’ diaries can objectively and accurately reflect the relief of constipation. Lactulose is an osmotic laxative that is recommended by the American College of Gastroenterology of Level A,3 and commonly used and recommended in China.19 This laxative is metabolized by the colonic bacteria20 to retain fluid in the large intestine or to draw fluid from the body into the bowel.21 In our trial, lactulose was taken orally during the whole circle, including the period of follow-up; consequently, the effects of deep and shallow needling during the treatment and follow-up periods can be more accurately revealed by using lactulose as the controlled intervention.
The therapeutic effects of deep and shallow needling are not superior to that of lactulose during week 1 to 4 in raising the mean weekly SBMs, with the increases of 2 (1.75), 2 (1.75), and 2 (2), respectively. Nonetheless, the therapeutic effects of deep and shallow needling persisted for 12 weeks after the acupuncture regimen had been stopped. The increases in the weekly SBMs were 2 (2), 2 (2.5) respectively through the deep needling at week 8 and week 16; 2 (3), 1.5 (2.5) through the shallow needling; and 1 (2), 1 (2) through lactulose. According to the Rome III criteria,22 the first defined symptom of functional constipation is a spontaneous defecating frequency <3 times/week, which is a complaint shared by many patients.23,24 Weekly SBMs evaluated during each time frame by deep needling were all ≥4 times, with the increases all ≥2 times. Moreover, the SBMs/week of the shallow needling group were all >3 times in each time frame. Figure 2 showed that weekly SBMs over the 4-week treatment period in 2 acupuncture groups exhibited rising trends, especially in the deep needling group. Whereas, during the 16-week treatment period of lactulose, weekly SBMs increased rapidly in the first 2 weeks, then started to decline from week 3. Further, the sustained effects of deep and shallow needling at week 8 and week 16 (the patients had already stopped the acupuncture treatment from week 5–16) were all better than using lactulose (the patients were still taking the medicine from week 5–16) in gaining SBMs (P < 0.05, respectively). These 12-week sustained effects of deep and shallow needling are clinically meaningful. In our previous clinical trial of deep needling that used the same definition of SBMs, the effects of deep needling in raising the SBMs/week could last for 6 months, remaining at 3.51 ± 1.42 times.10 In a trial conducted by Lu,25 the lasting effects of deep needling at ST25 for constipation can persist for 24 weeks, with the SBMs maintained at 5.67 ± 0.99 times/week. Nevertheless, osmotic laxatives, such as lactulose and polyethylene glycol (PEG), are generally thought to lack sustained effects.26 One trial of PEG 3350 for constipation reported that after 38.4 ± 14.1 days (nearly 5 weeks) of follow-up, the symptoms of constipation recurred in 29 out of 47 patients (61.7%).27 Trails using prokinetic agents also lack reports of sustained effects.28,29
In addition, searching for other drugs during an observing circle can also reflect the effect of intervention. In our trial, the emergency drug usage in the 2 acupuncture groups were both less than in the lactulose group (P < 0.01). The proportions of participants using emergency drugs in the lactulose group were 33.91%, 30.43%, and 29.57%, respectively, over week 1 to 4 and at week 8 and week 16. Still, these percentages were only 9.65%, 10.53%, and 17.11% in the deep needling group and 8.04%, 12.50%, and 14.29% in the shallow needling group. In 3 studies comparing the effect between PEG and lactulose, 18% of PEG patients required additional therapies and 30% of lactulose patients required extra treatments.26 The participants in our lactulose group took lactulose for 16 continuous weeks, and the chronic use of lactulose can indeed result in flatus and bacterial adaptation.30–32 Furthermore, 8.70% of patients in the lactulose group complained of abdominal discomfort and diarrhea caused by lactulose. Only 2.63% of patients in the deep needling group (P = 0.012 compared with lactulose) and 4.46% of patients in the shallow needling group complained of mild side effects, including moderate or more of pricking, mild fatigue, and subcutaneous hemorrhage, all of which were temporary.
In traditional acupuncture treatments, the depth of needling has an influence on the effects of acupuncture.9 In our trial, except as the depth of needling, the 2 acupuncture groups have the same acupoints, parameters of electroacupuncture, and course of treatment, which might benefit to blind the participants. The results suggest that there is no significant difference between deep and shallow needling groups in progress in intestinal function except for the change of weekly SBMs at week 4 and for the time to the first SBM. Moreover, the deep and shallow needling groups can both get more weekly SBMs after stopping acupuncture treatment than using lactulose during follow-up period.
Limitations
Lack of the mean value of primary outcomes during follow-up might bias the results; lack of fully blinding of participants in acupuncture and lactulose groups might also bias the results. Our 1-week screening period is a little bit shorter. Although a shorter screening period can increase the compliance of the participants, it might cause a little confounding of IBS. We have a standard training of all the manipulators, however, the depth of needling varied with patients’ body type. There would be a manipulating bias for that the needling was performed on different patients by different manipulators. There might also be a recall bias of the patients during follow-up period, because some of them may not record their BMs timely without the evaluator's monitoring. We used 3 different ways to enroll participants for the balance; nonetheless, we still missed some sources of patients. We did not recruit the participants from communities, so that the patients with lighter symptoms who did not care about their problems might pay little attention to the information from our hospitals. Besides, our 4-week treatment period might not be long enough for the interventions to show their effects completely. Although the results of our superiority-design trial did not reflect that the therapeutic effects of acupuncture were better than that of lactulose, these effects are clinically meaningful for the safety and 12-week sustained effects of deep needling and shallow needling.
CONCLUSIONS
Deep needling and shallow needling at ST25 can improve intestinal function remarkably and safely. Therapeutic effects of deep needling and shallow needling are not superior to that of lactulose; however, the sustained effects of deep and shallow needling after stopping the acupuncture treatments are superior to the therapeutic effect of lactulose, which might qualify the superiority of deep and shallow needling.
Acknowledgments
The design guide of our trial was Prof Lixing Lao (School of Chinese Medicine, the Chinese University of Hongkong). The diagnosis and differential diagnosis guide of functional constipation was Prof Yongdong Wu (Beijing Friendship Hospital, Capital Medical University) and Professor Huashan Li (Anorectal Department of Guang’anmen Hospital, China Academy of Chinese Medical Sciences). The guide for statistical analysis was Prof Xiaohua Zhou (Department of Biostatistics School of Public Health, University of Washington).
Footnotes
Abbreviations: BMs = Bowel Movements, CCS = Cleveland Clinic Scores, EA = electro-acupuncture, IBS = Irritable Bowel Syndrome, LOCF = last observation carried forward, m-ITT = modified intention-to-treat, PEG = polyethylene glycol, SBM = Spotaneous Bowel Movement, SBMs = Spotaneous Bowel Movements.
JW and BL are co-first authors.
Trial Registration: ClinicalTrials.gov ID: NCT00508482.
This trial was funded through the “12th five-year” National Science and Technology Pillar Program (2012BAI24B01) and the National Key Development Program for Basic Research (2011CB505202) by The Ministry of Science and Technology of the People's Republic of China.
ZL contributed to the design of this trial. JW and BL drafted the manuscript. BL, ZL, and YC reviewed the manuscript. BL conducted the statistical analysis. NL, JS, Lingling Wang, Liping Wang, YY, JL, and YW are in charge of the project implementation from 5 centers.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 2.Tack J, Muller-Lissner S. Treatment of chronic constipation: current pharmacologic approaches and future directions. Clin Gastroenterol Hepatol 2009; 7:502–508.quiz 496. [DOI] [PubMed] [Google Scholar]
- 3.American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005; 100 (s1):S1–S4. [DOI] [PubMed] [Google Scholar]
- 4.Wald A, Mueller-Lissner S, Kamm MA, et al. Survey of laxative use by adults with self-defined constipation in South America and Asia: a comparison of six countries. Aliment Pharmacol Ther 2010; 31:274–284. [DOI] [PubMed] [Google Scholar]
- 5.Ye F, Wang QM. Epidemiology of chronic constipation in China (Chinese). Chin J Clin Healthc 2010; 13:665–667. [Google Scholar]
- 6.Du WF, Yu L, Yan XK, et al. Met-analysis on randomized controlled clinical trials of acupuncture and moxibustion on constipation (Chinese). Zhongguo Zhen Jiu 2012; 32:92–96. [PubMed] [Google Scholar]
- 7.Feng H, Xiang Y. Rules of selecting acupoints for constipation (Chinese). J Clin Acupuncture Moxibustion 2003; 19:2–3. [Google Scholar]
- 8.Zhang T, Chon TY, Liu B, et al. Efficacy of acupuncture for chronic constipation: a systematic review. Am J Chin Med 2013; 41:717–742. [DOI] [PubMed] [Google Scholar]
- 9.Lin JG, Chou PC, Chu HY. An exploration of the needling depth in acupuncture: the safe needling depth and the needling depth of clinical efficacy. Evid Based Complement Alternat Med 2013; 2013:740508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng WN, Wang L, Liu ZS, et al. Analysis on follow-up efficacy and safety of slow transit constipation treated with individualized deep puncture at Tianshu (ST 25): a multi-central randomized controlled trial (Chinese). Zhongguo Zhen Jiu 2013; 33:865–869. [PubMed] [Google Scholar]
- 11.Chen S, Li XH, Li CH, et al. Briefing on the 61st academic conference of Japanese Acupuncture Association (Chinese). Zhongguo Zhen Jiu 2013; 33:543–546. [PubMed] [Google Scholar]
- 12.Wang MQ. Japanese silver-needle shallow and light puncture method (Chinese). Zhongguo Zhen Jiu 2001; 21:615–617. [Google Scholar]
- 13.64th WMA General Assembly. WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. 2014; http://www.wma.net/en/30publications/10policies/b3/ Accessed May, 2014 [Google Scholar]
- 14.Rome Foundation. Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. 2014; http://www.romecriteria.org/criteria/ Accessed May, 2014 [PubMed] [Google Scholar]
- 15.WHO Regional Office for the Western Pacific. WHO Standard Acupuncture Point Locations in the Western Pacific Region. Manila: Philippines; 2008. [Google Scholar]
- 16.Lee-Robichaud H, Thomas K, Morgan J, et al. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev 2010; CD007570. [DOI] [PubMed] [Google Scholar]
- 17.Lembo A, Camilleri M. Chronic constipation. N Engl J Med 2003; 349:1360–1368. [DOI] [PubMed] [Google Scholar]
- 18.Johanson JF, Wald A, Tougas G, et al. Effect of tegaserod in chronic constipation: a randomized, double-blind, controlled trial. Clin Gastroenterol Hepatol 2004; 2:796–805. [DOI] [PubMed] [Google Scholar]
- 19.The Gastrointestinal Dynamics Group of Digestive Disease Association affiliated to Chinese Medical Association, The Anus Colorectal Surgery Group of Surgery Association affiliated to Chinese Medicial Association. Diagnosis and treatment guideline of chronic constipation in China (Chinese). Chin J Gastroenterol 2013; 18:605–612. [Google Scholar]
- 20.Fritz E, Hammer HF, Lipp RW, et al. Effects of lactulose and polyethylene glycol on colonic transit. Aliment Pharmacol Ther 2005; 21:259–268. [DOI] [PubMed] [Google Scholar]
- 21.Carlin A, Justham D. A literature review of two laxatives: lactulose and polyethylene glycol. Br J Community Nurs 2011; 16:584.586, 588–590. [DOI] [PubMed] [Google Scholar]
- 22.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006; 130:1377–1390. [DOI] [PubMed] [Google Scholar]
- 23.Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol 2001; 96:3130–3137. [DOI] [PubMed] [Google Scholar]
- 24.Quigley EM, Vandeplassche L, Kerstens R, et al. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation—a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther 2009; 29:315–328. [DOI] [PubMed] [Google Scholar]
- 25.Lu DW. Evaluation of therapeutic effect of deep electro-acupuncture at Tianshu (ST25) for treatment of slow transit constipation (Chinese) [Master Degree]. Nanjing, China: Nanjing University of Chinese Medicine; 2009. [Google Scholar]
- 26.Gordon M, Naidoo K, Akobeng AK, et al. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev 2012; CD009118. [DOI] [PubMed] [Google Scholar]
- 27.Tran LC, Di Palma JA. Lack of lasting effectiveness of PEG 3350 laxative treatment of constipation. J Clin Gastroenterol 2005; 39:600–602. [DOI] [PubMed] [Google Scholar]
- 28.Emmanuel A, Cools M, Vandeplassche L, et al. Prucalopride improves bowel function and colonic transit time in patients with chronic constipation: an integrated analysis. Am J Gastroenterol 2014; 109:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueno N, Inui A, Satoh Y. The effect of mosapride citrate on constipation in patients with diabetes. Diabetes Res Clin Pract 2010; 87:27–32. [DOI] [PubMed] [Google Scholar]
- 30.Voskuijl W, de Lorijn F, Verwijs W, et al. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial. Gut 2004; 53:1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szilagyi A. Review article: lactose—a potential prebiotic. Aliment Pharmacol Ther 2002; 16:1591–1602. [DOI] [PubMed] [Google Scholar]
- 32.Attar A, Lémann M, Ferguson A, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999; 44:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]


