Abstract
The role of 18F-fuorodexoyglucose-positron emission tomography/computed tomography (18F-FDG-PET/CT) in the staging of Hodgkin lymphoma (HL) and aggressive B-cell non-Hodgkin lymphomas (NHL) has been demonstrated extensively. Nevertheless, the role of PET/CT in the diagnosis, staging, prognosis, and treatment evaluation of natural killer (NK)/T-cell lymphoma remains indeterminate.
To systematically review and meta-analyze the publications on the value of 18F-FDG-PET/CT in the diagnosis and staging of NK/T-cell lymphoma.
Pubmed, Embase, Cochrane Library, and some other database were searched for initial studies (last updated on May 8th, 2014).
The eligibility criteria were studies assessing the usefulness of PET/CT in the staging of NK/T-cell lymphoma, patients were diagnosed as NK/T-cell lymphoma through pathology, or clinical and imaging follow-up.
Sensitivities and specificities of 18F-FDG-PET/CT in individual studies were assessed. The summary receiver operating characteristic curve (sROC) and the area under the curve (AUC) were calculated. The “Meta-Disc 1.4” software was used for data analysis.
Eight studies, with a total of 135 NK/T-cell lymphoma patients, were included in this meta-analysis. In terms of the 6 studies with patient based data, the pooled sensitivity and specificity of PET/CT in the diagnosis of NK/T-cell lymphoma were 0.95 (95% CI: 0.89–0.98) and 0.40 (95% CI: 0.09–0.78), respectively. For lesion-based analysis, with 1546 lesions included, the pooled sensitivity and specificity of PET/CT in the staging of NK/T-cell lymphoma were 0.98 (95% CI: 0.96–0.99) and 0.99 (95% CI: 0.99–1.00), respectively. For the patient based data, the AUC and ∗Q index were 0.8537 and 0.7847, respectively. For lesion based data, the AUC and ∗Q index were 0.9959 and 0.9755, respectively.
The results of this current meta-analysis indicated that PET/CT could be used as a valuable diagnostic and staging tool for NK/T-cell lymphoma.
INTRODUCTION
Extranodal natural killer (NK)/T-cell lymphoma (ENKTL) is a newly recognized distinctive entity in the World Health Organization (WHO) classification of lymphomas.1 It accounts for less than 1% of all lymphomas in Western countries and approximately 3% to 9% of all lymphomas in Asia.2–4 ENKTL is an aggressive lymphoma with poor survival outcome and the cumulative probability of 5-year survival ranging from 37.9% to 49.5%.5 However, optimal treatment strategies have not been identified. Accurate staging plays a decisive role in the prognosis and treatment strategy of NK/T-cell lymphoma.6,7
18F-fuorodexoyglucose-positron emission tomography/computed tomography (18F-FDG-PET/CT) is a functional imaging test that has been widely used in the staging, prognosis, and treatment of Hodgkin lymphoma (HL) and various types of B-cell non-Hodgkin lymphomas (NHL).8–10 However, similar studies in T-cell and NK-cell lymphomas are relatively rare. Therefore, a systematic review aimed to evaluate the effect of PET/CT in the diagnosis and staging of NK/T-cell lymphoma was urgently needed. In this present study, we undertook a meta-analysis and systematic review to assess the role of 18F-FDG-PET/CT in the diagnosis and staging of NK/T-cell lymphoma.
MATERIALS AND METHODS
Literature Search
A systematic computer literature search was performed to identify studies assessing the value of PET or PET/CT in the diagnosis and staging of NK/T-cell lymphoma. The Pubmed, Embase, and Cochrane Library databases were searched with the following keywords (“PET” or “positron emission tomography,” “neutral killer/T-cell lymphoma” or “NK/T-cell lymphoma” or “lymphoma”). No start date limit was used. The search was last updated on May 2014. In addition, reference lists from the included studies were also hand searched. Ethical approval was not necessary for this meta-analysis, due to that all analyses were based on the results of previous published studies.
Inclusion and Exclusion Criteria
The inclusion criteria were: (1) Studies assessing the usefulness of PET/CT in the staging of NK/T-cell lymphoma, patients were diagnosed as NK/T-cell lymphoma through pathology, or clinical and imaging follow-up. (2) Article with the most detailed or the most recent article was chosen if data were presented in more than 1 article. (3) Articles with sufficient data to construct or calculate the true-positive (TP), false-positive (FP), true-negative (TN), false-negative (FN). (4) With full-text articles published in English.
The exclusion criteria were: (1) Publication did not aim to reveal the value of PET/CT in NK/T-cell lymphoma. (2) Publications without original data, such as case reports, letters, congress, comments, and reviews. (3) Vitro studies and animal experiments. (4) Studies with less than 5 patients with NK/T-cell lymphoma enrolled. (5) With less than 9 “yes” responses to the quality assessment of diagnosis accuracy studies (QUADAS).11 QUADAS is an evidence-based quality assessment tool used for assess the diagnostic accuracy of studies in systematic reviews.
Data Extraction and Quality Assessment
Two investigators independently extracted the data needed through screening the abstracts and full-text articles. Any differences were resolved by consensus. The criteria list recommended by the Cochrane Methods Working Group on Systematic Review of Screening and Diagnostic Tests was used. The QUADAS quality assessment tool was used to estimate the quality of the included studies.11
Statistical Analysis
Data on max based standardized uptake value (SUV), TP, TN, FP, and FN were calculated from the original data in the publications. Pool estimates of sensitivity and specificity were analyzed. In addition, the 95% CI was also constructed. The summary receiver operating characteristic curve (sROC) and the area under the curve (AUC) were calculated. sROC is a curve conducted with the sensitivity as y-axis and the “1-specificity” as x-axis. It reflects the continuous variables of sensitivity and specificity. The closer of sROC to the top left corner of coordinate axis, the higher of the accuracy of diagnostic studies. AUC is the area under sROC, which is used to assess the total efficiency of diagnosis. We also estimated the maximum joint sensitivity and specificity (Q index) to measure the overall diagnostic accuracy (the point on the sROC curve at which the sensitivity and specificity are equal). The patient-based data and lesion-based data were conducted, respectively. All data analyze were performed using the Meta-Disc software version 1.4. P-value of <0.05 were considered to be statistically significant.
RESULTS
Literature Search and Selection of Studies
According to the search strategy, a total of 744 papers were selected: 409 in Pubmed, 335 in Embase, 0 in Cochrane Library, and 0 by hand search (last updated on May 8th, 2014). After reading abstracts, we reviewed 85 studies in detail. Of these articles, 55 were excluded because: (1) Aim of these articles was not to reveal the value of PET/CT in the staging and prognosis of NK/T-cell lymphoma. (2) Insufficient data were reported to construct or calculate the TP, TN, FN, and FP or PFS, OS. (3) The number of “yes” response to the 14 QUADAS questions was less than 9. Finally, 8 studies with 135 patients with NK/T-cell lymphoma were selected for data extraction and analysis (Figure 1).
FIGURE 1.
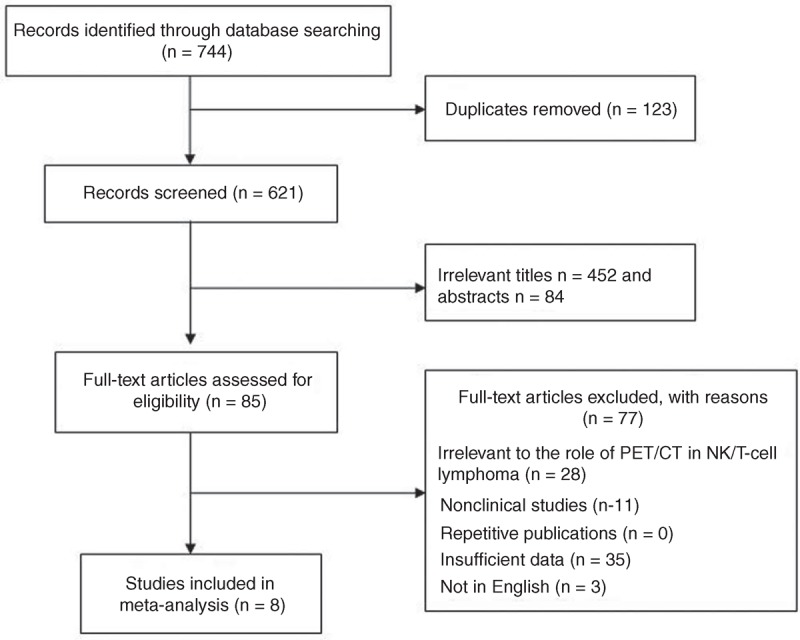
Studies evaluated for inclusion in this meta-analysis.
Study Design Characteristics and Publication Bias
Data were extracted by 2 authors and any differences were resolved by consensus. Data abstraction was not blinded to authors, institution, or source of publication. Characteristics of the included 8 studies were shown in Table 1. Among them, 6 studies concentrated on the patient based data (Table 2) and 6 studies focused on the lesion based data (Table 3) of the diagnostic value of PET/CT in NK/T-cell lymphoma.
TABLE 1.
Main Characteristics of the Included Studies
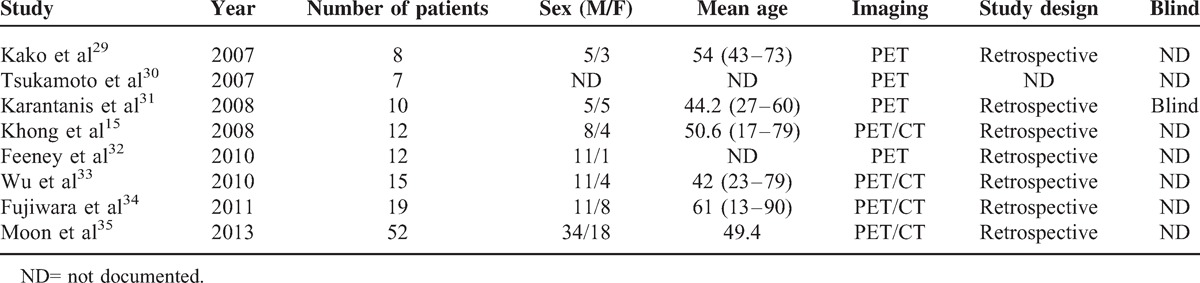
TABLE 2.
PET/CT in NK/T-Cell Lymphoma: Patient Based Data
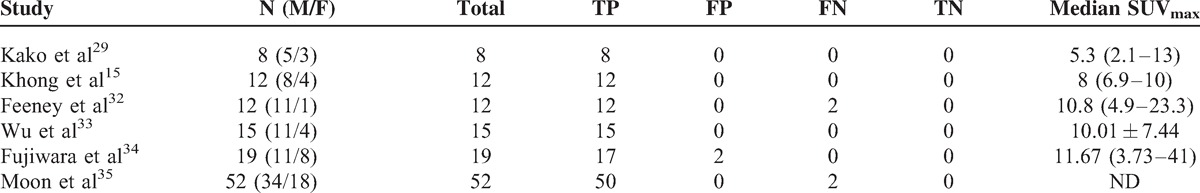
TABLE 3.
PET/CT in NK/T-Cell Lymphoma: Lesion Based Data
Summary Estimates of Sensitivity, Specificity, and Diagnostic Odds Ratio
In terms of the 6 studies with patient based data. The Spearman correlation coefficient was 0.507 (P = 0.305). These 2 data showed that no threshold effect existed in it. The pooled sensitivity and specificity of PET/CT in the diagnosis of NK/T-cell lymphoma were 0.95 (95% CI: 0.89–0.98) and 0.40 (95% CI: 0.09–0.78), respectively (Figure 2). The pooled positive LR and pooled negative LR were 1.63 (95% CI: 0.91–2.91) and 0.13 (95% CI: 0.05–0.38), respectively. The pooled DOR was 13.28 (95% CI: 2.60–67.82).
FIGURE 2.
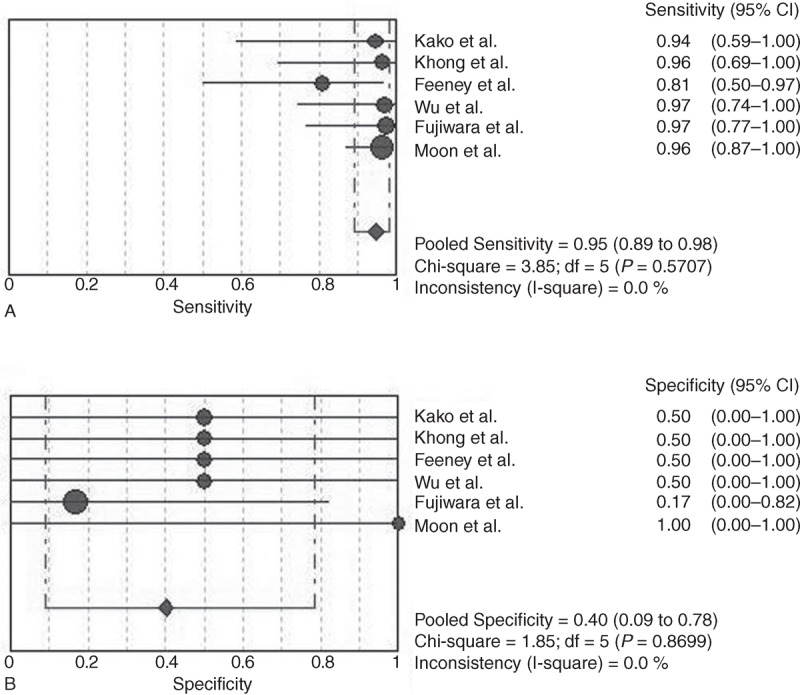
(A) Sensitivity and 95% confidence intervals for studies assessing the diagnostic accuracy of FDG PET in patients with NK/T-cell lymphoma. (B) Specificity and 95% confidence intervals for studies assessing the diagnostic value of PDG PET in patients with NK/T-cell lymphoma. ∗The diamond represents the 95% CI of the pooled estimate.
For lesion-based analysis, 6 studies were eligible for meta-analysis. The Spearman correlation coefficient was 0.086 (P = 0.872). No threshold effect was found. The pooled sensitivity and specificity of PET/CT in the staging of NK/T-cell lymphoma were 0.98 (95% CI: 0.96–0.99) and 0.99 (95% CI: 0.99–1.00), respectively (Figure 3). The pooled positive LR and pooled negative LR were 8.52 (95% CI: 0.23–312.09) and 0.03 (95% CI: 0.01–0.06), respectively. The pooled DOR was 268.66 (95% CI: 16.8–4296.47).
FIGURE 3.
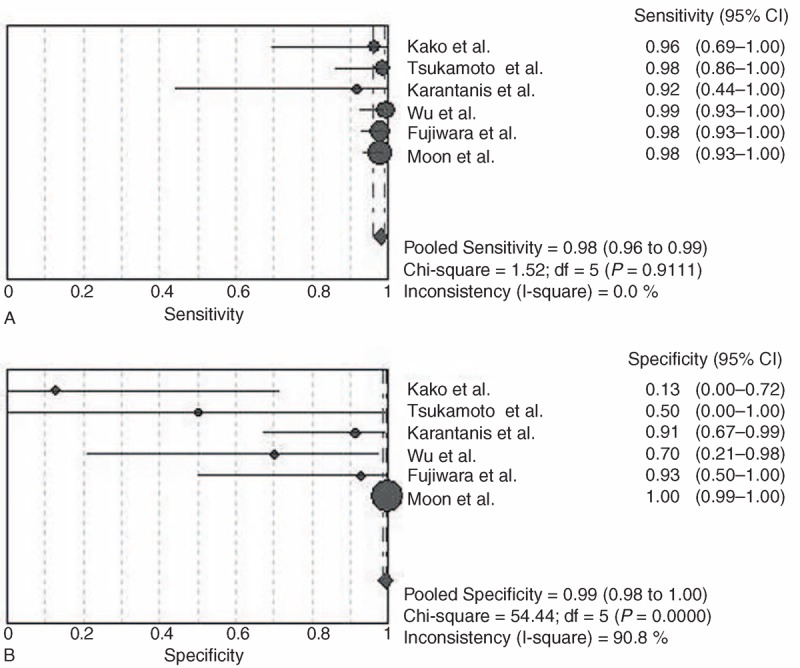
(A) Sensitivity and 95% confidence intervals for studies assessing the diagnostic accuracy of FDG PET with lesion based data. (B) Specificity and 95% CI for studies assessing the diagnostic value of PDG PET with lesion based data. ∗The diamond represents the 95% CI of the pooled estimate.
Heterogeneity Assessing
The presences of heterogeneity between studies were examined using the chi-square test. No heterogeneity was found in the sensitivity (Chi-square = 3.80, P = 0.5784) and specificity (Chi-square = 1.85, P = 0.8699) of PET/CT in the patients based data.
Summary ROC Curves and the ∗Q Index
The SROC curves and the ∗Q index for PET/CT in the diagnosis and staging of NK/T-cell lymphoma were shown in Figures 4 and 5. In the data based on patients, no heterogeneity and threshold effect were found. Therefore, we chose the fixed-effects model (Mantel–Haenszel) to analysis the SROC curves. The results showed that the AUC and ∗Q index were 0.8537 and 0.7847, respectively. For lesion-based data, the difference between b and 0 (P = 0.0132) is of statistical significance. Therefore, the Moses–Sphaprio–Littenberg method was used to construct the sROC. The AUC and ∗Q index were 0.9959 and 0.9755, respectively. This indicated that PET/CT is of great value in the detecting the lesions of NK/T-cell lymphoma.
FIGURE 4.
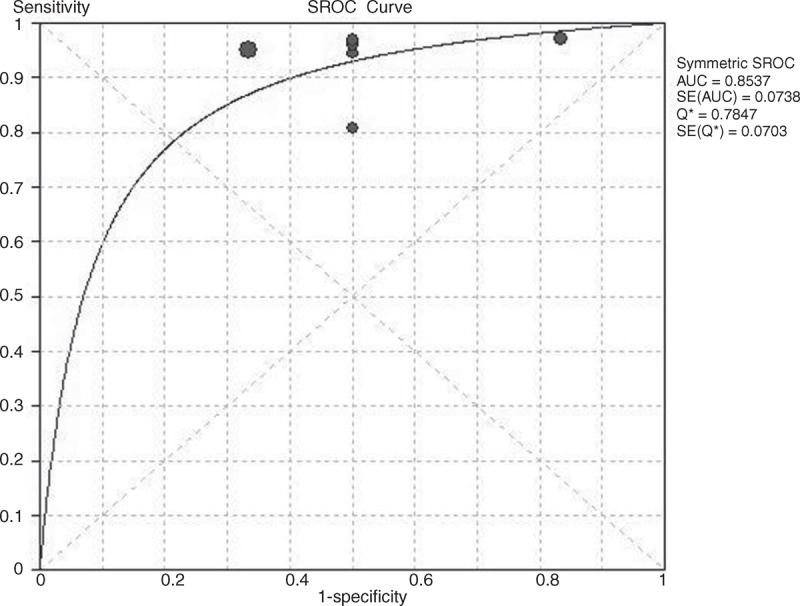
The SROC curves of PET/CT in the patient-based data of NK/T-cell lymphoma.
FIGURE 5.
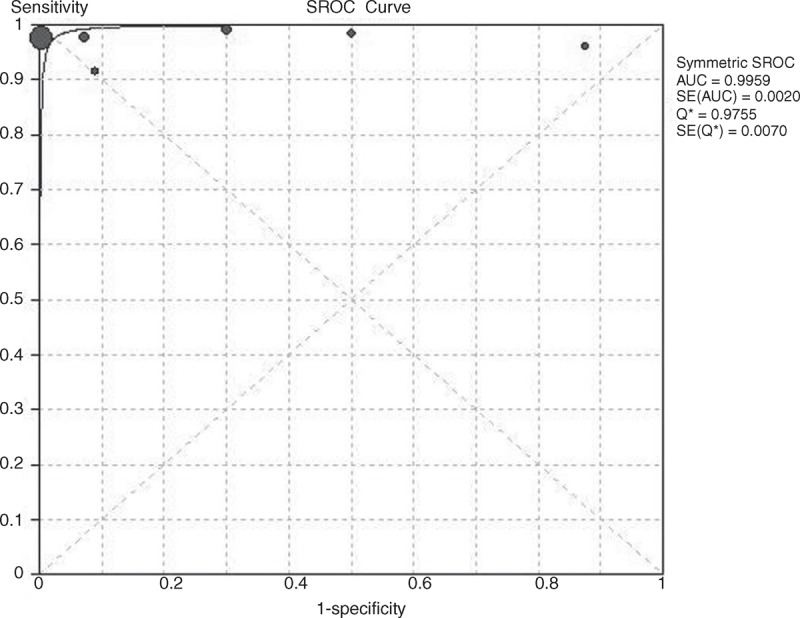
The SROC curves of PET/CT in the lesion-based data of NK/T-cell lymphoma.
DISCUSSION
The role of PET/CT in the staging of HL and aggressive B-cell NHL has been demonstrated extensively. Recently, several clinical studies have investigated the utility of PET/CT in the NK/T-cell lymphoma with some promising results in the diagnostic value of PET/CT in NK/T-cell lymphoma.12–14 Nevertheless, the role of PET/CT in the diagnosis, staging, prognosis, and treatment evaluation of NK/T-cell lymphoma remains indeterminate.
Due to the rarity of NK/T-cell lymphoma, limited investigations have been carried out in the diagnosis and staging of NK/T-cell lymphoma. Khong et al reported that the lesions of NK/T-cell lymphoma were FDG-PET avid in 2008.15 It was then indicated that the PET/CT was true positive in 5 cases, true negative in 15 cases, and 1 case unconfirmed of the total 21 lesions examined. The mean SUV(max) was relatively higher (16 g/mL) in nasal cavities than that in extranasal cases (10.9 g/mL).16 Furthermore, Suh et al identified that high FDG uptake was related to poor treatment responses and outcomes.17 It was recently suggested that PET/CT plays a more important role than MRI or PET alone in the staging of lymphoma.18 But, those studies were limited due to the low incidence of NK/T-cell lymphoma. The utility of PET/CT in the diagnosis and staging of this lymphoma is still unclear. Efforts are still needed to improve the staging accuracy of NK/T-cell lymphoma.
This systematic review and meta-analysis focused on evaluating the utility of PET/CT in the diagnosis and staging of NK/T-cell lymphoma. Eight studies with a total of 135 patients with NK/T-cell lymphoma were included. The results of this meta-analysis indicated that PET/CT has a high diagnostic accuracy in the evaluation of the staging in patients with NK/T-cell lymphoma. The pooled sensitivity was 0.95 and the pooled specificity was 0.4 in the patient based analysis. Moreover, the pooled positive and negative LR was 1.63 and 0.13, respectively. In the patient based data, the AUC was 0.8537 and the ∗Q was 0.7847. This illustrated that PET/CT is valuable in the diagnosis of NK/T-cell lymphoma. This conclusion was consistent with previous studies. However, the specificity is not very optimal in patient based data. On the other hand, we analyzed lesion-based data in patients with NK/T-cell lymphoma. The pooled sensitivity and the pooled specificity were 0.98 and 0.99, respectively. Moreover, the pooled DOR, the AUC and ∗Q index were 268.66, 0.9959, and 0.9755, respectively. The results demonstrated that PET/CT presented high sensitivity and specificity in detecting the NK/T-cell lymphoma related lesions. On the other hand, significant higher diagnostic accuracy was found in the lesion based analysis compared to that in the patient based analysis.
In the clinical practice, the treatment strategies of NK/T-cell lymphoma patients depend on the stage of disease. Patients with stage I/II NK/T-cell lymphoma showed more favorable prognosis than that with stage III/IV disease.19,20 Radiotherapy, sometimes combined with chemotherapy, is the main treatment strategy for stage I/II NK cell lymphomas.21,22 However, due to the highly aggressive and refractory of III/IV stage NK/T-cell lymphoma, chemotherapy acts as the mainstay choice of treatment. Most recently, the l-asparaginase based chemotherapeutic regimens, such as SMILE, have been proved to be effective in the treatment of advanced, relapsed, or refractory NK/T-cell lymphomas.23,24 In patients with advanced NK/T-cell lymphoma, this regimen showed a complete remission rate of 35% to 50% and an overall response rate of 74%.24 Nevertheless, due to the rarity of NK/T-cell lymphoma, the best treatment strategy is still unclear.
The prognostic role of International Prognostic Index (IPI) in NK/T-cell lymphoma has been validated in several studies.25 Recently, Glasgow Prognostic Score (GPS), a novel prognostic score based on CRP and albumin levels, was found to be superior to IPI in the prognosis of ENKL.26 But the IPI has also failed to predict survival in patients with ENKTCL.27 It has been revealed that PET/CT is a promising tool to diagnostic and staging of NK/T-cell lymphoma. Several investigations identified that almost all NK/T-cell lymphomas are FDG avid.15,28 Therefore, PET/CT represents a valuable addition to the staging procedures. Because of the rarity of NK/T-cell lymphoma, the role of PET/CT in the diagnosis and staging of NK/T-cell lymphoma is still indeterminate yet. Our results provided an analysis of the diagnostic performance of PET/CT in NK/T-cell lymphoma.
Nevertheless, there are several potential limitations during the meta-analysis conduction of diagnostic tests. Due to the limitations of the existing literature, further studies are needed to confirm the value of PET/CT in the prediction and treatment assessment of NK/T-cell lymphoma. In addition, other factors such as the retrospective design of studies and heterogeneity, are also involved in the limitations of this meta-analysis.
CONCLUSIONS
The findings from this meta-analysis demonstrate that PET/CT is a valuable tool in the diagnosis and staging for NK/T-cell lymphoma. However, it was not determined with certainty whether PET/CT is useful in the treatment response of NK/T-cell lymphoma. Furthermore, new parameters of PET should also be considered in the diagnosis of lymphoma. It is hopeful to add PET/CT to the routine staging workup of lymphoma.
Footnotes
Abbreviations: 18F-FDG-PET/CT = 18F-fuorodexoyglucose-positron emission tomography/computed tomography, 95% CIs = 95% confidence intervals, AUC = area under the curve, ENKTL = extranodal natural killer/T-cell lymphoma, FN = false-negative, FP = false-positive, HL = Hodgkin lymphoma, NHL = non-Hodgkin lymphomas, sROC = summary receiver operating characteristic curve, SUV = standardized uptake value, TN = true-negative, TP = true-positive, WHO = World Health Organization.
National Natural Science Foundation (No. 81270598; No. 31340009), Natural Science Foundations of Shandong Province (No. 2009ZRB14176, No. ZR2011HQ009, and No. ZR2012HZ003), Technology Development Projects of Shandong Province (No. 2008GG2NS02018 and No. 2010GSF10250), National Public Health Grand Research Foundation (No. 201202017), Program of Shandong Medical Leading Talent and Taishan Scholar Foundation of Shandong Province.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2009; 1:523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol 2005; 16:206–214. [DOI] [PubMed] [Google Scholar]
- 3.The world health organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int 2000; 50:696–702. [DOI] [PubMed] [Google Scholar]
- 4.Chen CY, Yao M, Tang JL, et al. Chromosomal abnormalities of 200 Chinese patients with non-Hodgkin's lymphoma in Taiwan: with special reference to T-cell lymphoma. Ann Oncol 2004; 15:1091–1096. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 2006; 24:612–618. [DOI] [PubMed] [Google Scholar]
- 6.Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol 2009; 147:13–21. [DOI] [PubMed] [Google Scholar]
- 7.Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 2009; 113:3931–3937. [DOI] [PubMed] [Google Scholar]
- 8.Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin's lymphoma. Ann Oncol 2002; 13:1356–1363. [DOI] [PubMed] [Google Scholar]
- 9.Kostakoglu L, Coleman M, Leonard JP, et al. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin's disease. J Nucl Med 2002; 43:1018–1027. [PubMed] [Google Scholar]
- 10.Hutchings M, Mikhaeel NG, Fields PA, et al. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol 2005; 16:1160–1168. [DOI] [PubMed] [Google Scholar]
- 11.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu HB, Wang QS, Wang MF, et al. Utility of 18F-FDG PET/CT for staging NK/T-cell lymphomas. Nucl Med Commun 2010; 31:195–200. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara H, Maeda Y, Nawa Y, et al. The utility of positron emission tomography/computed tomography in the staging of extranodal natural killer/T-cell lymphoma. Eur J Haematol 2011; 87:123–129. [DOI] [PubMed] [Google Scholar]
- 14.Moon SH, Cho SK, Kim WS, et al. The role of 18F-FDG PET/CT for initial staging of nasal type natural killer/T-cell lymphoma: a comparison with conventional staging methods. J Nucl Med 2013; 54:1039–1044. [DOI] [PubMed] [Google Scholar]
- 15.Khong PL, Pang CB, Liang R, et al. Fluorine-18fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol 2008; 87:613–621. [DOI] [PubMed] [Google Scholar]
- 16.Karantanis D, Subramaniam RM, Peller PJ, et al. The value of [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography in extranodal natural killer/T-cell lymphoma. Clin Lymphoma Myeloma 2008; 8:94–99. [DOI] [PubMed] [Google Scholar]
- 17.Suh C, Kang YK, Roh JL, et al. Prognostic value of tumor 18F-FDG uptake in patients with untreated extranodal natural killer/T-cell lymphomas of the head and neck. J Nucl Med 2008; 49:1783–1789. [DOI] [PubMed] [Google Scholar]
- 18.Wu LM, Chen FY, Jiang XX, et al. 1818F-FDG PET, combined FDG-PET/CT and MRI for evaluation of bone marrow infiltration in staging of lymphoma: a systematic review and meta-analysis. Eur J Radiol 2012; 81:303–311. [DOI] [PubMed] [Google Scholar]
- 19.Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia 2005; 19:2186–2194. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki R. Pathogenesis and treatment of extranodal natural killer/T-cell lymphoma. Semin Hematol 2014; 51:42–51. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Lu JJ, Ma X, et al. Combined chemoradiation for the management of nasal natural killer (NK)/T-cell lymphoma: elucidating the significance of systemic chemotherapy. Oral Oncol 2008; 44:23–30. [DOI] [PubMed] [Google Scholar]
- 22.Cheung MM, Chan JK, Lau WH, et al. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys 2002; 54:182–190. [DOI] [PubMed] [Google Scholar]
- 23.Tse E, Kwong YL. Practical management of natural killer/T-cell lymphoma. Curr Opin Oncol 2012; 24:480–486. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 2011; 29:4410–4416. [DOI] [PubMed] [Google Scholar]
- 25.Chim CS, Ma SY, Au WY, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood 2004; 103:216–221. [DOI] [PubMed] [Google Scholar]
- 26.Li YJ, Jiang WQ, Huang JJ, et al. The Glasgow Prognostic Score (GPS) as a novel and significant predictor of extranodal natural killer/T-cell lymphoma, nasal type. Am J Hematol 2013; 88:394–399. [DOI] [PubMed] [Google Scholar]
- 27.Aviles A, Diaz NR, Neri N, et al. Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol 2000; 22:215–220. [DOI] [PubMed] [Google Scholar]
- 28.Chan WK, Au WY, Wong CY, et al. Metabolic activity measured by F-18FDG PET in natural killer-cell lymphoma compared to aggressive B- and T-cell lymphomas. Clin Nucl Med 2010; 35:571–575. [DOI] [PubMed] [Google Scholar]
- 29.Kako S, Izutsu K, Ota Y, et al. FDG-PET in T-cell and NK-cell neoplasms. Ann Oncol 2007; 18:1685–1690. [DOI] [PubMed] [Google Scholar]
- 30.Tsukamoto N, Kojima M, Hasegawa M, et al. The usefulness of (18)F-fluorodeoxyglucose positron emission tomography ((18)F-FDG-PET) and a comparison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer 2007; 110:652–659. [DOI] [PubMed] [Google Scholar]
- 31.Karantanis D, Subramaniam RM, Peller PJ, et al. The value of [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography in extranodal natural killer/T-cell lymphoma. Clin Lymphoma Myeloma 2008; 8:94–99. [DOI] [PubMed] [Google Scholar]
- 32.Feeney J, Horwitz S, Gonen M, Schoder H. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol 2010; 195:333–340. [DOI] [PubMed] [Google Scholar]
- 33.Wu HB, Wang QS, Wang MF, et al. Utility of 18F-FDG PET/CT for staging NK/T-cell lymphomas. Nucl Med Commun 2010; 31:195–200. [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara H, Maeda Y, Nawa Y, et al. The utility of positron emission tomography/computed tomography in the staging of extranodal natural killer/T-cell lymphoma. Eur J Haematol 2011; 87:123–129. [DOI] [PubMed] [Google Scholar]
- 35.Moon SH, Cho SK, Kim WS, et al. The role of 18F-FDG PET/CT for initial staging of nasal type natural killer/t-cell lymphoma: a comparison with conventional staging methods. J Nucl Med 2013; 54:1039–1044. [DOI] [PubMed] [Google Scholar]


