Abstract
To investigate the clinical features of Behçet's disease (BD) complicated with thrombosis.
Medical records of patients with BD at Peking Union Medical College Hospital from 1993 to 2013 were reviewed to identify thrombosis.
Of the 766 patients with BD, 93 patients (16 female and 77 male) developed thrombosis. The most common thrombosis was extremity vein thrombosis (86.0%), including deep vein thrombosis (n = 78) and superficial thrombophlebitis (n = 4). The other thrombosis types associated with BD in descending frequency of order were: vena cava thrombosis (30.1%), pulmonary thromboembolism (15.1%), cerebral venous thrombosis (CVT) (12.9%), intracardiac thrombosis (8.6%), Budd–Chiari syndrome (7.5%), and renal vein thrombosis (4.3%), etc. Venous thrombosis is more frequent than arterial thrombosis, and most of patients (94.6%) experienced multiple thrombosis. A male predominance of extremity vein thrombosis and positive pathergy test, and a female predominance of CVT and genital ulcers were noted. All of these patients exhibited active disease during the emergence of thrombotic events. After treating with glucocorticosteroids, immunosuppressants, and/or anticoagulants, the thrombosis resolved in 89 patients. Three patients died from aneurysm rupture, myocardial infarction and Budd–Chiari syndrome, respectively. One patient with septic shock discontinued therapy during follow-up.
Thrombosis in BD patients is male predominance, mainly multiple and venous thrombosis is more common. Active disease patients are prone to thrombosis, which suggest the key role of immunosuppressive therapy for the complication.
INTRODUCTION
Behçet's disease (BD) is a chronic, relapsing, multisystem inflammatory disorder of unknown etiology and is associated with thrombogenicity. Vascular manifestations of BD, which consist of venous involvements (thrombosis, superficial phlebitis) and arterial involvements (aneurysm, stenosis, occlusion), have been added into the updated International Criteria for Behçet's Disease (ICBD), since they are one of the major characteristics of BD.1 Thrombosis is the most frequent vascular manifestation in BD and an important factor of adverse prognosis. In this study, we retrospectively analyzed 93 Chinese BD patients complicated with thrombosis, to summarize the clinical features of thrombosis in BD patients of Han ethnicity.
PATIENTS AND METHODS
Patients
We retrospectively analyzed medical record of BD patients with thrombosis from the Peking Union Medical College Hospital between December 1993 and December 2013. All of these patients fulfilled both 1990 International Study Group BD criteria2 and new ICBD.1 The diagnosis of thrombosis was based on the clinical assessments and imaging techniques findings, including Doppler ultrasound, phlebography, computed tomography angiography, and/or magnetic resonance angiography, in accordance with the criteria.3–7 Cardiac cavity thrombosis was diagnosed with transthoracic echocardiography.8 We also collected the demographic data, the interval between onset of BD and initial thrombotic event, related clinical features, laboratory parameters, treatment, and outcome. BD disease activity during the emergence of thrombosis event was assessed according to the BD Current Activity Form 2006 (BDCAF2006) (http://www.behcet.ws/pdf/BehcetsDiseaseActivityForm.pdf).
The institutional review board of PUMCH approved the study. Because the study was based on a review of medical records that had been obtained for clinical purposes, the requirement for written informed consent was waived.
Statistical Analysis
Statistical analysis was carried out using SPSS 16.0 for Microsoft Windows. Numerical data and categorical data were expressed as mean ± SD (range) and percentage, respectively. The Mann–Whitney U test is used to compare differences between means. The comparisons of various categorical clinical manifestations between two groups were evaluated using Pearson chi-square or Fisher exact test (when there were expected frequencies <5). All probabilities were two-sided, with P < 0.05 considered statistically significant.
RESULTS
Patient Characteristics
A total of 93 BD patients (77 male, 16 female) developed thrombosis, which accounted for 12.1% of the 766 BD patients (472 male, 294 female) hospitalized. Vascular thrombosis occurred more often in male patients (16.3% vs 5.4%, P < 0.01). Their ages at diagnosis of BD were 32 ± 11 years old (range: 16–66 years old) and their ages at first thrombotic events were 33 ± 10 years old (range: 15–66 years old). The interval between BD diagnosis and emergence of first thrombotic events was 0 to 15 years. Among the 93 BD patients with thrombosis, thrombosis developed after the diagnosis of BD in 30 patients. The median interval was 2 years (0.5–15 years). There were 12 patients who developed thrombosis prior to the diagnosis of BD with the average interval from 4 months to 4 years. BD was diagnosed concomitantly with thrombosis in 51 patients.
Types of Thrombosis
As shown in Table 1, the most common types of thrombosis were extremity vein thrombosis (n = 80; 86.0%), including deep vein thrombosis (DVT; n = 78) and superficial thrombophlebitis (n = 4). Lower extremity DVT was diagnosed in 69 patients (20 cases of left, 19 cases of right, and bilateral in 30 cases), while upper extremity DVT was found in 14 patients (5 cases of right, 3 cases of left, and 6 cases of bilateral). Other cases of thrombosis associated with BD in descending order of frequency were vena cava thrombosis (n = 28, 30.1%; 23 cases were in the inferior vena cava and 10 cases were in the superior vena cava), pulmonary thromboembolism (PTE; n = 14, 15.1%; 9 cases were accompanied with lower extremity DVT simultaneously), cerebral venous thrombosis (CVT; n = 12, 12.9%), intracardiac thrombosis (n = 8, 8.6%; 5 cases were only in the right cavity, 1 case was only in the left cavity, 2 cases were in both cavities of the heart, and 4 cases were accompanied by PTE). In addition, we found Budd–Chiari syndrome in 7 cases (7.5%), renal vein thrombosis in 4 cases (4.3%), lower extremity artery thrombosis in 3 cases (3.2%), superior mesenteric veins thrombosis in 2 cases (2.2%), and abdominal artery thrombosis, carotid artery aneurysm thrombosis, femoral aneurysm thrombosis, and abdominal aortic aneurysm thrombosis in 1 case each (1.1%).
TABLE 1.
Main Types of Thrombosis in BD Patients
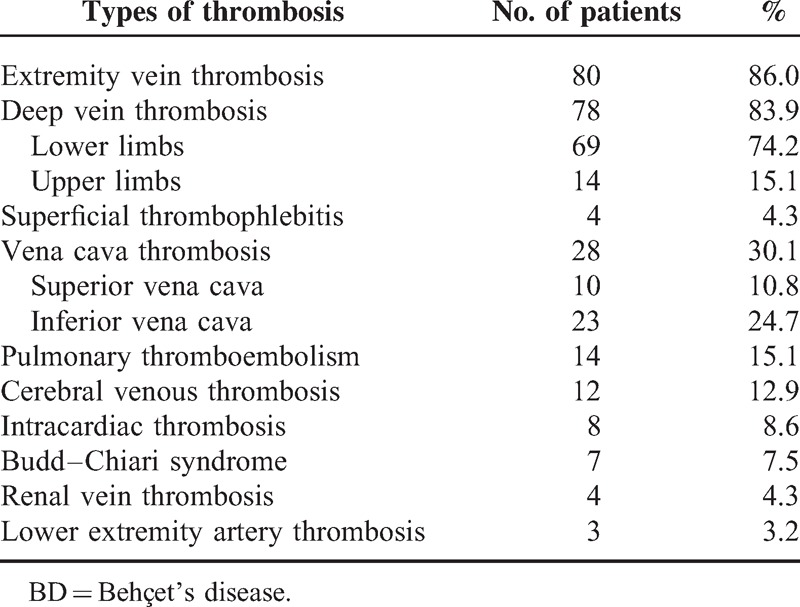
Among these cases, venous thrombosis was more common than arterial involvement, and most of patients (88/93, 94.6%) had multiple sites of thrombosis. Sixteen cases were complicated with aneurysms (abdominal aortic aneurysms in 8 cases, lower extremity artery aneurysms in 5 cases, pulmonary artery aneurysms in 3 cases, and carotid aneurysms and subclavian artery aneurysms in 1 case each); 5 cases were accompanied with artery stenosis and occlusion.
Systemic Manifestations
Oral ulceration was present in all patients. Other common findings included genital ulcers in 68 cases (73.1%), skin lesions in 67 cases (72.0%), positive pathergy test in 46 cases (49.5%), ocular involvement in 26 cases (28.0%), arthritis in 10 cases (10.8%), gastrointestinal involvement in 9 cases (9.7%), non-thrombotic neurologic involvement in 6 cases (6.5%), non-thrombotic cardiac involvement in 5 cases (5.4%), and epididymis/orchitis in 3 cases (3.2%).
Active BD was documented in all patients during thrombosis onset, with BDCAF2006 score 5.8 ± 2.0 (range: 3–9). The accompany symptoms of oral ulceration in 48 cases (51.6%), genital ulceration in 23 cases (24.7%), skin lesions in 38 cases (40.9%), positive pathergy test in 23 cases (24.7%), ocular involvement in 14 cases (15.1%), digestive ulceration in 7 cases (7.5%), arthritis in 4 cases (4.3%), neurologic involvement in 4 cases (4.3%), and epididymis/orchitis in 2 cases (2.2%).
Gender-Specific Clinical Features
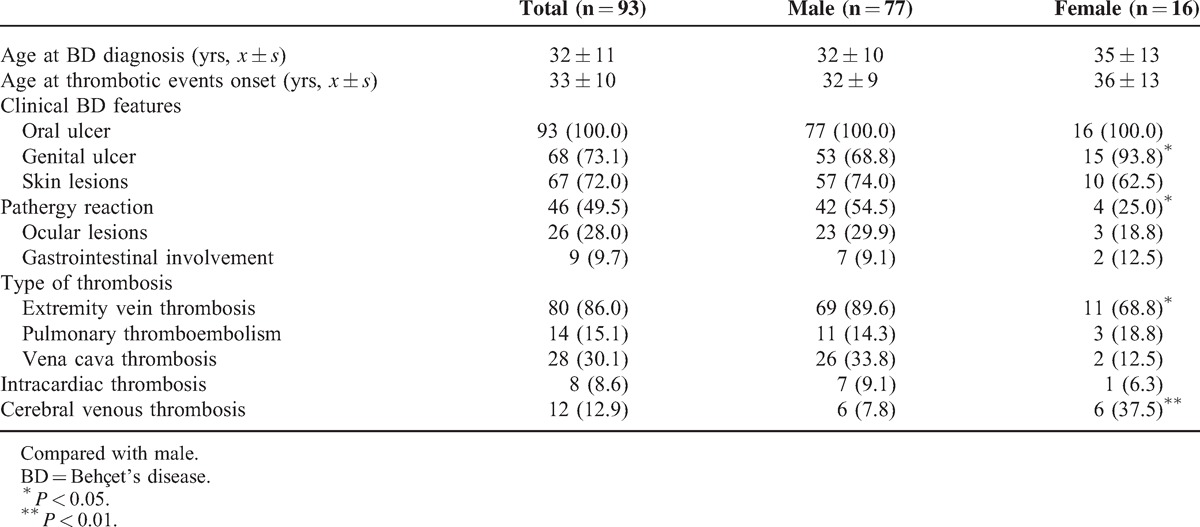
Patients were stratified by gender. Male patients showed more predominance in developing extremity vein thrombosis (89.6% vs 68.8%, P < 0.05), with positive pathergy test (54.5% vs 25.0%, P < 0.05), and less predominance in developing CVT (7.8% vs 37.5%, P < 0.01) and genital ulcers (68.8% vs 93.8%, P < 0.05) (Table 2).
TABLE 2.
The Influence of Sex on the Clinical Course of BD Patients Complicated With Thrombosis
Laboratory Examination
At the time of thrombosis development, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were significantly higher in 67.8% (59/87) and 96.2% (75/78) of patients, respectively. The mean ESR was 41 ± 33 mm/first hour and the mean CRP levels was 47 ± 51 mg/L.
Assays for anti-nuclear antibody (ANA), anti-double stranded DNA (anti-dsDNA) antibody, anti-extractable nuclear antigen (ENA) antibody, and anti-neutrophil cytoplasmic antibody (ANCA) were performed in all patients. Anti-dsDNA, anti-ENA antibody, and ANCA were all negative, while low titer of ANA was detected only in 7 patients. Human leukocyte antigen-B51 genotyping was positive in 11 out of 39 patients. Antiphospholipid antibodies including IgG type anti-cardiolipin antibody (ACL), IgG type anti-β2 glycosidoprotein 1 (β2GP1) antibody, and lupus anticoagulant (LA) were positive in 2/67, 1/32, and 5/54 patients, respectively. Activated partial thromboplastin time (APTT) and prothrombin time (PT) which were performed in 55 patients were almost in the normal range when thrombosis occurred. Deficiencies of protein C, protein S and antithrombin III and hyperhomocysteinemia was documented in 16.7% (2/12), 18.2% (2/11), 9.1% (1/11), and 35.7% (5/14) patients, respectively.
Treatment and Follow-Up
All patients received glucocorticosteroids and immunosuppressive agents. The initial glucocorticosteroids dosage was 10 mg to 60 mg/d of prednisone or equivalent dosage of other corticosteroids, with the average dose of 52 ± 11 mg. Thirteen patients had 1000 mg methylprednisolone pulse therapy for 3 days and then switched to oral prednisone 1 mg/kg/d and gradually tapered to 10 mg/d or less. In terms of immunosuppressant, cyclophosphamide was the most commonly used (83.7%, 77/92); others included thalidomide (n = 28), cyclosporine (n = 12), tripterygium glycosides (n = 11), colchicine (n = 8), hydroxychloroquine (n = 7), methotrexate (n = 6), and azathioprine (n = 1). Forty-nine patients received two or more immunosuppressants. Anti-platelet agents and/or anticoagulants were used in 82 patients. Among them, 76 patients received anticoagulants, 1 patient received anti-platelet agents, and 5 patients received a combination of anti-platelet agents and anticoagulants. Eleven cases with multiple artery aneurysms were received neither anti-platelet agents nor anticoagulants. Six patients with aneurysms and/or pseudoaneurysms and 2 patients with intracardiac thrombosis underwent surgical treatment.
After a median follow-up of 14 months (3–63 months), 89 patients improved as measured by decreased ESR and CRP levels (in 40 patients) and 4 patients died. One patient with DVT who was repeatedly hospitalized because of cardiac valvular disease complicated with infective endocarditis, and died from myocardial infarction despite of aggressive treatment of the underlying BD, anticoagulants and anti-infection therapy. One patient with vena cava thrombosis and multiple aneurysms received steroids and immunosuppressive agents and died from aneurysm rupture. One patient with Budd–Chiari syndrome died from hepatic failure, and another one complicated with septic shock and discontinued therapy.
DISCUSSION
Previous studies have demonstrated that vascular involvement is common in BD patients, affecting up to 40% of male patients; venous lesions have been reported to occur more frequently than arterial lesions.9,10 The prevalence of thrombosis in BD patients varies between 10% and 30%, in which multiple vessel beds are often affected.11 Fei et al12 have studied the prevalence and clinical characteristics of vascular involvement in Chinese BD patients from our center. Unlike Fei, our study focuses on clinical features of thrombosis in BD patients, including the location of DVT, disease activity on occurrence of thrombosis, and influence of gender on clinical course of BD patients with thrombosis. Based on our patient population, we demonstrated that the incidence of BD associated with thrombosis was 12.1% and it was more common in males (82.8%). Additionally, most of the patients had two or more sites of thrombosis. A male predominance of extremity vein thrombosis and positive pathergy test, and a female predominance of CVT and genital ulcers were noted. All of these patients exhibited active disease during the emergence of thrombotic events.
In the current study, the most common thrombotic manifestation was DVT in the extremities, and superficial thrombophlebitis occurred more often in male BD patients. As previously reported, DVT in the lower extremities often appears in the early stages of BD, the most common being venous thrombosis, accounting for 60% to 80% of all vascular involvement.9–11,13–14 The affected veins in descending order of frequency were the femoral, popliteal, and saphenous veins which could manifest as lower extremity pain, erythema with induration, edema and hyperpigmentation, intermittent claudication, and ulceration.15 The habitual recurrence of thrombosis in the legs often occurred prior to the involvement of other large major vessels.10 Compared with non-BD patients, the number of veins affected and the severity of damage were greater in the patients with thrombosis.15
PTE accounted for 15.1% of cases. It is generally believed that PTE is significantly related to DVT; they often occur concurrently and lower DVT in the extremities is the main reason of pulmonary embolism (PE). However, for BD patients, tight adhesions in the peripheral thrombosis of the venous walls seldom cause emboli in the lungs, while pulmonary vasculitis could result in intima injury and multiple pulmonary thrombi. Localized pulmonary vascular stenosis or occlusion could increase the resistance of pulmonary circulation, eventually lead to the destruction of pulmonary vascular structures, the formation of pulmonary artery aneurysms, and subsequent thrombosis in the arterial walls. However, sometimes it is difficult to distinguish in situ pulmonary thrombosis from PE, making the true frequency of pulmonary embolic disease unknown.15,16 Among the thirteen BD patients with PTE in our study, 3 patients had a pulmonary artery aneurysm concomitantly, and the other 2 patients had pulmonary artery stenosis and occlusion, which may suggest in situ pulmonary thrombosis formation.
Vena cava thrombosis was also a common case of thrombosis, accounting for 30.1% in our patients. Clinical manifestations of vena cava thrombosis varied according to anatomical localization. Chronic occlusion of the caval systems could lead to the appearance of prominent venous collaterals on the thoracic and abdominal walls. BD associated with superior vena cava thrombosis had a benign course with efficient collateral circulation.17 Budd–Chiari syndrome is a rare and serious complication of BD with a high mortality rate; it is estimated to occur in 1% to 3% of BD patients.16 Budd–Chiari syndrome is caused by occlusion of major hepatic veins, the adjacent inferior vena cava, or both, and manifests clinically as abdominal pain, ascites, edema in lower extremities and scrotum, and even hepatic failure in severe cases. In our study, in the 7 patients with Budd–Chiari syndrome, both the hepatic and inferior vena cava veins were affected.
CVT is one of the major neurological manifestations of BD, occurs in 8% of the BD patients and corresponds to 18% of the NBD.18 In the current study, CVT developed in 12 patients, and occurred more frequently in female BD patients with thrombosis. The most frequent symptoms were headache (91.7%), and the most frequent locations of CVT were occlusion of the transverse sinus (75%) and the superior sagittal sinus (66.7%), which is consistent with the literature reports.18,19 Short-term outcomes were good, as patients were treated with corticosteroids and immunosuppressants, such as cyclophosphamide, intrathecal dexamethasone, anticoagulants, and dehydration therapy; all 12 patients achieved clinical improvements.
Complication of intracardiac thrombosis is relatively rare in BD patients20,21; however, there is a high mortality rate associated with these complications. Intracardiac thrombosis predominantly affects male patients and it usually occurs in the right cavity; in addition, the pulmonary artery is often involved21 and the thrombosis often adheres to underlying endocardium or myocardium. Histological findings have revealed that there is an organizing thrombus containing inflammatory cell infiltrates with or without involvement of underlying cardiac tissue.21 In our study, intracardiac thrombosis tended to occur in the right heart; 4 patients had PTE. Detected by transthoracic echocardiography, the intracardiac thrombus was either attached to the free wall or the septum, varied in size, and sometimes multiplied in one chamber or affected multiple chambers. After treatment, in two cases, the intracardiac thrombus disappeared and, in another two cases, remained stable after surgery without recurrence; the thrombus decreased in size in the other three patients.
The underlying mechanism of thrombosis in BD patients remains elusive. Multiple prothrombotic factors have been studied, but the results were controversial. According to previous reports, there was no significant correlation between antiphospholipid antibodies (APL) and thrombosis in BD.22,23 PT and partial thromboplastin time (PTT) also could not contribute to thrombosis in BD.24 Few reports indicated that deficiencies of protein C and protein S might be the risk factors for BD thrombosis.25,26 The main factor responsible for the increased frequency of thrombosis in BD is thought to be endothelial damage/activation.27,28 In addition, abnormal fibrinolysis29 and altered platelet function30 have also been associated with the development of thrombosis in BD. Genetic risk factors also a cause of thrombosis in BD. Factor V Leiden (FVL), prothrombin, and methyltetrahydrofolate reductase (MTHFR) gene polymorphisms were reported to associate with thrombosis in BD. However, a systematic review and meta-analysis showed that FVL gene mutation (G1691A) was significantly associated with thrombosis in BD, but only relevant to Turkish patients. No significant association was found for the presence of prothrombin and MTHFR mutations and thrombosis in BD.31 Genetic predisposition may not be the same in different ethnic groups and geographical areas. Given the limitation of retrospective study, the genetic polymorphism information of our patients was not available.
The management of venous thrombosis in BD has remained controversial. In 2008, the European League Against Rheumatism published recommendations that endorsed the use of glucocorticoids and immunosuppressants (azathioprine, cyclophosphamide, and cyclosporine A) in the management of acute deep venous thrombosis in BD, while discouraging the use of antiplatelet, anticoagulant, and antifibrinolytic agents, due to the fact that tight adhesions of a peripheral thrombosis to venous walls rarely results in emboli in the lungs. In addition, anticoagulants could increase the risk of rupturing aneurysms which could cause fatal bleeding.32 Recently, a French study including 807 BD patients found that the use of immunosuppressants significantly reduced the risk of recurrent DVT. In our study, aspirin and/or anticoagulants were used in thrombotic patients who did not have pulmonary artery aneurysms.
Our study is a retrospective analysis of a case series, which may bring some potential limitations. As our hospital is the nominated national referred center for complicated rheumatic diseases, which may result in more severe cases enrolled in our study, we cannot exclude the possibility of selection bias in this study population. Moreover, conclusions are only limited to a Chinese population and not especially generalizable to other populations.
In conclusion, vascular thrombosis is an important complication in BD patients and occurs more often in male patients. Venous thrombosis is more frequent than arterial thrombosis, and most of patients experienced multiple thrombosis. Active disease patients are prone to thrombosis, which suggest the key role of immunosuppressive therapy for the complication. Future studies exploring the mechanism of thrombosis and its link to inflammation may provide important insights in predicting which patients may be at a higher risk for thrombosis to improve outcomes.
Footnotes
Abbreviations: ANA = anti-nuclear antibody, BD = Behçet's disease, BDCAF2006 = BD Current Activity Form 2006, CRP = C-reactive protein, CVT = cerebral venous thrombosis, CT = computed tomography, DVT = deep vein thrombosis, ESR = erythrocyte sedimentation rate, ICBD = International Criteria for BD, PTE = pulmonary thromboembolism.
Xiuhua Wu and Guohua Li contributed equally to this manuscript.
All authors made substantial contributions to the conception and design of this study. Guohua Li, Xinxiang Huang and Li Wang acquired the data. Xiuhua Wu performed the data analysis and interpretation and wrote the manuscript. Wenjie Zheng provided critical revisions of the manuscript. Yan Zhao and Wanli Liu also critically reviewed the manuscript and provided valuable input. All authors read and approved the final manuscript.
The authors declare no conflict of interest.
This study was supported by a grant from Chinese Medical Association (12040670367).
REFERENCES
- 1.Davatchi F, Assaad-Khalil S, Calamia KT, et al. The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014; 28:338–347. [DOI] [PubMed] [Google Scholar]
- 2.Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet 1990; 335:1078–1080. [PubMed] [Google Scholar]
- 3.Goodacre S, Stevenson M, Wailoo A, et al. How should we diagnose suspected deep-vein thrombosis? QJM 2006; 99:377–388. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Guan W, Chen D, et al. The value of CTPA for diagnosing acute pulmonary thromboembolism and the ensuing right ventricular dysfunction. Cell Biochem Biophys 2014; 69:517–522. [DOI] [PubMed] [Google Scholar]
- 5.Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:1158–1192. [DOI] [PubMed] [Google Scholar]
- 6.Asamov RE, Muminov ShM, Dadam’iants NG, et al. Diagnosis and treatment of inferior vena cava thrombosis. Ross Akad Med Nauk 2010; 10:59–63. [PubMed] [Google Scholar]
- 7.Adegboye VO, Ogunseyinde AO, Obajimi MO, et al. Superior vena cava obstruction: diagnosis, management and outcome. East Afr Med J 2008; 85:129–136. [PubMed] [Google Scholar]
- 8.Egolum UO, Stover DG, Anthony R, et al. Intracardiac thrombus: diagnosis, complications and management. Am J Med Sci 2013; 345:391–395. [DOI] [PubMed] [Google Scholar]
- 9.Kural-Seyahi E, Fresko I, Seyahi N, et al. The long-term mortality and morbidity of Behçet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore) 2003; 82:60–76. [DOI] [PubMed] [Google Scholar]
- 10.Calamia KT, Schirmer M, Melikoglu M. Major vessel involvement in Behçet's disease: an update. Curr Opin Rheumatol 2011; 23:24–31. [DOI] [PubMed] [Google Scholar]
- 11.Gaffo AL. Thrombosis in vasculitis. Best Pract Res Clin Rheumatol 2013; 27:57–67. [DOI] [PubMed] [Google Scholar]
- 12.Fei Y, Li X, Lin S, et al. Major vascular involvement in Behçet's disease: a retrospective study of 796 patients. Clin Rheumatol 2013; 32:845–852. [DOI] [PubMed] [Google Scholar]
- 13.Sarica-Kucukoglu R, Akdag-Kose A, Kayaball M, et al. Vascular involvement in Behçet's disease: a retrospective analysis of 2319 cases. Int J Dermatol 2006; 45:919–921. [DOI] [PubMed] [Google Scholar]
- 14.Düzgün N, Ateş A, Aydintuğ OT, et al. Characteristics of vascular involvement in Behçet's disease. Scand J Rheumatol 2006; 35:65–68. [DOI] [PubMed] [Google Scholar]
- 15.Seyahi E, Yurdakul S. Behçet's syndrome and thrombosis. Mediterr J Hematol Infect Dis 2011; 3:e2011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Springer J, Villa-Forte A. Thrombosis in vasculitis. Curr Opin Rheumatol 2013; 25:19–25. [DOI] [PubMed] [Google Scholar]
- 17.Hamuryudan V, Melikoglu M. Yazici Y, Yazici H. Vascular involvement in Behçet's syndrome. Behcet's Syndrome 1st ed.New York: Springer; 2010. 115–134. [Google Scholar]
- 18.Aguiar de Sousa D, Mestre T, Ferro JM. Cerebral venous thrombosis in Behçet's disease: a systematic review. J Neurol 2011; 258:719–727. [DOI] [PubMed] [Google Scholar]
- 19.Saadoun D, Wechsler B, Resche-Rigon M, et al. Cerebral venous thrombosis in Behçet's disease. Arthritis Rheum 2009; 61:518–526. [DOI] [PubMed] [Google Scholar]
- 20.Mogulkoc N, Burgess MI, Bishop PW. Intracardiac thrombus in Behçet's disease: a systematic review. Chest 2000; 118:479–487. [DOI] [PubMed] [Google Scholar]
- 21.Geri G, Wechsler B, ThiHuong du L, et al. Spectrum of cardiac lesions in Behçet disease: a series of 52 patients and review of the literature. Medicine (Baltimore) 2012; 91:25–34. [DOI] [PubMed] [Google Scholar]
- 22.Mader R, Ziv M, Adawi M, et al. Thrombophilic factors and their relation to thromboembolic and other clinical manifestations in Behçet's disease. J Rheumatol 1999; 26:2404–2408. [PubMed] [Google Scholar]
- 23.Harman E, Sayarlıoglu M, Harman M, et al. The evaluation of coagulation parameters and vessel involvement in Behcet's disease. A clinical experience of Behcet's disease: study of 152 cases. Acta Med Iran 2013; 51:215–223. [PubMed] [Google Scholar]
- 24.Sengül N, Demirer S, Yerdel MA, et al. Comparison of coagulation parameters for healthy subjects and Behçet disease patients with and without vascular involvement. World J Surg 2000; 24:1584–1588. [DOI] [PubMed] [Google Scholar]
- 25.Navarro S, Ricart JM, Medina P, et al. Activated protein C levels in Behçet's disease and risk of venous thrombosis. Br J Haematol 2004; 126:550–556. [DOI] [PubMed] [Google Scholar]
- 26.Kwon SR, Lim MJ, Park SG, et al. Decreased protein S activity is related to the disease activity of Behcet's disease. Rheumatol Int 2006; 27:39–43. [DOI] [PubMed] [Google Scholar]
- 27.Haznedaroglu IC, Ozcebe OI, Ozdemir O, et al. Impaired haemostatic kinetics and endothelial function in Behçet's disease. J Intern Med 1996; 240:181–187. [DOI] [PubMed] [Google Scholar]
- 28.Chambers JC, Haskard DO, Kooner JS. Vascular endothelial function and oxidative stress mechanisms in patients with Behçet's syndrome. J Am Coll Cardiol 2001; 37:517–520. [DOI] [PubMed] [Google Scholar]
- 29.Ricart JM, Ramon LA, Vaya A, et al. Fibrinolytic inhibitor levels and polymorphisms in Behçet disease and their association with thrombosis. Br J Haematol 2008; 141:716–719. [DOI] [PubMed] [Google Scholar]
- 30.Akar S, Ozcan MA, Ates H, et al. Circulated activated platelets and increased platelet reactivity in patients with Behçet's disease. Clin Appl Thromb Hemost 2006; 12:451–457. [DOI] [PubMed] [Google Scholar]
- 31.Chamorro AJ, Marcos M, Hernández-García I, et al. Association of allelic variants of factor V Leiden, prothrombin and methylenetetrahydrofolate reductase with thrombosis or ocular involvement in Behçet's disease: a systematic review and meta-analysis. Autoimmun Rev 2013; 12:607–616. [DOI] [PubMed] [Google Scholar]
- 32.Hatemi G, Silman A, Bang D, et al. EULAR recommendations for the management of Behçet disease. Ann Rheum Dis 2008; 67:1656–1662. [DOI] [PubMed] [Google Scholar]


