Abstract
Purpose:
The purpose of this study was to determine whether it is possible to differentiate benign from malignant thyroid nodules according to the proportion of sponge-like appearance within the nodules.
Methods:
A total of 201 thyroid nodules containing sponge-like appearance from 195 patients (157 women and 38 men) were included this study. Each thyroid nodule was classified into one of three grades by real-time ultrasonography (US) based on the areas with a sponge-like appearance within nodule: grade I had sponge-like areas occupying <50%; grade II, between 50% and 75%; and grade III, >75%. We evaluated whether a correlation existed between these grades and cytopathologic diagnoses.
Results:
Of the 201 nodules, 196 were benign and five were malignant, and according to the US classification, 101 nodules were grade I, 45 were grade II, and 55 were grade III. Of the five malignant nodules, four were grade I, and one was grade II. No statistically significant difference was found in the rate of malignancy between grade III and grades I and II, due to insufficient statistical power. A sponge-like appearance was correlated with follicles filled with colloid and cholesterol granules in benign nodules and with papillary fronds around the dilated cystic spaces in malignant nodules.
Conclusion:
No malignancies were found in thyroid nodules with >75% sponge-like appearance. Due to the overall low incidence of malignancy and the limited number of patients, a statistically significant difference could not be found in the prevalence of malignancy depending on the proportion of sponge-like areas within the nodule.
Keywords: Thyroid nodule; Cysts; Diagnosis, differential; Ultrasonography
Introdution
Thyroid nodules are very common in clinical practice. The incidence of thyroid nodules is 10%-67% in adults undergoing ultrasonography (US). However, most thyroid nodules are benign, and only a small percentage (9.2%-14.8%) of nodules are malignant [1-3]. Since the late 1990s, many studies have reported specific US features of thyroid nodules that may predict malignancy [3-8]. A few reports have evaluated the specific US features of benign thyroid nodules that do not require US-guided fine needle aspiration (FNA) [9-13]. In light of previous research on this topic, determining specific US findings capable of differentiating benign thyroid nodules from malignant nodules is helpful in avoiding unnecessary FNA or frequent follow-up US.
Areas of spongiform appearance (also known as “honey-comb” or “puff pastry”) in thyroid nodules are usually characterized by clustered, similarly sized, microcystic spaces separated by thin echogenic septa. Thyroid nodules with these features have previously been regarded as benign [10,13]. According to the definition presented by Moon et al. [14], thyroid nodules containing microcystic components that occupy more than half of the entire nodule volume are considered to have a spongiform appearance, and it was suggested that this feature, in combination with isoechogenicity, is specific for benign nodules. Bonavita et al. [15] described a “puff-pastry” pattern as diffuse internal linear cysts. They suggested that the entire nodule must be spongiform and that the nodule cannot be hypervascular; according to their definition, a hypervascular nodule is not a spongiform nodule, even if the entire nodule has a spongiform appearance. No consensus currently exists regarding the terminology used to describe spongiform patterns in thyroid nodules, and no studies have attempted to find correlations between US findings and pathologic outcomes in thyroid nodules with a sponge-like portion or clustered multiple small cysts [4,9,13]. For this reason, we hypothesized that a sponge-like appearance or the presence of clustered multiple small cysts in thyroid nodules might be a typical benign feature in and of itself, regardless of other US features. In order to evaluate this hypothesis, we assessed the pathologic findings of thyroid nodules with varying degrees of sponge-like appearance.
Materials and Methods
Patients
Our Institutional Review Board approved this prospective study, and informed consent was obtained from each participant prior to the beginning of the study. From August 2006 to February 2009, one radiologist (J.Y.K.) performed thyroid US and made prospective US diagnoses for nodules with a sponge-like appearance in 273 patients. Of these 273 patients, 195 patients (157 women and 38 men; age range, 17 to 78 years; mean age, 51.8 years) with 201 sponge-like nodules with a maximum diameter >1 cm were enrolled in this study. The exclusion criteria for thyroid nodules with a sponge-like appearance were as follows: (1) a diameter <1 cm, because in such cases it is difficult to discriminate the sponge-like component within the nodule; (2) the absence of an indication for FNA, such as a probable benign nodule <2 cm in diameter; (3) patients who refused FNA or repeat FNA; and (4) nodules that were not diagnosed by cytology or pathology.
All 201 sponge-like nodules were cytopathologically diagnosed by consecutive US-guided FNA at least twice within a 2-year period or postoperatively in cases where surgery was performed following the initial thyroid US for any reason, such as cytologic findings suspicious for malignancy in the sponge-like nodule or concomitant malignancy of another thyroid nodule.
Thyroid US
Thyroid US was performed by a single radiologist with a high-resolution ultrasound instrument (HDI 5000, Philips Medical System, Bothell, WA, USA) equipped with a 7-12-MHz linear array transducer. A nodule with a sponge-like appearance was defined as exhibiting clustered, similarly sized, microcystic spaces separated by thin echogenic septa or a solid portion within the nodule, irrespective of the proportion of such areas within the nodule or other combined US findings. Based on the thyroid US findings, each sponge-like nodule was classified into one of three grades by the same radiologist immediately after real-time US examination. The grades were determined according to the proportion of the sponge-like area within the nodule by measuring the maximum diameter of the nodule and the sponge-like area in the two-dimensional images: grade I nodules were defined as having <50% of their volume made up by sponge-like areas, while the corresponding proportions were defined as 50%-75% in grade II nodules and >75% in grade III nodules (Fig. 1). The US findings of each sponge-like nodule were evaluated according to the following criteria: size (maximal dimension), shape (ovoid to round, taller than wide, or irregular), margin (smooth, ill-defined, or spiculated), internal echogenicity (hypoechoic, isoechoic, or hyperechoic compared to the background thyroid tissue), the presence of calcifications (microcalcifications, macrocalcifications, or rim calcification), and the presence of tiny echogenic foci with comet-tail artifacts (colloid microcrystals). Microcalcifications were defined as tiny, hyperechoic foci (<1 mm in size) with no comet-tail artifacts or posterior shadow on real-time US [14].
Fig. 1. Grading of sponge-like nodules.
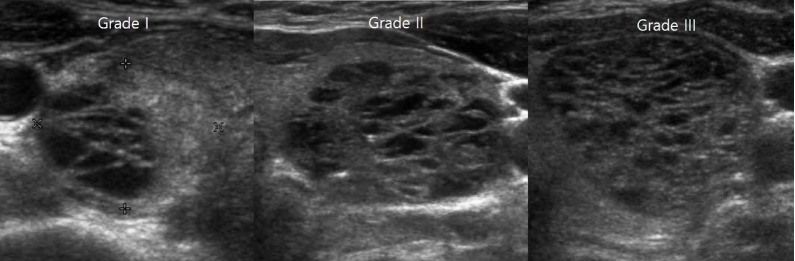
The grades were determined according to the proportion of areas with a sponge-like appearance within each nodule. Grade I was defined as <50%, grade II as between 50% and 75%, and grade III as >75%.
US-Guided FNA and Reference Standard
US-guided FNA was performed on 200 sponge-like nodules of 194 patients without local anesthesia. One patient with a sponge-like nodule underwent total thyroidectomy after real-time US examination because of a coincidental malignant nodule. US-guided FNA was performed with a 23-gauge needle attached to a 10-mL disposable plastic syringe. The target of FNA was the solid portion of the sponge-like nodule. Samples obtained from the aspiration biopsy were expelled on glass slides and smeared. For each sample, four to five slides fixed in 95% ethanol were sent to the Department of Pathology for Papanicolaou staining. The cytopathologists were not present on site during the biopsy. Two cytopathologists interpreted the FNA slides. Malignant nodules were confirmed surgically, while nodules that were diagnosed as benign during the first FNA were confirmed as benign nodules by means of surgery or repeat FNA.
Of 201 sponge-like nodules, US-guided FNA was performed on 200 nodules, while surgery was performed on one nodule after US. Of the 200 nodules that underwent FNA, 192 were benign and five were suspicious for malignancy according to the initial results, while one had atypical cytology and two had unsatisfactory cytology. The findings were confirmed through surgery for 19 nodules and through repeat FNA for 182 nodules. The mean interval between initial and repeat FNA was 18.6 months (range, 3 to 36 months) for the 182 FNA-confirmed thyroid nodules. The interval between the first and repeat FNA was more than three months in all patients to avoid nuclear atypia related to aspiration (Fig. 2). We evaluated the cytopathologic features of both the benign and malignant nodules with a sponge-like appearance.
Fig. 2. Flow chart of the study group.
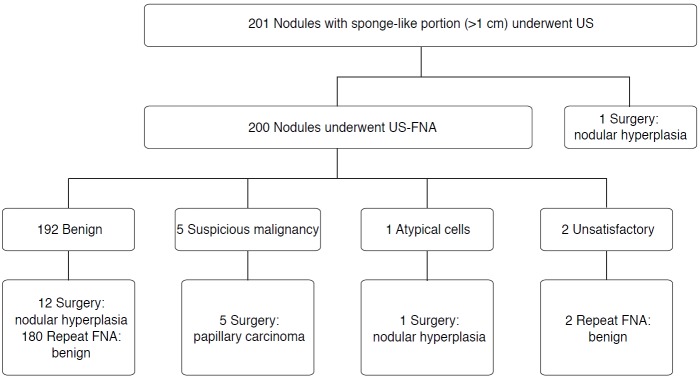
US, ultrasonography; FNA, fine needle aspiration.
Statistical Analysis
Statistical analysis was performed with a SPSS ver. 16 for Windows (SPSS Inc., Chicago, IL, USA). Each spongiform grade was analyzed to determine its association with the benignity or malignancy of the nodules. P-values of <0.05 were considered to indicate statistical significance. The chi-squared test and the Fisher exact test were used to compare categorical variables, and the Student’s t-test was used to compare quantitative variables. In addition, multiple logistic regression analysis was performed to determine whether any of the US findings under evaluation could independently predict malignancy.
Results
Of the 201 sponge-like nodules, 196 were benign (14 confirmed by surgery, 182 confirmed by repeated cytology) and five were malignant nodules, as confirmed by surgery. Surgery was performed to excise all of the malignant nodules, and pathologic diagnoses confirmed them to be papillary carcinomas. The distribution of nodules according to the grades that reflected the proportion of sponge-like areas was as follows: 101 nodules (50.2%) were grade I, 45 (22.4%) were grade II, and 55 (27.4%) were grade III. The size of the nodules ranged from 1.0 to 5.6 cm (mean size, 1.62 cm). Common US findings in the sponge-like nodules were ovoid to round shape (99%, n=199), smooth margins (66%, n=133), isoechogenicity (89%, n=179), and colloid microcrystals (82%, n=165).
The prevalence of malignant nodules in grade I was 4% (four of 101 nodules) and 2.2% in grade II (one of 45 nodules). No malignant nodules were found in grade III. However, no statistically significant difference was found in the rate of malignancy between the nodules in which >75% of the volume was occupied by sponge-like areas (grade III) and those in which <75% of the volume was occupied by sponge-like areas (grade I and II), due to insufficient statistical power (power value, 0.55).
Of the 196 benign nodules, 97 were grade I, 44 were grade II, and 55 were grade III. Common US findings in the benign sponge-like nodules were ovoid to round shape (n=194), smooth margins (n=130), isoechogenicity (n=178), and the presence of colloid microcrystals (n=161). Calcifications were only found in 11 nodules; microcalcifications were found in five nodules, macrocalcifications in one nodule, both microcalcifications and macrocalcifications in one nodule, and rim calcification in four nodules (Fig. 3A, B). Of the five malignant nodules, four were grade I, and one was grade II. The US findings in the malignant sponge-like nodules were as follows: ovoid to round shape (n=5), smooth margin (n=3) or ill-defined margin (n=2), hypoechogenicity (n=4) or isoechogenicity (n=1), and microcalcifications (n=3) (Fig. 4A, B).
Fig. 3. Benign sponge-like thyroid nodule in a 46-year-old woman.
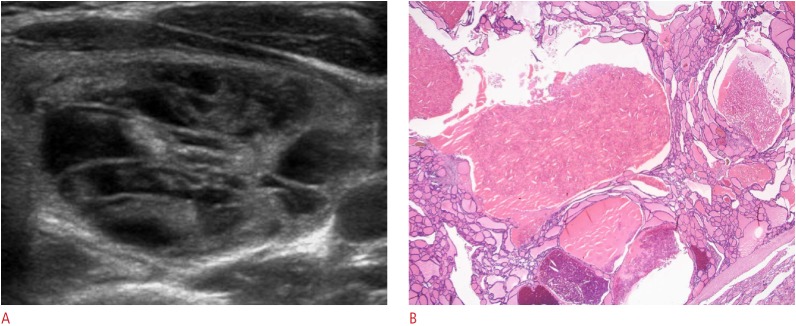
A. Axial view of the left thyroid lobe shows a sponge-like nodule, >75% of the volume of which is occupied by areas with a sponge-like appearance (grade III) with a smooth margin, ovoid shape, and isoechogenicity. B. Photomicrographic section shows multiple variably sized nodular follicles filled with old colloid and cholesterol granules caused by the condensation of colloid microcrystals (H&E, ×40). This nodule was diagnosed as nodular hyperplasia.
Fig. 4. Malignant sponge-like nodule in a 55-year-old woman.
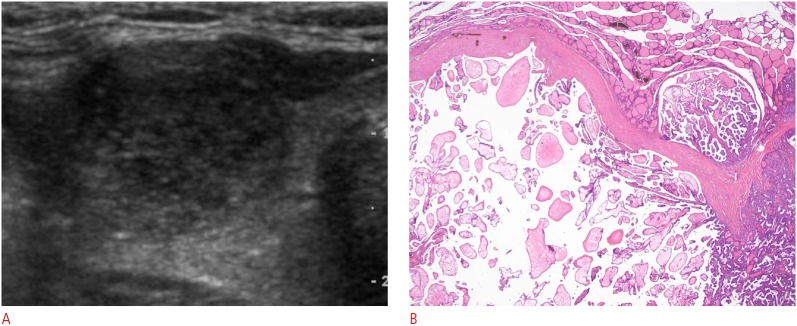
A. Axial view of the right thyroid lobe shows a sponge-like nodule, <50% of the volume of which consists of areas with a sponge-like appearance (grade I), with a smooth margin, ovoid shape, hypoechogenicity, and microcalcifications. B. Photomicrographic section shows cystic dilatation of the interpapillary space and papillary fronds within the dilated cystic spaces (H&E, ×40). This nodule was diagnosed as papillary carcinoma.
No statistically significant difference was found in the size of the benign and malignant sponge-like nodules. The rate of malignancy according to the US features of thyroid nodules were as follows: 19.6% in nodules with an ovoid to round shape, 2.3% in nodules with a smooth margin, 0.5% in isoechoic nodules, 20% in hypoechoic nodules, and 33.3% in nodules with microcalcifications. The US features of the benign and malignant sponge-like nodules were compared using the Fisher exact test. Significant differences between the two groups were found with regard to hypoechogenicity (P<0.001) and the presence of microcalcifications (P=0.001). The US findings are summarized in Table 1.
Table 1.
Sonographic features of 201 benign and malignant sponge-like nodules
| Characteristic | Benign (n=196, 97.5%) | Malignant (n=5, 2.5%) | P-value |
|---|---|---|---|
| Mean maximum diameter (range, cm) | 1.6 (1.0-5.6) | 1.8 (1.0-3.3) | 0.780 |
| Proportion of sponge-like appearance | 0.424 | ||
| Grade I (<50%) | 97 (49.5) | 4 (80.0) | |
| Grade II (50%-75%) | 44 (22.4) | 1 (20.0) | |
| Grade III (>75%) | 55 (28.1) | 0 | |
| Shape | >0.999 | ||
| Ovoid to round | 194 (98.9) | 5 (100) | |
| Taller than wide | 2 (1.0) | 0 | |
| Margin | >0.999 | ||
| Smooth | 130 (66.3) | 3 (60.0) | |
| III-defined | 65 (33.2) | 2 (40.0) | |
| Spiculated | 1 (0.5) | ||
| Echogenicity | <0.001 | ||
| Isoechoic | 178 (90.1) | 1 (20.0) | |
| Hypoechoic | 16 (8.2) | 4 (80.0) | |
| Hyperechoic | 2 (1.0) | ||
| Colloid microcrystals | >0.999 | ||
| Presence | 161 (82.1) | 4 (80.0) | |
| Absence | 35 (17.9) | 1 (20.0) | |
| Calcificationsa) | 0.001 | ||
| Absence | 185 (94.4) | 2 (40.0) | |
| Microcalcifications | 6 (3.1) | 3 (60.0) | |
| Macrocalcifications | 2 (1.0) | 0 | |
| Rim calcification | 4 (2.0) | 0 |
Values are presented as number (%).
One benign sponge-like nodule had microcalcifications and macrocalcifications.
We performed follow-up US of benign sponge-like nodules more than 3 years after the last FNA. On the follow-up US of 182 benign nodules, 19 nodules had increased in size by at least 2 mm in two or more dimensions, 15 nodules had decreased in size, and 149 nodules showed no significant changes. Repeat FNA was performed in the 19 nodules that had increased in size and the cytological results were benign in all cases.
The pathologic features of benign and malignant nodules with a sponge-like appearance were significantly different in a limited number of cases. The sponge-like areas in benign nodules were composed of multiple follicles filled with old colloid and cholesterol granules formed by condensation of colloid microcrystals (Fig. 3). However, the sponge-like areas of malignant nodules revealed papillary fronds around the dilated cystic spaces, resulting from edematous fibrovascular stromas (Figs. 4, 5).
Fig. 5. Malignant sponge-like nodule in a 38-year-old man.
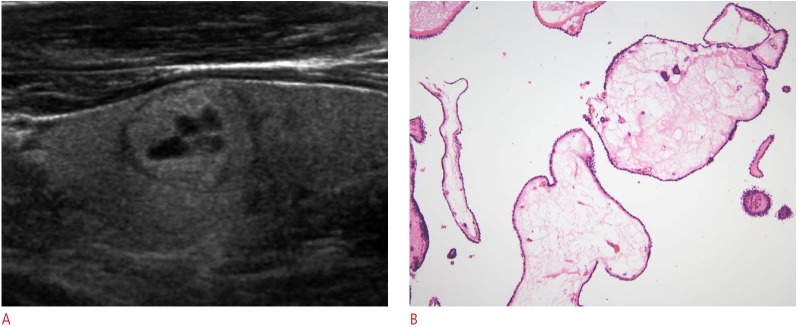
A. Longitudinal view of the left thyroid lobe shows a sponge-like nodule, <50% of the volume of which consists of areas with a sponge-like appearance (grade I), with a smooth margin, ovoid shape, and isoechogenicity. B. Photomicrographic section shows edematous changes in the core of the papillary fronds lining, with papillary carcinoma cells (H&E, ×200). This nodule was diagnosed as papillary carcinoma.
Discussion
US is the modality of choice for imaging thyroid nodules. US findings of thyroid nodules are diagnostically important and play a major role in determining appropriate clinical management [4,6,16-19]. Although US features indicating malignant nodules are of concern to clinicians, most thyroid nodules are benign. Our objective was to determine the typical US findings of benign nodules that do not require FNA [5,6,8,16,20,21].
In daily practice, we frequently find a focal spongiform appearance within benign thyroid nodules. In previous studies, spongiform nodules were defined as having spongiform areas comprising more than half of the entire nodule volume with background isoechogenicity, or as having a spongiform area filling the entire nodule without hypervascularity [13,14]. According to these criteria, nodules in which <50% of the volume is comprised by sponge-like areas or sponge-like nodules without background isoechogenicity were considered as solid nodules. We hypothesized that the sponge-like appearance itself may be a valuable finding characterizing benign nodules and that it may be possible to differentiate benign nodules from malignant nodules according to the proportion of areas with a sponge-like appearance within the thyroid nodule.
In our study, all of the grade III sponge-like nodules (with sponge-like areas comprising >75% of the total nodule volume, n=55) were benign, while malignant sponge-like nodules usually had a relatively small area with a sponge-like appearance (grades I and II, <75% of the total nodule volume comprised by areas with a sponge-like appearance), and such nodules would have been regarded as solid according to the criteria articulated in previous reports. Our results were consistent with previous studies. Nodules with a relatively large proportion of areas with a sponge-like appearance (>75%) have high specificity and lower sensitivity for benignity (Table 2). However, no statistically significant difference was found in the rate of malignancy according to the proportion of areas with a sponge-like appearance within the nodule. Our results had insufficient statistical power (power value, 0.55), likely due to the small sample size.
Table 2.
Diagnostic accuracy for benignity according to the proportion of areas with a sponge-like appearance within a nodule
| Characteristic | Sensitivity | Specificity | Negative predictive value | Positive predictive value | Accuracy |
|---|---|---|---|---|---|
| Grade II and III (nodules with >50% sponge-like appearance) | 99/196 (50.5) | 4/5 (80) | 4/101 (3.9) | 99/100 (99) | 103/201 (51.2) |
| Grade III (nodules with >75% sponge-like appearance) | 55/196 (28) | 5/5 (100) | 5/146 (3.4) | 55/55 (100) | 60/201 (29.8) |
Values are presented as number (%).
Statistical power is the probability of correctly rejecting the null hypothesis. The size of the study population has an effect on the statistical power; as the population of the study increases, so does the power. It is difficult to obtain statistical significance in small populations, even if a significant difference does actually exist. Therefore, a large-scale study is necessary to obtain sufficient statistical power, which would require at least 95 grade III nodules and 190 nodules belonging to grades I and II (for a P-value of 0.05 and a power of 0.8).
Four of the five malignant sponge-like nodules had US features characteristic of malignancy, such as hypoechogenicity (n=4) and microcalcifications (n=3). Hypoechogenicity and microcalcifications were US features that showed a statistically significant ability to differentiate benign and malignant sponge-like nodules (Table 1). In multiple logistic regression analysis for the detection of malignant nodules, microcalcifications (P=0.011) and hypoechogenicity (P=0.012) were determined to be significant US findings (Table 3). Therefore, concomitant malignant US features, such as hypoechogenicity and microcalcifications, may be helpful in differentiating between benign and malignant nodules with a sponge-like appearance.
Table 3.
Results of multiple logistic regression analysis for the detection of malignancy
| Characteristic | β Coefficient | Odds ratio | 95% Confidence interval | P-value |
|---|---|---|---|---|
| Hypoechogenicity | 3.205 | 22.458 | 2.026-300.061 | 0.012 |
| Microcalcification | 3.112 | 24.654 | 2.038-247.420 | 0.011 |
| Less than 50% sponge-like appearance | 0.077 | 1.080 | 0.075-15.486 | 0.955 |
In the pathologic evaluation of a limited number of nodules with a sponge-like appearance, different pathologic features were found in benign and malignant nodules. The sponge-like appearance of malignant nodules involved papillary fronds within the dilated cystic spaces and edematous changes in the core of the papillary fronds. Cystic dilatation of the interpapillary space is a relatively common finding in large-sized malignancies. However, edematous changes in the core of the papillae are not common. We speculate that these pathologic findings have rarely been presented as sponge-like appearance in the US examination of malignant nodules [22].
The results of this study are subject to some limitations. First, this study was limited by its small sample size and by using data from a single institution. Second, our results were limited by the fact that most benign nodules were diagnosed on the basis of repeat FNA. Third, the pathologic evaluation of nodules with a sponge-like appearance was limited by the small number of cases. Further large-scale studies are required to confirm the pathologic features of nodules with a sponge-like appearance. Fourth, we did not evaluate Doppler images of the sponge-like nodules. Finally, since a single investigator interpreted the US findings, interobserver variability in the interpretation of the sponge-like appearance and US characteristics was not evaluated.
In conclusion, no malignancies were found in thyroid nodules in which >75% of the volume was occupied by sponge-like areas. However, our study found no statistically significant difference in the rate of benignity and malignancy of nodules depending on the proportion of the volume of a nodule occupied by areas with a sponge-like appearance, due to the overall low incidence of malignancy in a limited number of patients. Further large-scale studies are required to examine the diagnostic value of assessing the proportion of the volume of thyroid nodules occupied by areas with a sponge-like appearance.
Acknowledgments
This study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Brander A, Viikinkoski P, Nickels J, Kivisaari L. Thyroid gland: US screening in a random adult population. Radiology. 1991;181:683–687. doi: 10.1148/radiology.181.3.1947082. [DOI] [PubMed] [Google Scholar]
- 2.Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, Lee CH, Kim SY, Jeon WK, Kang JH, An SK, et al. Radiologic and pathologic findings of nonpalpable thyroid carcinomas detected by ultrasonography in a medical screening center. J Ultrasound Med. 2008;27:215–223. doi: 10.7863/jum.2008.27.2.215. [DOI] [PubMed] [Google Scholar]
- 4.Katz JF, Kane RA, Reyes J, Clarke MP, Hill TC. Thyroid nodules: sonographic-pathologic correlation. Radiology. 1984;151:741–745. doi: 10.1148/radiology.151.3.6718735. [DOI] [PubMed] [Google Scholar]
- 5.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 6.Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004;23:1455–1464. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- 7.Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US features of thyroid malignancy: pearls and pitfalls. Radiographics. 2007;27:847–860. doi: 10.1148/rg.273065038. [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Kim EK, Park SI, Kim BM, Kwak JY, Kim SJ, et al. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. Radiographics. 2008;28:1869–1886. doi: 10.1148/rg.287085033. [DOI] [PubMed] [Google Scholar]
- 9.Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003;22:1027–1031. doi: 10.7863/jum.2003.22.10.1027. [DOI] [PubMed] [Google Scholar]
- 10.Reading CC, Charboneau JW, Hay ID, Sebo TJ. Sonography of thyroid nodules: a "classic pattern" diagnostic approach. Ultrasound Q. 2005;21:157–165. doi: 10.1097/01.ruq.0000174750.27010.68. [DOI] [PubMed] [Google Scholar]
- 11.Moon WJ, Kwag HJ, Na DG. Are there any specific ultrasound findings of nodular hyperplasia ("leave me alone" lesion) to differentiate it from follicular adenoma? Acta Radiol. 2009;50:383–388. doi: 10.1080/02841850902740940. [DOI] [PubMed] [Google Scholar]
- 12.Virmani V, Hammond I. Sonographic patterns of benign thyroid nodules: verification at our institution. AJR Am J Roentgenol. 2011;196:891–895. doi: 10.2214/AJR.10.5363. [DOI] [PubMed] [Google Scholar]
- 13.Bonavita JA. Sonographic patterns of benign thyroid nodules. AJR Am J Roentgenol. 2012;198:W102–W103. doi: 10.2214/AJR.11.7737. [DOI] [PubMed] [Google Scholar]
- 14.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation: multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 15.Bonavita JA, Mayo J, Babb J, Bennett G, Oweity T, Macari M, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009;193:207–213. doi: 10.2214/AJR.08.1820. [DOI] [PubMed] [Google Scholar]
- 16.Cappelli C, Castellano M, Pirola I, Gandossi E, De Martino E, Cumetti D, et al. Thyroid nodule shape suggests malignancy. Eur J Endocrinol. 2006;155:27–31. doi: 10.1530/eje.1.02177. [DOI] [PubMed] [Google Scholar]
- 17.Yuan WH, Chiou HJ, Chou YH, Hsu HC, Tiu CM, Cheng CY, et al. Gray-scale and color Doppler ultrasonographic manifestations of papillary thyroid carcinoma: analysis of 51 cases. Clin Imaging. 2006;30:394–401. doi: 10.1016/j.clinimag.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 18.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 19.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12:1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacevic DO, Skurla MS. Sonographic diagnosis of thyroid nodules: correlation with the results of sonographically guided fine-needle aspiration biopsy. J Clin Ultrasound. 2007;35:63–67. doi: 10.1002/jcu.20287. [DOI] [PubMed] [Google Scholar]
- 21.Tae HJ, Lim DJ, Baek KH, Park WC, Lee YS, Choi JE, et al. Diagnostic value of ultrasonography to distinguish between benign and malignant lesions in the management of thyroid nodules. Thyroid. 2007;17:461–466. doi: 10.1089/thy.2006.0337. [DOI] [PubMed] [Google Scholar]
- 22.Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB., Jr Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med. 2003;22:1083–1090. doi: 10.7863/jum.2003.22.10.1083. [DOI] [PubMed] [Google Scholar]


