Abstract
Purpose:
The aim of this study was to identify the optimal ultrasound (US) parameters for gene and drug delivery.
Methods:
In order to target SkBr3, which is a breast cancer cell overexpressing the Her2 receptor, trastuzumab (Herceptin) was used. Micobubble-nanoliposome complex (MLC) was mixed with trastuzumab and stored overnight. Finally, MLC was combined with Her2Ab. A US device equipped with a 1-MHz probe was used for delivery to the cell. Several parameters, including intensity (w/cm2), time (minutes), and duty cycle (%), were varied within a range from 1 w/cm2, 1 minute, and 20% to 2 w/cm2, 2 minutes, and 60%, respectively. A confocal laser scanning microscope (CLSM) was used to confirm the delivery of MLC to the cells after US treatment.
Results:
MLC with fluorescent dyes and trastuzumab was synthesized successfully. By delivering MLC with Her2Ab to cells, the targeting effect of trastuzumab with MLC was confirmed by CLSM. The cell membranes showed green (fluorescein isothiocyanate) and red (Texas red) fluorescence but treatments with MLC without Her2Ab did not show any fluorescence. Optimal conditions for US-mediated delivery were 1 or 2 w/cm2, 2 minutes, and 60% (uptake ratio, 95.9% for 1 w/cm2 and 95.7% for 2 w/cm2) for hydrophobic materials and 2 w/cm2, 2 minutes, and 60% (uptake ratio, 95.0%) for hydrophilic materials.
Conclusion:
The greater the strength, duty cycle, and period of US application within the tested range, the more efficiently the fluorescent contents were conveyed.
Keywords: Microbubbles; Liposomes; Therapy, image-guided; Ultrasound
Introduction
For the delivery of therapeutic materials into cells, various methods have been investigated, including electroporation, hydrodynamic delivery, ballistic delivery, and microinjection [1]. Delivery by ultrasound (US) has been evaluated to be an improved option [2]. US has many advantages, such as lack of invasiveness, safety, speed, and reduced cost. In addition, delivery by US has taken center stage in a new paradigm of image-guided therapy because the therapeutic contents can be loaded in microbubbles (MB) [3]. A great deal of interest surrounds the use of US and MB for cancer therapy. In addition to high-resolution imaging, US shows powerful potential for image-guided therapy by MB-mediated delivery. The synergetic effect of US and MB used to carry US contrast agents makes sonoporation and delivery to cancer cells simple. Despite the many studies on US-mediated gene and drug delivery using MB [4], the conditions under which US-mediated delivery is most effective have not been determined [5]. Thus, the aim of this study is to optimize US treatment conditions to maximize the efficacy of delivery to cancer cells.
Materials and Methods
Preparation of MB-Nanoliposome-Her2Ab Complex (MLC-Her2Ab)
To prepare MB including green fluorescent dye (fluorescein isothiocyanate, FITC, Sigma-Aldrich, St. Louis, MO, USA) and hydrophobic gas (SF6 gas, Dong-A Industrial Gas, Seoul, Korea), DPPC (1,2-dihexadecanoyl-sn-glycero-3-phosphocholine, Avanti, Alabaster, AL, USA), DPPE (1,2-dipalmitorysn-glycero-3-phosphoethanolamine, Sigma-Aldrich), and DSPE-PEG-SPDP (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[PDP(polyethylene glycol)-2000], Avanti) were used [6]. H2O was poured on film composed of DPPC, DPPE, DSPE-PEG-SPDP, and FITC and transferred to a hermetic vial (1.5-mL vial in vial file, Clr, PTFE Lnr, Wheaton, Millville, NJ, USA). After filling the vial with SF6 gas, MB-FITC was prepared by using an amalgamator (JS 2001MX, KIMS, Incheon, Korea). Nanoliposome-Texas red was produced using red fluorescent dye (Texas red, Sigma-Aldrich), DPPC, and DPPE. Texas red in H2O was poured on the DPPC and DPPE film, and it was sonicated by a bath-type sonicator (4020P, Kodo Technical Research, Hwaseong, Korea). Unloaded Texas red was washed out by centrifugation (13,000 rpm, 5 minutes, twice). MB-FITC-nanoliposome-Texas red complex (MLC) was prepared by reacting nanoliposome-Texas red with MB-FITC for 2 hours. The unreacted components were washed out by centrifugation (13,000 rpm, 5 minutes, twice). After reaction of trastuzumab (Herceptin, Roche, Basel, Switzerland) with MLC and centrifugation (13,000 rpm, 5 minutes, twice), MB-FITC-nanoliposome-Texas red-Her2Ab complex (MLC-Her2Ab) was prepared.
Cancer Cell Culture
One of the most common breast cancer cell lines, SkBr3, was cultured using RPMI media (Life Technologies, Frederick, MD, USA), 10% fetal bovine serum (Life Technologies), and 1% penicillin-streptomycin (37°C, 5% CO2; Life Technologies). In order to treat cells with MLC-Her2Ab, the cells were seeded into a 1-well chamber slide (SPL, Pocheon, Korea).
US Conditions
After delivering MLC-Her2Ab to cells (3 hours, 37°C, 5% CO2), the MLC-Her2Ab that remained unattached to the cells was washed out by fresh media twice. For US treatment of cells, a sonoporator (Sonidel, Dublin, Ireland) equipped with a 1-MHz probe was used. According to the intensity (w/cm2), time (min), and duty cycle (%) of the US machine, the cells were divided into eight groups: group A, 1 w/cm2, 1 minute, 20%; group B, 1 w/cm2, 1 minute, 60%; group C, 1 w/cm2, 2 minutes, 20%; group D, 2 w/cm2, 1 minute, 20%; group E, 1 w/cm2, 2 minutes, 60%; group F, 2 w/cm2, 1 minute, 60%; group G, 2 w/cm2, 2 minutes, 20%; and group H, 2 w/cm2, 2 minutes, and 60%. We also evaluated a control group, a group without antibody, and a group without US. The group without anti'body was treated with MLC without Her2Ab. The group without US was treated with MLC-Her2Ab, but no US was applied.
Analysis of Targeting and Delivery Effect
To confirm the targeting effect of Her2Ab and delivery effect with various US parameters, a confocal laser scanning microscope (CLSM; Leica, Wetzlar, Germany) was used. To analyze FITC (excitation, 488 nm; emission, 500-570 nm) and Texas red (excitation, 594 nm; emission, 600-680 nm), we used 20× and 63× lenses. By using Leica Application Suite Advanced Fluorescence Lite, the software provided from the CLSM manufacturer, the fluorescence intensities inside and outside of the cell membrane were analyzed quantitatively. The experiments were performed in triplicate under equivalent conditions.
Statistical Analysis
One-way analysis of variance (ANOVA) was performed to evaluate the differences of fluorescence intensities in each group. All analyses were performed using IBM SPSS Statistics for Windows software, ver. 20 (IBM Co., Armonk, NY, USA).
Results
MLC-Her2Ab complex was successfully synthesized. Fig. 1 contains an image of a US phantom study of MLC-Her2Ab. Compared with distilled water (Fig. 1B), the US image of MLC-Her2Ab (Fig. 1A) shows very high echogenicity. Fig. 2 presents the set-up for the in vitro experiment using the US device. On a clean bench, the 1-MHz probe of the US generator was fixed by a support and clamp and placed on the cell dish containing cells. Fig. 3 shows the CLSM results from the control group, the group without Her2Ab, the group without US, and groups A-D. The control group contained only cells without any US insonication nor the addition of MLC-Her2Ab. The group without Her2Ab contained cells treated by MLC without Her2Ab. The group without US contained cells treated by MLC-Her2Ab but without US treatment. The control and group without antibody showed no signal; on the other hand, the group without US showed fluorescent signals around the cells. The results of the above three groups indicate that MLC-Her2Ab was successfully synthesized and the targeting effect of MLC-Her2Ab to the cells was strongly selective. The group without antibody treated with MLC only did not show any fluorescence intensity. On the other hand, the group without US treated with MLC-Her2Ab showed some fluorescence intensity, mainly outside of the cells, which suggests that MLC-Her2Ab attached to the outer surface of the cells. The three parameters of US treatment were intensity (range, 1 to 2 w/cm2), time (range, 1 to 2 minutes), and duty cycle (range, 20% to 60%). Group A was exposed to the lowest of each US parameter and group B was exposed to an intensified duty cycle only. Groups C and D underwent intensified time and intensity, respectively. Fig. 4 shows the CLSM results of the groups that were exposed to two or three intensified parameters at once. Fig. 5 shows the fluorescence intensity ratios. The basic US parameters were intensity, 1 w/cm2; time, 1 minute; and duty cycle, 20%. With exposure to these basic US conditions, the proportion of the fluorescence intensity inside increased from 4.7% to 9.5% with FITC and from 5.6% to 14.5% with Texas red. By changing the duty cycle from 20% to 60%, the proportion of the fluorescence intensity on the inside increased from 9.5% to 91.0% with FITC and from 14.5% to 92.5% with Texas red. By changing the treatment time from 1 to 2 minutes, the proportion of the fluorescence intensity inside the cells increased from 9.5% to 50.5% with FITC and from 14.5% to 38.5 with Texas red. When we changed the intensity from 1 to 2 w/cm2, the proportion of the fluorescence intensity inside increased from 9.5% to 86.0% with FITC and from 14.5% to 76.7% with Texas red. Among the three parameters, the parameter that was most important to optimize was the duty cycle since the fluorescence intensity ratio of group B was highest among groups A to D (Fig. 3). For delivery of FITC (i.e., the hydrophobic contents of MB), the groups with the most effective parameters were group E (1 w/cm2, 2 minutes, 60%) and group H (2 w/cm2, 2 minutes, 60%) (one-way ANOVA, P<0.001, Tukey’s honestly significant difference [HSD] was used as a post-hoc test). The fluorescence intensity ratios (inside the cells/outside the cells) were 95.9:4.1 in group E and 95.7:4.3 in group H (green, for FITC). Furthermore, for delivery of Texas red (i.e., the hydrophilic contents of the nanoliposomes), the most effective parameters were those of group H (2 w/cm2, 2 minutes, 60%) (one-way ANOVA, P<0.001, with Tukey’s HSD used as a post-hoc test). The fluorescence intensity ratio was 95.0:5.0.
Fig. 1. Phantom ultrasonogram of MB-FITC-nanoliposome-Texas red-Her2Ab complex (MLC-Her2Ab) and distilled water.
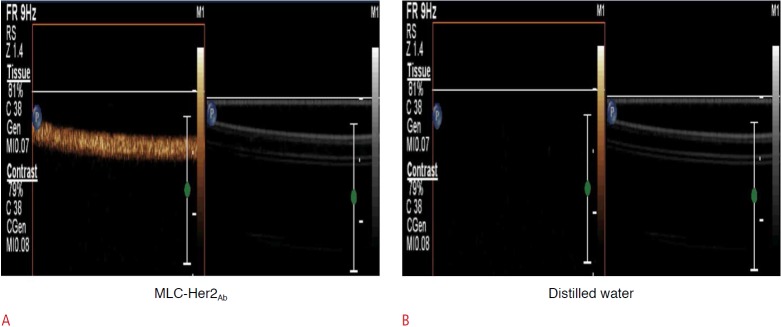
Compared with the distilled water (B), the image of MLC-Her2Ab (A) indicates high echogenicity.
Fig. 2. Set-up of ultrasound (US) experiments.

On a clean bench, the probe of the US generator was fixed by a support and clamp onto a cell culture dish.
Fig. 3. Comparison of confocal laser scanning microscope images of SkBr3 treated by MB-FITC-nanoliposome-Texas red-Her2Ab complex (MLC-Her2Ab) in groups.
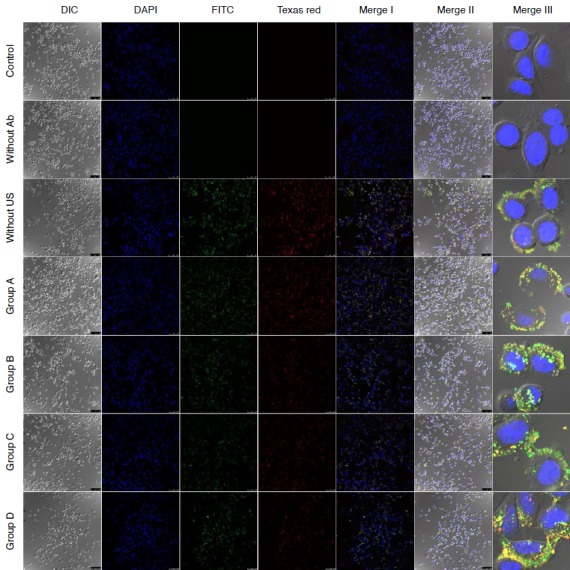
Merge I images are combined images of DAPI, FITC, and Texas red mode. Merge II images are combined images of differential interference contrast (DIC) and Merge I mode. Merge III images are magnified images of Merge II. The group without Her2Ab shows no fluorescence intensity. This is similar to the control group. The group without ultrasound (US) shows some fluorescence intensity, mainly in the region outside of SkBr3. Groups A-D showed some fluorescence intensity both outside and inside SkBr3 in a ratio depending on the US conditions of intensity, time, and duty cycle: group A, 1 w/cm2, 1 minute, 20% duty cycle; group B, 1 w/cm2, 1 minute, 60% duty cycle; group C, 1 w/cm2, 2 minutes, 20% duty cycle; group D, 2 w/cm2, 1 minutes, 20% duty cycle (DIC, DAPI, FITC, Texas red, Merge I, and Merge II, ×20; Merge III, ×63).
Fig. 4. Comparison of confocal laser scanning microscope images of SkBr3 treated by MB-FITC-nanoliposome-Texas red-Her2Ab complex (MLC-Her2Ab) in groups.
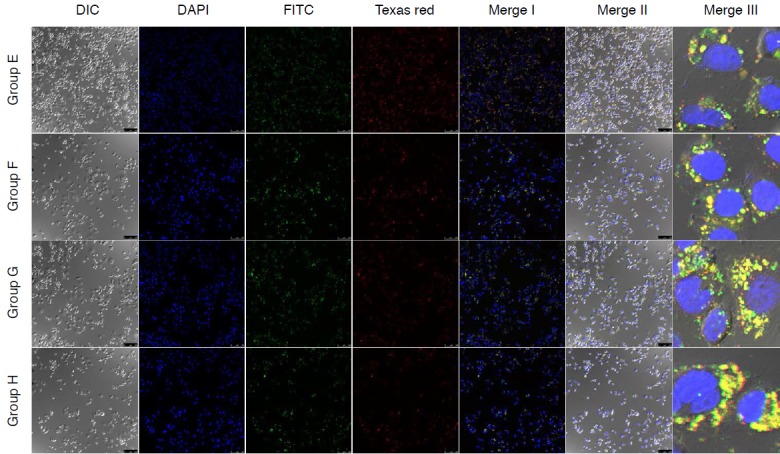
Groups E-H show some varying fluorescence intensity outside and inside SkBr3 depending on the ultrasound intensity, time, and duty cycle parameters: group E, 1 w/cm2, 2 minute, 60% duty cycle; group F, 2 w/cm2, 1 minute, 60% duty cycle; group G, 2 w/cm2, 2 minutes, 20% duty cycle; group H, 2 w/cm2, 2 minutes, 60% duty cycle. DIC, differential interference contrast (DIC, DAPI, FITC, Texas red, Merge I, and Merge II, ×20; Merge III, ×63).
Fig. 5. Graph showing the fluorescence intensity (%) of confocal laser scanning microscope results by ultrasound (US) parameters.
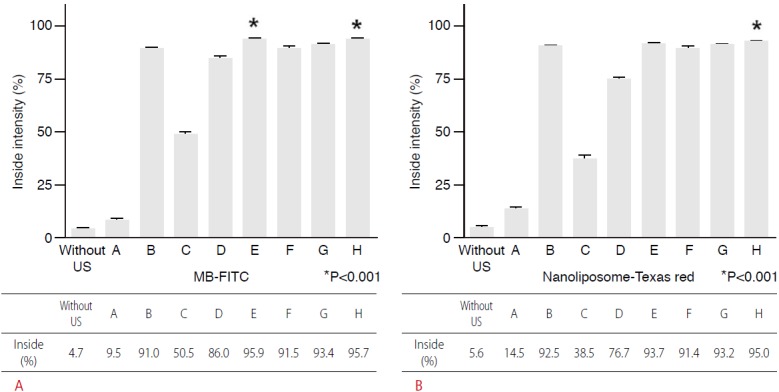
From group A to D, the most influential parameter of US is duty cycle (group B), the second parameter is intensity (group D), and the next parameter is time (group C). A. For MB-FITC, the groups with the most parameters are group E (1 w/cm2, 2 minutes, 60%) and group H (2 w/cm2, 2 minutes, 60%) (asterisk indicates the most effective conditions, one-way ANOVA, P<0.001). B. In addition, for nanoliposome-Texas red, the most effective conditions were found in group H (2 w/cm2, 2 minutes, 60%) (asterisk indicates the most effective conditions, one-way ANOVA, P<0.001).
Discussion
MB, which are used as a contrast agent for US imaging in clinical diagnostics, comprise a gas-filled core and a shell of lipids or proteins. They range in size from 1 to 100 μm. Their gas component can generate echogenicity triggered by the US device because of the tendency of the layer to absorb or reflect US waves and nonlinear oscillation. The optimization of a variety of US conditions has a significant impact on the utilization of therapeutic US in many clinical settings [7]. Researchers have reported that sonoporation, the formation of temporary pores in the cell membrane, enhanced endocytosis. Qin et al. [8] reported that sonoporation achieved disruption of the plasma membrane for delivery of fluorescent dye in HeLa cancer cells. Delalande et al. [9] and Fan et al. [10] noted that the use of US and MB for sonoporation is being considered an emerging method for achieving localized drug and gene delivery. Owing to the variety of US conditions used and corresponding MB behavior, optimization of US conditions is essential [11-13]. When MB are transiently exposed to the proper US intensity, time, and duty cycle, the MB contract or collapse, simplifying delivery. Fan et al. [14] reported US parameters in experiments including acoustic pressures of 0.06-0.6 MPa, pulse repetition frequency of 10 Hz-1 KHz, and duty cycles of 0.016%-20%. The total duration of US application was 1 second. Comparison of the delivery outcomes indicated that high acoustic pressure was the most powerful parameter for intracellular delivery. For Yan et al. [15], the parameters were times from 5 seconds to 60 seconds and acoustic pressures from 0.35 to 1.0 MPa. The duty cycle of US application was 50%. The above results are meaningful, but they provide no precise protocol for the most effective delivery in vitro. In this study, we focused on setting US conditions for the most efficient delivery to cells. We used a US generator, a sonoporator (SP100, Sonidel), equipped with a 1-MHz probe. The radius of the probe (bottom) was 75 mm. This US generator had variable controls for time, intensity, and duty cycle. A duty cycle is defined as the percentage of one period in which a signal is active. For example, if an electrical device runs for 50 out of 100 seconds, its duty cycle is 50%. For our study, we synthesized MLC including FITC in MB and Texas red in nanoliposome and binding Her2Ab. Because FITC is hydrophobic, it should be contained in MB, whereas Texas red is hydrophilic, so it should be contained in nanoliposomes. As can be seen in Fig. 1A, our MLC-Her2Ab showed high echogenicity, showing the possibilities of US as a contrast agent, and furthermore, as a vehicle for gene and drug delivery. As shown in Fig. 3, we verified the high targeting effect of MLC-Her2Ab to cells. When cells were treated with MLC without Her2Ab, no fluorescence appeared (“without antibody group” of Fig. 3). In contrast, when cells were treated with MLC-Her2Ab, intense green (FITC) and red (Texas red) fluorescence appeared around the cell membrane (“without US group” of Fig. 3). This shows that MB-mediated delivery can be more effective with an active targeting strategy. Tanizaki et al. [16] and Weigelt et al. [17] reported on the targeting effect of Her2Ab to SkBr3. Depending on various US conditions, we confirmed the delivery effect to the inside of the cell membrane after US. The US parameters were intensity (w/cm2), time (seconds), and duty cycle (%). We found that the most powerful US parameter was duty cycle, followed by intensity, and then time (Fig. 5). A synergetic effect among parameters also existed. Group E (1 w/cm2, 2 minutes, 60% duty cycle) (Fig. 4) showed the most powerful effect of FITC delivery to the cells. FITC in MB can substitute for many other hydrophobic therapeutic materials. To deliver hydrophobic therapeutic materials, for example, paclitaxel for therapy, the conditions of 1 w/cm2, 2 minutes, 60% duty cycle were found to be most suitable in our experiments. Group H (2 w/cm2, 2 minutes, 60% duty cycle) (Fig. 4) showed the most powerful effect for FITC and Texas red co-delivery to cells. Texas red, which was carried by nanoliposomes, can be used as a substitute for many hydrophilic therapeutic materials such as doxorubicin or genetic therapeutic materials. To deliver hydrophobic and hydrophilic therapeutic materials for therapy, the parameters of 2 w/cm2, 2 minutes, and 60% duty cycle were the best among those we tested.
This study has several limitations. First, our experiments were limited to in vitro studies. Therefore, future experiments should investigate optimal parameters for in vivo conditions. Second, our parameters depended on the US machine, so we had only a few options for settings; for more exact information regarding optimal conditions, we need to measure the acoustic pressure at each setting.
In spite of these limitations, we confirmed that MB-FITC-nanoliposome-Texas red-Her2Ab complex (MLC-Her2Ab) was successfully synthesized and, in an in vitro study, an active targeting method, such as attaching Her2Ab, was useful in MB-mediated delivery. Results with varying US parameters in vitro verified that the duty cycle played the most powerful role in delivery into cancer cells in our experiments. Our results should provide a basis for establishing reference parameters for performing gene and drug delivery studies using MB and US under in vitro conditions. Future improvements in US techniques combined with new developments of contrast agents containing therapeutic materials such as drugs and genes will make US a more powerful therapy modality in addition to its role as an imaging modality.
Acknowledgments
This work was funded by the National Research Foundation of Korea (the Basic Science Research Program 2010-0009271).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Wells DJ. Gene therapy progress and prospects: electroporation and other physical methods. Gene Ther. 2004;11:1363–1369. doi: 10.1038/sj.gt.3302337. [DOI] [PubMed] [Google Scholar]
- 2.Newman CM, Lawrie A, Brisken AF, Cumberland DC. Ultrasound gene therapy: on the road from concept to reality. Echocardiography. 2001;18:339–347. doi: 10.1046/j.1540-8175.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 3.Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ. 2001;322:1222–1225. doi: 10.1136/bmj.322.7296.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrie A, Brisken AF, Francis SE, Tayler DI, Chamberlain J, Crossman DC, et al. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999;99:2617–2620. doi: 10.1161/01.cir.99.20.2617. [DOI] [PubMed] [Google Scholar]
- 5.Miller DL, Quddus J. Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast-agent gas bodies. Ultrasound Med Biol. 2000;26:661–667. doi: 10.1016/s0301-5629(99)00170-2. [DOI] [PubMed] [Google Scholar]
- 6.Yoon YI, Kwon YS, Cho HS, Heo SH, Park KS, Park SG, et al. Ultrasound-mediated gene and drug delivery using a microbubble-liposome particle system. Theranostics. 2014;4:1133–1144. doi: 10.7150/thno.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JB, Wansaicheong G, Merton DA, Forsberg F, Goldberg BB. Contrast-enhanced ultrasound imaging: state of the art. J Med Ultrasound. 2005;13:109–126. [Google Scholar]
- 8.Qin P, Xu L, Hu Y, Zhong W, Cai P, Du L, et al. Sonoporation-induced depolarization of plasma membrane potential: analysis of heterogeneous impact. Ultrasound Med Biol. 2014;40:979–989. doi: 10.1016/j.ultrasmedbio.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Delalande A, Kotopoulis S, Postema M, Midoux P, Pichon C. Sonoporation: mechanistic insights and ongoing challenges for gene transfer. Gene. 2013;525:191–199. doi: 10.1016/j.gene.2013.03.095. [DOI] [PubMed] [Google Scholar]
- 10.Fan Z, Kumon RE, Park J, Deng CX. Intracellular delivery and calcium transients generated in sonoporation facilitated by microbubbles. J Control Release. 2010;142:31–39. doi: 10.1016/j.jconrel.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hensel K, Siepmann M, Haendschke K, Emmelmann S, Daigeler A, Hauser J, et al. Monitoring of insonicated microbubble behavior and their effect on sonoporation supported chemotherapy of fibrosarcoma cells. IFMBE Proc. 2009;22:1422–1425. [Google Scholar]
- 12.Lentacker I, De Cock I, Deckers R, De Smedt SC, Moonen CT. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv Drug Deliv Rev. 2014;72:49–64. doi: 10.1016/j.addr.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Lin CR, Chen KH, Yang CH, Cheng JT, Sheen-Chen SM, Wu CH, et al. Sonoporation-mediated gene transfer into adult rat dorsal root ganglion cells. J Biomed Sci. 2010;17:44. doi: 10.1186/1423-0127-17-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Z, Chen D, Deng CX. Characterization of the dynamic activities of a population of microbubbles driven by pulsed ultrasound exposure in sonoporation. Ultrasound Med Biol. 2014;40:1260–1272. doi: 10.1016/j.ultrasmedbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan F, Li L, Deng Z, Jin Q, Chen J, Yang W, et al. Paclitaxel-liposome-microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J Control Release. 2013;166:246–255. doi: 10.1016/j.jconrel.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Tanizaki J, Okamoto I, Takezawa K, Tsukioka S, Uchida J, Kiniwa M, et al. Synergistic antitumor effect of S-1 and HER2-targeting agents in gastric cancer with HER2 amplification. Mol Cancer Ther. 2010;9:1198–1207. doi: 10.1158/1535-7163.MCT-10-0045. [DOI] [PubMed] [Google Scholar]
- 17.Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


