Abstract
Objectives. To estimate the consultation incidence of OA using population-based health care data in England and compare OA incidence figures with those derived in other countries.
Methods. A population-based health care database (Consultations in Primary Care Archive) in England was used to derive the consultation incidence of OA (overall and by joint site) using the maximum available run-in period method. These estimates, and their distribution by age and sex, were compared with those published from population-based health care databases in Canada, the Netherlands and Spain. A novel age-stratified run-in period method was then used to investigate whether the consultation incidence has been increasing over time in younger adults.
Results. The annual consultation incidence of OA (any joint) was 8.6/1000 persons ≥15 years of age (95% CI 7.9, 9.3) [6.3 (95% CI 5.5, 7.1) in men and 10.8 (95% CI 9.8, 12.0) in women]. Incidence increased sharply between 45 and 64 years of age, peaking at 75–84 years. The joint-specific incidence was 1.4 (95% CI 1.1, 1.7), 3.5 (95% CI 3.1, 3.9) and 1.3 (95% CI 1.1, 1.6) for hip OA, knee OA and hand OA, respectively. The estimates and their distribution by age and sex were broadly consistent with international estimates. Between 2003 and 2010, incidence in those aged 35–44 years increased from 0.3 to 2.0/1000 persons.
Conclusion. Newly diagnosed cases of OA in England occur in 9 in 1000 at-risk adults each year, similar to other international estimates. Although lower, the consultation incidence proportion in younger adults appears to have increased in the past decade.
Keywords: osteoarthritis, incidence, population-based health care data, consultation
Rheumatology key messages
Approximately 9 in 1000 persons aged ≥15 years is newly diagnosed with OA each year in England.
An increase in England in newly diagnosed OA observed between 2003 and 2010 in 35- to 44-year-olds warrants further investigation.
Introduction
OA accounts for 3% of all years lived with disability in the high-income countries [1]. An ageing population, increasing prevalence of risk factors such as obesity and increasing strain on health budgets, including costs of increasing numbers of patients undergoing joint arthroplasty [2, 3], suggest a growing challenge for population health, the National Health Service and the UK economy [4].
A key recommendation of the UK Chief Medical Officer is the need for better information in musculoskeletal disorders [5]. Alongside the National Joint Registry (http://www.njrcentre.org.uk/) and bespoke surveys and cohorts, population-based health care databases based on anonymized, routinely collected clinical information recorded in the electronic health record are important sources of such information, whose advantages in terms of representativeness, cost, size and continuity over time are well recognized.
Data from general practice—the setting where most cases of OA are assessed and managed—has been used to estimate the consultation prevalence of OA (the proportion of the population with a diagnosed consultation over a defined period of time) in several countries. Fewer studies [6–10], and indeed none from the UK, have estimated consultation incidence (the rate of new cases presenting to general practice). Yet incidence rates are an important measure of occurrence in the population, responding more quickly to changes in risk factors and being less influenced by disease duration [11]. Previous estimates from Canadian provincial health care databases would suggest an annual consultation incidence for OA in men and women of 9.3–12.2/1000 and 11.0–17.4/1000, respectively [7, 9, 10]. Recent estimates from a large regional primary care database in Spain found a 3-fold higher incidence of knee OA than hip or hand OA (6.5 vs 2.4 and 2.1/1000 persons >45 years of age) [8]. However, direct international comparisons are limited by different OA case definitions and population strata. In addition, these previous estimates cannot be assumed to be representative of current rates in the UK since consultation incidence proportions for OA may be influenced by cohort and period effects, some of which may be particular to location (e.g. the effect of the UK Quality and Outcomes Framework on coding of OA since 2004).
One of the methodological challenges in estimating incidence is how to ensure prevalent cases (those with previous OA consultations) are excluded from the calculation. This is achieved in many databases by using a run-in period (disease-free observation period [12], look-back period [13] or clearance period [14]), which for OA can be at least 9 years [15] (e.g. one needs to look back to 2001 to be confident that a patient consulting with OA in 2010 is receiving that diagnosis for the first time). One disadvantage of this is that databases must be established with satisfactory data quality for many years before trends in incidence can begin to be described [16]. However, we propose that prevalent cases can be effectively excluded using shorter run-in periods in younger age groups since they are less likely to have a long prior history of OA consultations. Therefore our study had two main objectives: first, to estimate the consultation incidence of OA (overall and separately for hip, knee and hand) by applying a maximum available (10 year) run-in period to data from a local primary care database in North Staffordshire, England and compare OA incidence figures with those derived in other countries; second, in the same database, to develop a novel age-stratified run-in period method and demonstrate its application by testing the hypothesis that there has been an increase in the consultation incidence proportion of diagnosed OA among younger adults (ages 35–44 years; i.e. the 10 year age band below the typical age stated in guidelines for clinical diagnosis of OA [17]). Although the incidence of OA in this age group is lower than in older age groups, increasing incidence of OA over time in this age group could be of particular concern in the context of increasing childhood obesity.
Methods
Data source
The Consultations in Primary Care Archive (CiPCA), which contains all recorded consultation data by general practitioners (GPs) and practice nurses from 11 general practices in North Staffordshire, England between 2000 and 2010 (total practice population consisted of 94 955 people in 2010) [16]. North Staffordshire is more deprived than England as a whole, although the CiPCA practices cover both deprived and less deprived areas. In England, general practice provides the first point of access to the National Health Service for most non-emergency care and also provides continuing care for many chronic diseases. The vast majority of the population are registered at a general practice [18, 19]. Within CiPCA practices, 97% of contacts with a GP are assigned a morbidity code and practices undergo an annual cycle of assessment, feedback and training in morbidity coding [20]. A similar annual primary care consultation prevalence figure for OA from CiPCA practices has been shown compared with those derived from national UK [21] and international (Swedish [16]) databases. Moreover, in CiPCA, secondary care information (e.g. hospital letters) is obtained and coded at the discretion of the practices, thus completeness varies by practice.
Ethical approval for the CiPCA database was given by the North Staffordshire Research Ethics Committee to download, store and analyse anonymized medical records information for research use from participating general practices in CiPCA (reference 03/04). Patients are informed by a poster at their GP’s practice and by leaflet that the practice is a Keele research practice and that their anonymized records (with identifiable information removed) may be used for research, and that they can opt out if they wish by informing the practice staff. Therefore no separate ethical approval was required for our study.
Case definition
An OA consultation was defined as a Read code starting with N05 (OA and allied disorders), equivalent to ICD9 codes beginning with 715. Knee, hip and hand OA were each defined by Read code lists drawn up through a consensus process involving local GPs (code lists are available upon request from the authors [22]). A single medical contact with an OA diagnosis is a standard definition used in most previous studies [6, 23] and is preferred in previous validation studies to case definitions based on multiple contacts, referrals and prescription records [24, 25]. Prieto-Alhambra et al. [8] report that only 1.3% of cases defined in this way are subsequently given an alternative diagnosis.
Estimating consultation incidence using maximum available run-in period
The overall annual consultation incidence of OA and incidence rates by age (defined as age on 31 December 2010) and sex were estimated for the calendar year 1 January 2010 to 31 December 2010. The numerator for the incidence calculation was defined as those with a recorded OA code in 2010 with no prior OA code and complete registration in the previous 10 years. Hence prevalent cases who were diagnosed with OA during the 10 year run-in period from 1 January 2000–31 December 2009 were not eligible to be included in the numerator. Each patient continuously registered in 2010 and for the run-in period (1 January 2000–31 December 2009) was included in the denominator, with exclusion of those who were prevalent cases in the run-in period. The incidence proportions were then corrected for the loss of patients with <10 years registration (supplementary Tables S1–S24, available at Rheumatology Online). A previous study of consultation incidence of OA using health administrative data in British Columbia, Canada concluded that a run-in period of 9 years [10] was generally sufficient to remove prevalent cases. We verified that the 10 year run-in period (the maximum available within CiPCA) was sufficient to observe a stable estimate of consultation incidence in CiPCA by rerunning the analyses varying the run-in period from 0 to 9 years (see supplementary Fig. S1, available at Rheumatology Online).
Using the maximum run-in period method, the consultation incidence of OA was estimated for the total population, among people ≥15 years of age and among people ≥45 years of age, as was consultation incidence of OA by gender and specific age. All analyses were repeated for OA in three selected body regions. We then compared the consultation incidence of OA in women with that in men using overall (age-adjusted) and age-specific female:male incidence rate ratios (IRRs) with 95% CIs using Poisson regression.
Comparison with international estimates
An earlier rapid literature review by us identified six original English-language articles that reported potentially comparable OA incidence estimates from 10 databases in Canada, the Netherlands and Spain [6, 8–10, 26] (supplementary Table S25, available at Rheumatology Online). Using tables and plots we compared these international estimates with those obtained in the present study using the maximum available run-in period method in CiPCA. To facilitate the comparisons, direct standardization was used, in which the published age-stratified incidence rates from other international studies were applied to the age–sex distribution of the CiPCA population.
Trend in consultation incidence of OA in younger adults
A novel age-stratified run-in period method (supplementary data, the time series regression model for deriving age-stratified run-in periods section, and supplementary Fig. S2, available at Rheumatology Online) was used to investigate the recent trend in annual consultation incidence of OA among adults aged 35–44 years. Since this age group had the lowest minimum run-in period of all age groups, more data points were available to evaluate trends. The annual incidences in 2003–10 were estimated and the trend was observed by fitting a log-linear model with Wald test to test for linear trend. To increase the stability of the rates with a minimum loss of information, 3 year moving average incidence proportions for 2005–10 were estimated by using the mean annual number of incident cases and mean denominator population over the 3 years. Statistical analyses were carried out using Stata 12.0 (StataCorp, College Station, TX, USA).
Results
On 1 January 2010, 94 955 people (48 237 women, 46 718 men) including 1953 people with a recorded OA diagnosis (1251 women, 702 men) were registered in CiPCA.
Annual consultation incidence of OA
Based on the maximum available run-in period method, the annual consultation incidence of OA at any joint for persons aged ≥15 years was 8.6/1000 persons (95% CI 7.9, 9.3) [6.3 (95% CI 5.5, 7.1) for men and 10.8 (95% CI 9.8, 12.0) for women]. In those aged ≥45 years, the corresponding estimates were 16.1 (95% CI 14.8, 17.5), 12.0 (95% CI 10.5, 13.7) and 20.1 (95% CI 18.1, 22.2), respectively (Table 1). The consultation incidence proportions, expressed per 1000 persons aged ≥15 years, were 3.5 (95% CI 3.1, 3.9) for knee OA, 1.4 (95% CI 1.1, 1.7) for hip OA and 1.3 (95% CI 1.1, 1.6) for hand OA.
Table 1.
Annual consultation incidence (per 1000 persons) of OA (any joint and by selected body region): CiPCA 2010a
| Total population |
Population ≥15 years of age |
Population ≥45 years of age |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Numerator | Denominator | IR (95% CI) | Numerator | Denominator | IR (95% CI) | Numerator | Denominator | IR (95% CI) | ||
| OA | Total | 627 | 87 458 | 7.2 (6.6, 7.8) | 627 | 73 207 | 8.6 (7.9, 9.3) | 597 | 37 067 | 16.1 (14.8, 17.5) |
| Men | 230 | 43 907 | 5.2 (4.6, 6.0) | 230 | 36 637 | 6.3 (5.5, 7.1) | 221 | 18 358 | 12.0 (10.5, 13.7) | |
| Women | 396 | 43 548 | 9.1 (8.2, 10.0) | 396 | 36 567 | 10.8 (9.8, 12.0) | 376 | 18 707 | 20.1 (18.1, 22.2) | |
| Hip OA | Total | 109 | 92 587 | 1.2 (1.0, 1.4) | 109 | 78 336 | 1.4 (1.1, 1.7) | 102 | 42 099 | 2.4 (2.0, 2.9) |
| Men | 40 | 45 916 | 0.9 (0.6, 1.2) | 40 | 38 646 | 1.0 (0.7, 1.4) | 40 | 20 306 | 2.0 (1.4, 2.7) | |
| Women | 69 | 46 671 | 1.5 (1.2, 1.9) | 69 | 39 690 | 1.7 (1.4, 2.2) | 62 | 21 793 | 2.8 (2.2, 3.6) | |
| Knee OA | Total | 267 | 90 695 | 2.9 (2.6, 3.3) | 267 | 76 444 | 3.5 (3.1, 3.9) | 260 | 40 229 | 6.5 (5.7, 7.3) |
| Men | 149 | 45 138 | 3.3 (2.8, 3.9) | 149 | 37 868 | 3.9 (3.3, 4.6) | 145 | 19 543 | 7.4 (6.3, 8.7) | |
| Women | 156 | 45 557 | 3.4 (2.9, 4.0) | 156 | 38 576 | 4.0 (3.4, 4.7) | 153 | 20 684 | 7.4 (6.3, 8.7) | |
| Hand OA | Total | 103 | 92 712 | 1.1 (0.9, 1.3) | 103 | 78 461 | 1.3 (1.1, 1.6) | 100 | 42 219 | 2.4 (1.9, 2.9) |
| Men | 24 | 46 125 | 0.5 (0.3, 0.8) | 24 | 38 855 | 0.6 (0.4, 0.9) | 22 | 20 510 | 1.1 (0.7, 1.6) | |
| Women | 80 | 46 588 | 1.7 (1.4, 2.1) | 80 | 39 607 | 2.0 (1.6, 2.5) | 79 | 21 710 | 3.6 (2.9, 4.5) | |
aCalculated using the maximum available (10 year) run-in period method. CiPCA: Consultations in Primary Care Archive; IR: incidence rate (annual consultation).
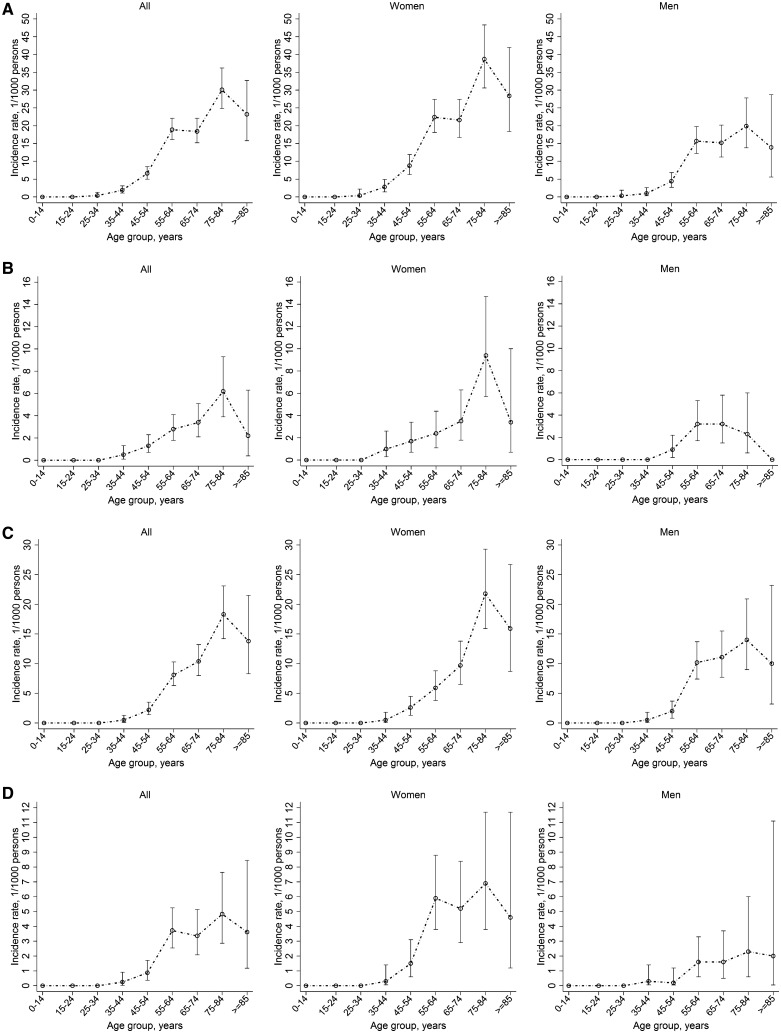
Age- and gender-specific consultation incidence estimates are shown in Fig. 1 (see also supplementary Table S26, available at Rheumatology Online). Similar patterns of age-specific consultation incidence were found for OA at any joint, hip, knee and hand OA: a progressive increase from age 25 to 34 years, with the steepest change in slope for age group 55–64 years, peaking at 75–84 years, with a slight decrease in the age group ≥85 years. The pattern of age-specific consultation incidence was similar between men and women at any joint, knee and hand OA except hip OA, which showed an earlier peak in men than in women. The consultation incidence of OA was significantly higher in women, with the exception of knee OA: age-adjusted IRR 1.6 (95% CI 1.3, 1.9) for OA at any joint, 1.6 (95% CI 1.0, 2.5) for hip OA, 1.0 (95% CI 0.7, 1.2) for knee OA and 3.3 (95% CI 2.0, 5.4) for hand OA. There was no strong evidence that the female:male IRRs differed across age strata. At age 55–64 years, a higher consultation incidence of knee OA was noted among men compared with women (10.2/1000 persons vs 5.9/1000 persons), although all such age- and sex-stratified joint-specific estimates were based on small numbers (see supplementary Table S27, available at Rheumatology Online).
Fig. 1.
Annual age-specific consultation incidence (per 1000 persons) of OA (any joint and by selected body region), overall and by gender: CiPCA 2010 [calculated using the maximum available (10 year) run-in period method]
(A) OA (any joint); (B) hip OA; (C) knee OA; (D) hand OA. CiPCA: Consultations in Primary Care Archive.
Comparison with international estimates
The estimates from CiPCA were broadly comparable to previously published international studies (Table 2), but with some exceptions. While the overall estimate for the total population fell within the range reported recently for Dutch general practice research networks [26], in the population ≥45 years of age the estimates from CiPCA were substantially lower than those reported from the Medical Service Plan health administrative database in British Columbia [10]. Incidence continued to rise sharply with age >50 years in the British Columbia study, in contrast to the slowing or levelling off seen in CiPCA and another provincial health administrative database from Alberta, Canada (supplementary Fig. S3A, available at Rheumatology Online) [9]. Estimates for hip and knee OA were noticeably higher in CiPCA than from the second Dutch National Survey of General Practice in 2001. In contrast, there was generally fairly close agreement between CiPCA and a large Catalan general practice database [8] for joint-specific consultation incidence estimates for the population aged ≥40–45 years as well as similar age and gender patterns (supplementary Fig. 3B, available at Rheumatology Online). An exception was the relatively high consultation incidence in CiPCA for knee OA among men.
Table 2.
Comparison of crude consultation incidence estimates (per 1000 persons) with previously published international estimates
| Current studya | Prieto-Alhambra et al. [8] | van den Dungen et al. [23] | Kopec et al. [7] | Sun et al. [9] | Van der Waal et al. [6] | Kopec et al. [10] | |
|---|---|---|---|---|---|---|---|
| Database | CiPCA | SIDIAP | Various GPRN | MSP | AHCIP | DNSGP-2 | MSP |
| Location | North Staffordshire | Catalonia | The Netherlands | British Columbia | Alberta | The Netherlands | British Columbia |
| Year | 2010 | 2006–10 | 2007 | 2003–04 | 2002 | 2001 | 2000–01 |
| Consultation incidence estimate | |||||||
| OA, total population | 7.2 | 5.4–9.7 | — | — | 11.7 | ||
| OA, total population, men | 5.2 | 12.2 | 9.3 (11.14) | 10.0 (13.93) | |||
| OA, total population, women | 9.1 | 17.4 | 11.0 (12.70) | 13.4 (19.31) | |||
| OA, population aged ≥15 years | 8.6 | 14.7 | |||||
| OA, population aged ≥45 years | 16.1 | 29.7 | |||||
| Hip OA, total population | 1.2 | 0.9 | |||||
| Hip OA, total population, men | 0.9 | 0.6 | |||||
| Hip OA, total population, women | 1.5 | 1.2 | |||||
| Hip OA, population aged ≥45 years | 2.4 | 2.1b (2.22) | |||||
| Hip OA, population aged ≥45 years, men | 2.0 | 1.7b (1.84) | |||||
| Hip OA, population aged ≥45 years, women | 2.8 | 2.4b (2.56) | |||||
| Knee OA, total population | 2.9 | 1.5 | |||||
| Knee OA, total population, men | 3.3 | 0.9 | |||||
| Knee OA, total population, women | 3.4 | 2.0 | |||||
| Knee OA, population aged ≥45 years | 6.5 | 6.5b (6.92) | |||||
| Knee OA, population aged ≥45 years, men | 7.4 | 4.6b (4.83) | |||||
| Knee OA, population aged ≥45 years, women | 7.4 | 8.4b (8.89) | |||||
| Hand OA, population aged ≥45 years | 1.1 | 2.4b (2.62) | |||||
| Hand OA, population aged ≥45 years, men | 0.5 | 1.3b (1.36) | |||||
| Hand OA, population aged ≥45 years, women | 1.7 | 3.5b (3.77) |
aCiPCA estimates are those using the maximal available run-in period method. bEstimates for population aged ≥40 years. In SIDIAP [8], AHCIP [9] and MSP [10], age-stratified incidence was available, allowing indirect standardization for comparison with CiPCA by using the age-stratified population size in CiPCA. The standardized overall estimates are presented in the parentheses. AHCIP: Alberta Health Care Insurance Plan; CIPCA: Consultations in Primary Care Archive; DNSGP-2: Second Dutch National Survey of General Practice; GPRN: General Practice Research Networks; MSP: Medical Service Plan; SIDIAP: Sistema d‘Informació per al Desenvolupament de l‘Investigació en Atenció Primària;
Age-stratified run-in period method
The age-stratified run-in period method resulted in estimates very similar to those obtained from the maximum run-in period method when restricted to the population ≥15 years or ≥45 years (supplementary Table S28, available at Rheumatology Online). For example, the consultation incidence of OA estimated by the age-stratified run-in period method was 9.2 (95% CI 8.5, 9.9) for OA at any joint, 1.2 (95% CI 1.0, 1.4) for hip OA, 3.6 (95% CI 3.2, 4.0) for knee OA and 1.1 (95% CI 0.9, 1.4) for hand OA among those ≥15 years of age.
Trend in consultation incidence of OA in younger adults
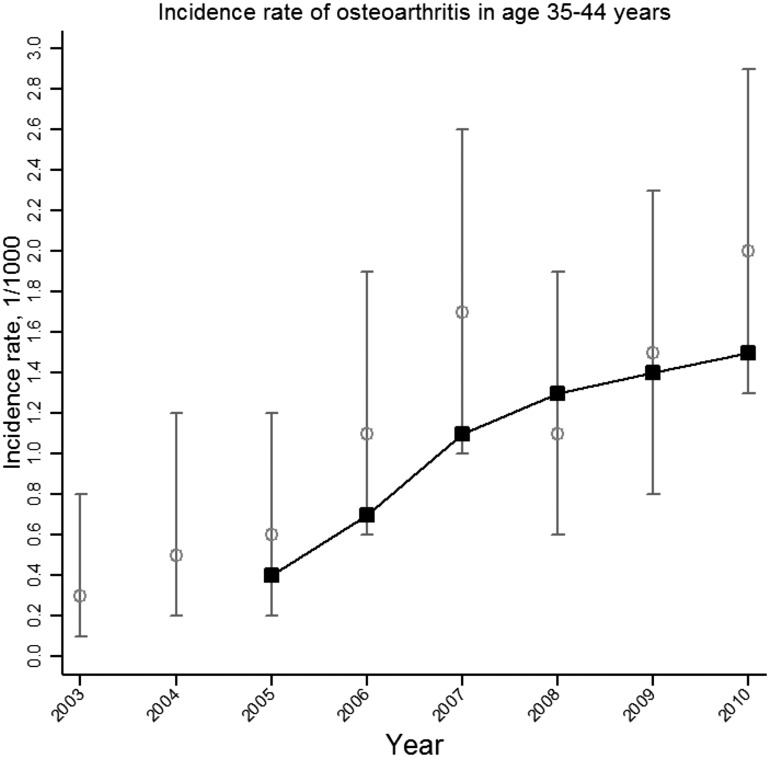
The annual consultation incidence proportions of OA in those 35–44 years of age from 2003 to 2010 are presented graphically in Fig. 2, along with the 3 year moving average. The trend analysis revealed a linear increasing trend (P = 0.0173) from an estimated annual consultation incidence of 0.3/1000 (95% CI 0.1, 0.8) in 2003 to 2.0/1000 (95% CI 1.3, 2.9) in 2010.
Fig. 2.
Annual consultation incidence (per 1000 persons) of OA (any joint) among people aged 35–44 years between 2003 and 2010: CiPCA 2010 (calculated using the age-stratified run-in period method)
CiPCA: Consultations in Primary Care Archive. Grey bars represent annual consultation incidence; black line represents 3-year moving average.
Discussion
To our knowledge, this is the first study to estimate the consultation incidence for OA using UK population-based health care data. We found that 1 in 100 adults is newly diagnosed with OA during the course of a year, rising to 3% of adults aged 75–84 years. Our novel age-stratified method for determining incidence revealed a trend of increasing incidence of recorded cases of OA among adults aged 35–44 years.
Our estimates were generally within the range reported by previous international studies, although an area of disagreement concerns the relationship between OA consultation incidence and age. Previous studies have reported conflicting findings, showing either a steep, continuous increase in incidence with age [10] or a marked decline from the age of 60–79 years [9]. Our study suggested a plateau in incidence rates for men at age 65–74 years and an increase in women up to age 75–84 years. The exact reason for the differences in findings between studies is unclear and further studies in other databases using comparable methods are required. Differences in coding behaviour between UK primary care and Canadian administrative data, differences in the size of the denominator population for the very old age groups, the inclusion of comprehensive linked hospital data [10] and the use of a power function to model very long run-in periods [9] in the Canadian datasets may each contribute. In the UK, health conditions that are incentivized within the General Medical Services contract are likely to be more frequently diagnosed and recorded when present than non-incentivized conditions [27]. A consequence of this could be underrecording of OA and an apparent reduction in the consultation incidence proportion of OA, particularly in older adults with multimorbidity [28]. However, a levelling off or decline in incidence rates for hand, knee and hip OA after the age of 80 years has been reported in US data using case definitions based on symptoms and radiographs [29]. Nevertheless, as has been argued before [15, 30], this pattern of declining incidence in later life must be cautiously interpreted since it may reflect biases due to competing risks, that is, individuals who would have been at high risk of incident OA in later life [30] may also have a higher mortality rate and thus may be lost to follow-up.
The observed trend in incidence among those 35–44 years old is potentially significant but must be interpreted with caution. An OA diagnosis at this age is both uncommon and potentially significant, with the age threshold conventionally used for a working diagnosis of OA being 45 years [31], implying that this younger age group with early onset has the prospect of several decades of living with the condition. However, the consultation incidence of diagnosed OA plainly does not equate to the incidence of pathology or even of symptoms [32, 33]. Instead, consultation incidence represents an event in the course of a patient’s experience of associated symptoms and is determined by their decision to consult and the GP’s decision to make and record the diagnosis of OA. Observed trends may be due to changes in the thresholds for these decisions and other aspects of data quality. Although there is evidence from other primary care databases in the UK that the number of recorded general practice consultations per person-year has been increasing [34, 35], this has been less marked in younger age groups and the magnitude of this appears unlikely to explain the trend we observed. More diligent recording of secondary diagnoses and a lowering of the threshold among practitioners for making the diagnosis of OA (as opposed to using non-specific codes such as knee pain) may contribute to this trend. However, we observed the same trend of increasing incidence in adults aged 35–44 years when restricting the analysis to diagnoses made in primary care (data not shown). Between 1996–97 and 2003–04, Kopec et al. [7] found no evidence of an increased consultation incidence of OA in persons <40 years of age in British Columbia. However, we cannot exclude the possibility that our findings reflect a true secular change between 2003 and 2010 in the incidence of OA in adults aged 35–44 years. These cohorts grew up during periods of increasing prevalence of obesity in England [36–38], which would be expected to increase the risk of OA [39]. The contribution of injury, another important determinant of OA in younger ages, is unclear due to a lack of available data on representative populations.
The higher incidence of knee OA in men than in women in the 55–64 year age band was unexpected. This may be a chance finding based on multiple comparisons and relatively small numbers of incident cases within each age–gender stratum, although it is consistent with previous consultation prevalence in this age group [40] and radiographical OA [41] in the population.
Administrative databases and electronic health care records data are increasingly recognized as key resources for chronic disease research and surveillance [42, 43]. OA is underrepresented in these fields given its importance to population health [1, 4]. One recurrent concern, shared with all secondary uses of data recorded principally for administrative or clinical purposes, is the validity of OA case definitions. A variety of approaches have been undertaken in our and other databases internationally, including manual review of anonymized free text in the health record, typically adjudicated by one or more clinicians [44–46], comparison of rates in different databases [17, 21], comparison with independent patient self-reports [24, 25, 47] and clinical or imaging assessment [48]. As with other data sources [49], incidence and prevalence estimates derived from administrative and health record data are sensitive to the particular case definition adopted [10]. While a single record of a consultation with an OA diagnostic code has been supported in some of these studies [24, 25, 47], and is the most commonly used case definition, it must nevertheless be acknowledged as naive in that it assumes no misclassification errors [50]. Uncertainty around our estimates is likely to be greater than implied by the CIs and, together with the relatively small size of the database, means that our findings should be replicated in other similar databases. If confirmed, an increase in newly diagnosed cases of OA in younger adulthood may have important but quite different implications depending on whether it reflects a lowering of the diagnostic threshold or the earlier onset of OA. Distinguishing between these two interpretations warrants further investigation. Secondary care information (e.g. hospital letters) is recorded and coded in CiPCA, but the completeness of this information will vary by practice. While it is unlikely (due to the generally long run-in periods that we have used) that many patients with an existing diagnosis of OA during the run-in period would not be identified in CiPCA, the number of new cases of OA in 2010 may be conservative since there may be some patients diagnosed in secondary care who were not recorded with OA in CiPCA.
Our estimates of the rate and age–sex distribution of new OA diagnoses, showing broad consistency with incidence rates derived internationally, help to address an information gap on OA occurrence in the UK. A novel method, designed to more efficiently use data within routine electronic health care databases, has provided preliminary data on a trend likely to be of public health concern but which these data alone cannot answer conclusively.
Supplementary Material
Acknowledgements
We thank the Keele GP Research Partnership and the informatics team at the Arthritis Research UK Primary Care Centre. The CiPCA database is funded by the North Staffordshire Primary Care Research Consortium and Keele University Research Institute for Primary Care and Health Sciences.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organisation for Economic Co-operation and Development. Health at a glance 2011: OECD indicators. Paris: OECD Publishing, 2011. [Google Scholar]
- 3.Culliford DJ, Maskell J, Beard DJ, et al. Temporal trends in hip and knee replacement in the United Kingdom: 1991 to 2006. J Bone Joint Surg Br 2010;92:130–5. [DOI] [PubMed] [Google Scholar]
- 4.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 5.Davies SC. Annual report of the Chief Medical Officer: Volume One, 2011 – On the state of the public’s health. London: Department of Health, 2012. [Google Scholar]
- 6.van der Waal JM, Bot SD, Terwee CB, et al. The incidences of and consultation rate for lower extremity complaints in general practice. Ann Rheum Dis 2006;65:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopec JA, Rahman MM, Sayre EC, et al. Trends in physician-diagnosed osteoarthritis incidence in an administrative database in British Columbia, Canada, 1996–1997 through 2003–2004. Arthritis Rheum 2008;59:929–34. [DOI] [PubMed] [Google Scholar]
- 8.Prieto-Alhambra D, Judge A, Javaid MK, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014;73:1659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Gooch K, Svenson LW, Bell NR, Frank C. Estimating osteoarthritis incidence from population-based administrative health care databases. Ann Epidemiol 2007;17:51–6. [DOI] [PubMed] [Google Scholar]
- 10.Kopec JA, Rahman MM, Berthelot JM, et al. Descriptive epidemiology of osteoarthritis in British Columbia, Canada. J Rheumatol 2007;34:386–93. [PubMed] [Google Scholar]
- 11.Rothman KJ, Greenland S, Lash TL. Modern epidemiology, 3rd edn Philadelphia, PA: Lippincott, Williams & Wilkins, 2008. [Google Scholar]
- 12.Gershon AS, Wang C, Wilton AS, Raut R, To T. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population-based study. Arch Intern Med 2010;170:560–5. [DOI] [PubMed] [Google Scholar]
- 13.Gershon AS, Guan J, Wang C, To T. Trends in asthma prevalence and incidence in Ontario, Canada, 1996–2005: a population study. Am J Epidemiol 2010;172:728–36. [DOI] [PubMed] [Google Scholar]
- 14.Asghari S, Courteau J, Carpentier AC, Vanasse A. Optimal strategy to identify incidence of diagnostic of diabetes using administrative data. BMC Med Res Methodol 2009;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am 2013;39:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan KP, Joud A, Bergknut C, et al. International comparisons of the consultation prevalence of musculoskeletal conditions using population-based healthcare data from England and Sweden. Ann Rheum Dis 2014;73:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute for Health and Clinical Excellence (NICE). Osteoarthritis care and management in adults.http://www.nice.org.uk/guidance/cg177/resources/guidance-osteoarthritis-pdf (12 June 2015, date last accessed). [PubMed] [Google Scholar]
- 18.The King’s Fund. General practice in England: an overview. http://www.kingsfund.org.uk/sites/files/kf/general-practice-in-england-overview-sarah-gregory-kings-fund-september-2009.pdf (12 June 2015, date last accessed). [Google Scholar]
- 19.Bowling A. Research methods in health. Buckingham, UK: Open University Press, 1997. [Google Scholar]
- 20.Porcheret M, Hughes R, Evans D, et al. Data quality of general practice electronic health records: the impact of a program of assessments, feedback, and training. J Am Med Inform Assoc 2004;11:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan K, Clarke AM, Symmons DP, et al. Measuring disease prevalence: a comparison of musculoskeletal disease using four general practice consultation databases. Br J Gen Pract 2007;57:7–14. [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan KP, Kadam UT, Hayward R, Porcheret M, Young C, Croft P. Annual consultation prevalence of regional musculoskeletal problems in primary care: an observational study. BMC Musculoskelet Disord 2010;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res 2010;62:460–4. [DOI] [PubMed] [Google Scholar]
- 24.Lix L, Yogendran M, Burchill C, et al. Defining and validating chronic diseases: an administrative data approach. Winnipeg, MB, Canada: Manitoba Centre for Health Policy, 2006. [Google Scholar]
- 25.Lix L, Yogendran M, Mann J. Defining and validating chronic diseases: an administrative data approach-an update with ICD-10-CA https://www.researchgate.net/profile/Marina_Yogendran/publication/242284353(26 June 2015, date last accessed). [Google Scholar]
- 26.van den Dungen C, Hoeymans N, Boshuizen HC, et al. The influence of population characteristics on variation in general practice based morbidity estimations. BMC Public Health 2011;11:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doran T, Kontopantelis E, Valderas JM, et al. Effect of financial incentives on incentivised and non-incentivised clinical activities: longitudinal analysis of data from the UK Quality and Outcomes Framework. BMJ 2011;342:d3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedson J, Kadam U, Muller S, Peat G. Does comorbid disease influence consultation for knee problems in primary care? Prim Health Care Res Dev 2011;12:322–8. [DOI] [PubMed] [Google Scholar]
- 29.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum 1995;38:1134–41. [DOI] [PubMed] [Google Scholar]
- 30.Nuesch E, Dieppe P, Reichenbach S, et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The care and management of osteoarthritis in adults [CG59]. London: National Institute for Health and Care Excellence, 2008. [Google Scholar]
- 32.Birrell F, Croft P, Cooper C, et al. Radiographic change is common in new presenters in primary care with hip pain. PCR Hip Study Group. Rheumatology 2000;39:772–5. [DOI] [PubMed] [Google Scholar]
- 33.Bedson J, Jordan K, Croft P. The prevalence and history of knee osteoarthritis in general practice: a case-control study. Fam Pract 2005;22:103–8. [DOI] [PubMed] [Google Scholar]
- 34.Hippisley-Cox J, Fenty J, Heaps M. Trends in consultation rates in general practice 1995 to 2006: analysis of the QRESEARCH database. Nottingham, UK: QRESEARCH, 2007. [Google Scholar]
- 35.Haynes K, Bilker WB, Tenhave TR, Strom BL, Lewis JD. Temporal and within practice variability in the health improvement network. Pharmacoepidemiol Drug Saf 2011;20:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamatakis E, Primatesta P, Chinn S, Rona R, Falascheti E. Overweight and obesity trends from 1974 to 2003 in English children: what is the role of socioeconomic factors? Arch Dis Child 2005;90:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rennie KL, Jebb SA. Prevalence of obesity in Great Britain. Obes Rev 2005;6:11–2. [DOI] [PubMed] [Google Scholar]
- 38.Howel D. Trends in the prevalence of abdominal obesity and overweight in English adults (1993–2008). Obesity 2012;20:1750–2. [DOI] [PubMed] [Google Scholar]
- 39.Wills AK, Black S, Cooper R, et al. Life course body mass index and risk of knee osteoarthritis at the age of 53 years: evidence from the 1946 British birth cohort study. Ann Rheum Dis 2012;71:655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arthritis Research UK. Osteoarthritis in general practice. Data and perspectives, 2013. http://www.arthritisresearchuk.org/arthritis-information/data-and-statistics/osteoarthritis/data-on-knee-oa.aspx (8 August 2014, date last accessed). [Google Scholar]
- 41.Lacey RJ, Thomas E, Duncan RC, Peat G. Gender difference in symptomatic radiographic knee osteoarthritis in the Knee Clinical Assessment – CAS(K): a prospective study in the general population. BMC Musculoskelet Disord 2008;9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol 2005;58:323–37. [DOI] [PubMed] [Google Scholar]
- 43.Birtwhistle R, Keshavjee K, Lambert-Lanning A, et al. Building a pan-Canadian primary care sentinel surveillance network: initial development and moving forward. J Am Board Fam Med 2009;22:412–22. [DOI] [PubMed] [Google Scholar]
- 44.Gabriel SE, Crowson CS, O’Fallon WM. A mathematical model that improves the validity of osteoarthritis diagnoses obtained from a computerized diagnostic database. J Clin Epidemiol 1996;49:1025–9. [DOI] [PubMed] [Google Scholar]
- 45.Harrold LR, Yood RA, Straus W, et al. Challenges of estimating health service utilization for osteoarthritis patients on a population level. J Rheumatol 2002;29:1931–6. [PubMed] [Google Scholar]
- 46.Kadhim-Saleh A, Green M, Williamson T, Hunter D, Birtwhistle R. Validation of the diagnostic algorithms for 5 chronic conditions in the Canadian Primary Care Sentinel Surveillance Network (CPCSSN): a Kingston Practice-based Research Network (PBRN) report. J Am Board Fam Med 2013;26:159–67. [DOI] [PubMed] [Google Scholar]
- 47.Prieto-Alhambra D, Nogues X, Javaid MK, et al. An increased rate of falling leads to a rise in fracture risk in postmenopausal women with self-reported osteoarthritis: a prospective multinational cohort study (GLOW). Ann Rheum Dis 2013;72:911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahman M, Aghanjanian J, Kopec J, Cibere J. Validation of osteoarthritis diagnosis in administrative data using a clinically and radiologically defined population-based cohort of osteoarthritis. Osteoarthritis Cartilage 2008;16(Suppl 4):S150. [Google Scholar]
- 49.Pereira D, Peleteiro B, Araujo J, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19:1270–85. [DOI] [PubMed] [Google Scholar]
- 50.Ladouceur M, Rahme E, Pineau CA, Joseph L. Robustness of prevalence estimates derived from misclassified data from administrative databases. Biometrics 2007;63:272–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.