Abstract
Background
As access to antiretroviral therapy (ART) expands, increasing numbers of older patients will start treatment and require specialised long-term care. However the impact of age in ART programs in resource-constrained settings is poorly understood. South Africa has the second largest population of older (≥50 years) people in sub-Saharan Africa. The HIV epidemic is also ageing rapidly and the country has one of the highest HIV population prevalences worldwide. This study explored the effect of age on mortality on ART in South Africa and whether this effect was mediated by baseline immunologic status.
Methods
IeDEA-SA is a regional collaboration which combines routine observational data from large ART programmes across Southern Africa. This study was a retrospective cohort analysis of adults starting ART from 2004-2013 in six large South African cohorts: two primary care clinics, three hospitals and a large rural cohort. The primary outcome was mortality; secondary outcomes were loss to follow-up (LTF), immunologic and virologic responses. Patients' vital status was ascertained through linkage to the National Population Register. Inverse probability weighting was used to correct mortality for LTF. Mortality was estimated using Cox's proportional hazards and competing risks regression. The interaction between baseline CD4+ cell count and age was tested. Immunologic responses were graphed by age and duration on ART.
Findings
83 566 patients were followed for 174 640 patient-years. Patients were predominantly female, especially in the younger age groups: 81% (18 819/23 258) of patients 16-29 years and 66% (12 812/19 372) of those aged 30-34. Mortality increased with age in a dose response, mediated by baseline immunologic status. Patients with CD4 counts <50 cells/μL were a particularly high risk group, comprising 14% of all older patients starting ART. The percentage of older patients enrolling increased with successive calendar years from 6% (290/4 999) in 2004 to 10% (35/9 657) in 2012/13, comprising 9% of total enrolment (7 295/83 566). By analysis closure, 14% of all patients still alive and in care were ≥50 years old.
Interpretation
Health services need re-orientation towards diagnosing and starting ART in older individuals. Policies are needed for long-term care of older people with HIV.
Funding
Supported by NIH (NIAID), USAID & South African Centre for Epidemiological Modelling & Analysis.
1 Background
The world's population is ageing rapidly. By 2050, the World Health Organisation (WHO) estimates that there will be two billion elderly individuals (defined as ≥60 years old) worldwide, 80% living in developing countries (1). South Africa has the second largest population of older people in sub-Saharan Africa (SSA) (2). These numbers are expected to increase. By 2025, nearly 5.23 million South Africans will be older than 60 years of age (3). In addition to this rapid ageing in the general population, the HIV epidemic is ageing and the total number of HIV-positive individuals older than 50 years in South Africa may triple in the next 30 years (4). South Africa also has the largest ART programme worldwide, with an estimated 2.3 million individuals still on treatment in the public sector by March 2013 (5). The country is now facing the challenges of a successful ART programme in the middle of a major demographic transition. As access to treatment expands, increasing numbers of older patients may start ART and require long-term care.
Initiating and retaining older individuals on ART has major public health implications. Older people are not generally targeted for HIV prevention and testing (6). Health care workers are less likely to ask about sexual practices and to diagnose HIV in older individuals. As a result older people may start treatment with more advanced HIV disease than younger people. In addition, older people are more likely to have co-morbidities and may require more specialised care than younger patients (7). Studies suggest that HIV mimics the effects of ageing in the immune system, compounded by long-term ART toxicity and interactions with co-medications for other age-related conditions (8). Poorer outcomes on ART have been reported in older adults compared to younger adults (9).
To date there has been limited research on age in ART programmes in resource-constrained settings (10-13) and mortality has been estimated from standard patient record systems which are known to miss a high proportion of deaths (14, 15). Using linkages with the South African vital registration system, the International epidemiologic Databases to Evaluate AIDS-Southern Africa (IeDEA-SA) collaboration is able to correct mortality estimates for loss to follow-up (LTF), providing a unique opportunity to explore the long-term outcomes of a large number of older individuals starting ART in South Africa since 2004. We investigated the association between age and mortality risk and whether this effect was modified by pre-ART immunologic status.
2 Methods
2.1 Study design and population
IeDEA-SA is a regional collaboration which combines routine observational data from large ART programmes across Southern Africa This study was a retrospective cohort analysis of data from South African cohorts of IeDEA-SA providing ART services in three of the most populous provinces (KwaZulu-Natal, Gauteng and Western Cape). Cohorts are predominantly government-funded and follow standardised national ART guidelines. Patients are broadly representative of patients accessing public sector ART in rural and urban centres.
2.2 Data sources
Using a standardised data transfer format, participating IeDEA-SA cohorts transfer anonymised, routinely-collecteddata to the IeDEA-SA data centre at the University of Cape Town. This study included data from six cohorts: Gugulethu and Khayelitsha primary care clinics and Tygerberg hospital (Western Cape province); McCord Hospital and Hlabisa, a large rural cohort of 17 primary health care clinics (KwaZulu-Natal), and Themba Lethu, a large urban public hospital in Gauteng. All ART-naïve HIV-positive adults (16-80 years old) who started ART 2004-2013 were eligible for inclusion.
2.3 Statistical analyses
Cleaning, coding and analysis of data were done in Intercooled Stata 13.1 (STATA Corporation, College Station, TX). Patients were followed from ART initiation to the earliest of: death, LTF, transfer out (TFO) or alive at analysis closure. The primary outcome was death. LTF, immunologic and virologic responses were secondary outcomes. LTF was defined as no contact with the health facility for six months (17). The analysis excluded patients enrolled up to six months prior to database closure to allow the LTF definition to be met. Given that a high proportion of LTF patients were likely to have died, we used inverse probability weighting to correct mortality for deaths misclassified as LTF (18). LTF patients with ID numbers were linked to the National Population Register to confirm vital status and date of death if deceased, and up-weighted to represent all LTF. TFO was defined by sites and TFOs were censored at the date of TFO.
Patients were analysed by age group: 16-29 years old and 5-year groups thereafter. Summary baseline characteristics (median, interquartile range (IQR) and proportions) were described. The number and proportion of patients dead, the median follow-up times to death and the rates and incidence of mortality were reported by age. The proportions of patients ≥50 years old at ART initiation and at analysis closure were reported by calendar year of enrolment.
Cox's proportional hazards models were used to assess crude and adjusted associations between patient characteristics and outcomes. Models were adjusted for baseline patient characteristics (gender, CD4+ cell count, WHO stage, haemoglobin, TB, weight and site of ART initiation) and stratified by duration on ART. Assuming that data were likely missing at random, we used multiple imputation (19) by chained equation methods (20) to impute missing baseline covariates. We multiply imputed ten times CD4+ cell count (baseline, 12-month and 24-month), baseline WHO stage, weight, haemoglobin and tuberculosis (yes/no). We explored immunologic response by reporting and graphing CD4+ cell counts at baseline, 12, 24 and 36 months on ART. We reported the percentage of virally suppressed (viral load (VL) measurement <400 copies/ml) among all patients with VLs at 12, 24 and 36 months. We assessed whether the effect of age on mortality risk was modified by baseline CD4+ cell count by recategorising age (16-39, 40-49 and 50+ years) and CD4+ cell counts (<50, 50-199 and ≥200 cells/μl). We included their interaction in the models and graphed the hazard ratios of this interaction. We compared estimates of mortality and LTF using Kaplan-Meier and competing risks methods and explored possible heterogeneity between cohorts by comparing median ages, numbers (%) of patients ≥50 years old at ART initiation and at analysis closure, and crude hazard ratios for the effect of age on mortality, by cohort.
2.4 Role of the funding source
This study was supported by funding from the United States (US) National Institutes of Health Grant 5U01AI069924, US Agency for International Development Cooperative Agreement AID 674-A-12-00029, and the South African Centre for Epidemiological Modelling and Analysis. The content of this publication is solely the authors' responsibility and does not necessarily reflect the views or policies of the funders or the US Government. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3 Results
Overall 84 078 eligible ART-naïve adults started ART in these cohorts from 2004-2013. Of these, 512 were excluded due to: missing/invalid dates (n=504) and unknown sex (n=8). The analysis included 83 566 patients followed for 174 640 person-years (pyrs). The median (IQR) follow-up time was 2.13 (0.77-4.13) pyrs.
The proportion of patients enrolled in successive age groups decreased from 23 258/83 566 (28%) among 16-29 year olds to 492/83 566 (1%) among those older than 65 years (Table 1). Patients were predominantly female (54 638/83 566, 65%), especially in the younger age groups: 18 819/23 258 (81%) of patients aged 16-29 and 12 812/19 372 (66%) of those aged 30-34 years. The median CD4+ cell counts were similar in patients aged 16-29 (137 cells/μL, IQR 62-210) and 65+ years old (135 cells/μL, IQR 75-208), as were the proportions of patients in WHO stages III and IV (35 vs 34%). Among patients with TB measurements at ART initiation, the proportions with TB decreased in older compared with younger patients.
Table 1. Baseline characteristics, year of ART initiation, patient outcomes and mortality rates by age at enrolment.
| Characteristic | Age categories (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16-29 | 30-34 | 35-39 | 40-44 | 45-49 | 50-54 | 55-59 | 60-64 | 65+ | Overall | |
| Patients enrolled, n (%) | 23258 (28) | 19372 (23) | 16231 (19) | 10616 (13) | 6794 (8) | 4006 (5) | 1991 (2) | 806 (1) | 492 (1) | 83566 (100) |
| Females, n (%) | 18819 (81) | 12812 (66) | 9411 (58) | 5975 (56) | 3770 (55) | 2165 (54) | 1040 (52) | 399 (50) | 247 (50) | 54638 (65) |
| CD4+ cell count, median cells/μL (IQR) | 137 (62-210) | 120 (152-188) | 117 (51-186) | 120 (53-186) | 124 (59-190) | 128 (64-195) | 125 (60-190) | 130 (66-195) | 135 (75-208) | 125 (56-192) |
| CD4+ cell count, categorical, n(%) | ||||||||||
| 0-49 | 3692 (16) | 3696 (19) | 3182 (20) | 2002 (19) | 1188 (17) | 615 (15) | 326 (16) | 111 (14) | 51 (10) | 14863 (18) |
| 50-99 | 3002 (13) | 2863 (15) | 2514 (15) | 1634 (15) | 1003 (15) | 652 (16) | 300 (15) | 117 (14) | 91 (19) | 12176 (15) |
| 100-199 | 6657 (29) | 5613 (29) | 4646 (29) | 3177 (30) | 2114 (31) | 1210 (30) | 637 (32) | 269 (33) | 143 (29) | 24466 (29) |
| 200-349 | 4030 (17) | 2826 (15) | 2267 (14) | 1471 (14) | 979 (14) | 649 (16) | 278 (14) | 136 (17) | 83 (17) | 12719 (15) |
| 350-499 | 377 (2) | 246 (1) | 232 (1) | 156 (1) | 118 (2) | 66 (2) | 32 (2) | 11 (1) | 10 (2) | 1248 (2) |
| ≥500 | 196 (1) | 120 (1) | 118 (1) | 78 (1) | 62 (1) | 40 (1) | 28 (1) | 4 (1) | 9 (2) | 655 (1) |
| Missing | 5304 (23) | 4008 (21) | 3272 (20) | 2098 (20) | 1330 (20) | 774 (19) | 390 (20) | 158 (20) | 105 (21) | 17439 (21) |
| TB at ART start, n(%) | 1725 (17) | 1730 (20) | 1467 (19) | 982 (18) | 633 (17) | 321 (15) | 155 (13) | 64 (14) | 35 (12) | 7112 (18) |
| Missing | 12896 (55) | 10533 (54) | 8337 (51) | 5178 (49) | 3118 (46) | 1807 (45) | 837 (42) | 333 (41) | 189 (38) | 43228 (52) |
| Anaemia, n(%) | ||||||||||
| none | 3011 (13) | 2925 (15) | 2740 (17) | 1913 (18) | 1286 (19) | 784 (20) | 411 (21) | 134 (17) | 81 (16) | 13285 (16) |
| mild | 4299 (18) | 3613 (19) | 3131 (19) | 2173 (20) | 1447 (21) | 896 (22) | 452 (23) | 173 (21) | 115 (23) | 16299 (20) |
| moderate | 2828 (12) | 2505 (13) | 2133 (13) | 1381 (13) | 945 (14) | 524 (13) | 275 (14) | 125 (16) | 74 (15) | 10790 (13) |
| severe | 1051 (5) | 782 (4) | 640 (4) | 422 (4) | 227 (3) | 127 (3) | 49 (2) | 26 (3) | 16 (3) | 3340 (4) |
| Missing | 12069 (52) | 9547 (49) | 7587 (47) | 4727 (45) | 2889 (43) | 1675 (42) | 804 (40) | 348 (43) | 206 (42) | 39852 (48) |
| Year of ART initiation, n(%) | ||||||||||
| 2004 | 1419 (28) | 1335 (27) | 1030 (21) | 583 (12) | 342 (7) | 178 (4) | 69 (1) | 27 (1) | 16 (0) | 4999 (100) |
| 2005 | 1976 (29) | 1745 (26) | 1339 (20) | 799 (12) | 527 (8) | 242 (4) | 108 (1) | 39 (1) | 18 (0) | 6793 (100) |
| 2006 | 2291 (27) | 2181 (26) | 1629 (19) | 1162 (14) | 623 (7) | 375 (4) | 167 (2) | 59 (1) | 34 (0) | 8521 (100) |
| 2007 | 2265 (26) | 2129 (24) | 1737 (20) | 1166 (13) | 714 (8) | 378 (4) | 202 (2) | 67 (1) | 38 (0) | 8696 (100) |
| 2008 | 2751 (27) | 2347 (23) | 1990 (20) | 1293 (13) | 841 (8) | 506 (5) | 244 (2) | 94 (1) | 63 (1) | 10129 (100) |
| 2009 | 2892 (26) | 2615 (23) | 2169 (19) | 1473 (13) | 997 (9) | 588 (5) | 289 (3) | 121 (1) | 57 (1) | 11201 (100) |
| 2010 | 3605 (30) | 2664 (22) | 2322 (19) | 1440 (12) | 938 (8) | 589 (5) | 319 (3) | 127 (1) | 84 (1) | 12088 (100) |
| 2011 | 3304 (29) | 2346 (20) | 2148 (19) | 1492 (13) | 956 (8) | 639 (6) | 347 (3) | 150 (1) | 100 (1) | 11482 (100) |
| 2012 & 2013 | 2755 (29) | 2010 (21) | 1867 (19) | 1208 (13) | 856 (9) | 511 (5) | 246 (3) | 122 (1) | 82 (1) | 9657 (100) |
| Weight, median kg, (IQR) | 60 (52-69) | 61 (54-70) | 62 (55-71) | 62 (54-72) | 62 (54-72) | 62 (54-71) | 62 (54-72) | 61 (53-71) | 60 (51-70) | 61 (54-70) |
| Outcome at analysis closure, n(%) | ||||||||||
| Deaths | 1817 (8) | 1746 (9) | 1556 (10) | 1124 (11) | 767 (11) | 515 (13) | 275 (14) | 136 (17) | 93 (19) | 8029 (10) |
| Transferred out | 2749 (12) | 2128 (11) | 1672 (10 | 1060 (10) | 667 (10) | 385 (10) | 187 (9) | 73 (9) | 40 (8) | 8961 (11) |
| Lost to follow-up | 6828 (29) | 5229 (27) | 4068 (25) | 2536 (24) | 1500 (22) | 815 (20) | 417 (21) | 186 (23) | 88 (18) | 21667 (26) |
| Alive | 11864 (51) | 10269 (53) | 8935 (55) | 5896 (56) | 3860 (57) | 2291 (57) | 1112 (56) | 411 (51) | 271 (55) | 44909 (54) |
| Mortality rate per 100 pyrs | 3.1 | 3.2 | 3.6 | 4.0 | 4.5 | 5.6 | 6.6 | 9.4 | 11.4 | 3.7 |
| Median days to death | 749 | 863 | 795 | 799 | 746 | 700 | 606 | 473 | 420 | 777 |
After correction for LTF, at analysis closure, 8 029/83 566(10%) had died, 8 961 (11%) were TFO, 21 667 (26%) were LTF and 44 909 (54%) were alive and in care (Table 1). Deaths increased from 8% (1 817/23 258) of patients aged 16-29 years to 19% (93/492) of patients aged 65+ years while the percentage of patients LTF decreased from 29% (6 828/23 258) to 18% (88/492) in the same age groups. The median duration of follow-up was 777 (IQR 280-1510) person-days and decreased with older age. The overall mortality rate was 3.7/100 pyrs), increasing from 3.1 to 11.4/100 pyrs across the age groups (Table 1). At 12, 24 and 36 months the cumulative incidence of mortality was 6.9% (6.7-7.1), 9.2% (8.9-9.4) and 10.8% (10.6-11.1) respectively, with slightly lower estimates derived from competing risks analysis (Supplementary Table S1).
The percentage of patients aged ≥50 years at ART initiation increased from 6% (290/4 999) in 2004 to 10% (35/9 657) in 2012/13, comprising 9% of total enrolment (7 295/83 566) (Supplementary Table S2). Within cohorts, this ranged from 1 473/23 713 (6%) in Khayelitsha to 2 167/19 946 (11%) in Hlabisa (Supplementary Table S3). At analysis closure, 6 304/44 909 (14%) patients alive and in care were aged ≥50 years, ranging from 1 479/14 096 (10%) in Khayelitsha to 16% in the large cohorts of Themba Lethu (1 850/11 433) and Hlabisa (2 253/19 946).
In univariate analysis, there was a dose response between increasing age and the hazard of death (Table 2). Compared with patients 16-29 years, the crude hazard was slightly higher in those 35-39 years (HR 1.16, 95% CI 1.08-1.24) and three-fold higher in patients aged ≥65 years old (HR 3.00, 95% CI 2.43-3.70). These associations were attenuated but persisted in multivariable analysis adjusted for baseline characteristics (aHR 2.52, 95% CI 2.01-3.17, 65+ vs. 16-29 years old). The effect of age on mortality persisted over duration on ART but with less precise estimates (Table 2). Other baseline characteristics that increased the adjusted risk of mortality were male gender (aHR 1.40, 95% CI 1.33-1.48), WHO stages III/IV vs. I & II (aHR 2.37, 95% CI 2.09-2.70, Stage IV vs. I & II) and having any level of anaemia compared with none. Compared with patients starting ART at a CD4+ cell count <50 cells/μL, the hazard of mortality was lowest in patients enrolling at 200-349 cells/μL (aHR 0.38, 95% CI 0.33-0.43). Having TB at ART initiation increased the risk of death in crude but not in multivariable analysis (aHR 0.82, 95% CI 0.76-0.90). In a sensitivity analysis limited to cohorts that collected ID numbers, the point estimates were similar but less precise. In analysis to explore heterogeneity, the point estimates for the effect of age on the hazard of mortality were similar across all cohorts (Supplementary Table S3).
Table 2. Crude and adjusted* mortality after multiple imputation, overall and by duration on ART.
| OVERALL | 0-12 months | 12-24 months | 24-36 months | ||
|---|---|---|---|---|---|
| HR | aHR*** | aHR | aHR | aHR | |
| Age (years) | |||||
| 16-29 | 1 | 1 | 1 | 1 | 1 |
| 30-34 | 1.06 (0.99-1.14) | 1.03 (0.96-1.10) | 1.05 (0.90-1.22) | 0.99 (0.73-1.35) | 0.83 (0.56-1.23) |
| 35-39 | 1.16 (1.08-1.24) | 1.12 (1.04-1.20) | 1.14 (0.99-1.32) | 1.11 (0.82-1.50) | 0.83 (0.57-1.22) |
| 40-44 | 1.29 (1.20-1.40) | 1.24 (1.14-1.34) | 1.26 (1.09-1.46) | 1.14 (0.84-1.55) | 0.78 (0.53-1.17) |
| 45-49 | 1.45 (1.33-1.58) | 1.35 (1.23-1.48) | 1.37 (1.17-1.60) | 1.39 (1.01-1.91) | 0.87 (0.57-1.32) |
| 50-54 | 1.73 (1. 57-1.92) | 1.65 (1.48-1.84) | 1.52 (1.28-1.80) | 1.63 (1.16-2.27) | 1.26 (0.82-1.93) |
| 55-59 | 1.94 (1.70-2.20) | 1.86 (1.63-2.14) | 1.89 (1.57-2.27) | 1.85 (1.30-2.66) | 1.87 (1.20-2.92) |
| 60-64 | 2.63 (2.19-3.16) | 2.49 (2.04-3.06) | 2.04 (1.63-2.54) | 1.77 (1.11-2.82) | 2.44 (1.45-4.10) |
| 65+ | 3.00 (2.43-3.70) | 2.52 (2.01-3.17) | 2.79 (2.21-3.53) | 3.21 (2.04-5.06) | 2.06 (1.07-3.98) |
| Male gender | 1.62 (1.55-1.70) | 1.40 (1.33-1.48) | 1.28 (1.19-1.37) | 1.38 (1.20-1.59) | 1.74 (1.45-2.10) |
| CD4+ cell count (cells/μL) | |||||
| <50 | 1 | 1 | 1 | 1 | 1 |
| 50-99 | 0.64 (0.60-0.68) | 0.71 (0.65-0.78) | 0.60 (0.55-0.65) | 0.82 (0.69-0.97) | 0.91 (0.72-1.17) |
| 100-199 | 0.39 (0.36-0.41) | 0.54 (0.80-0.59) | 0.40 (0.37-0.44) | 0.61 (0.52-0.72) | 0.85 (0.68-1.06) |
| 200-349 | 0.24 (0.22-0.26) | 0.38 (0.33-0.43) | 0.30 (0.26-0.35) | 0.53 (0.41-0.68) | 0.60 (0.42-0.86) |
| 350-499 | 0.34 (0.27-0.44) | 0.52 (0.41-0.65) | 0.43 (0.31-0.59) | 0.64 (0.34-1.22) | 1.25 (0.58-2.70) |
| ≥500 | 0.26 (0.18-0.37) | 0.44 (0.30-0.63) | 0.39 (0.25-0.62) | 0.40 (0.13-1.26) | 1.60 (0.59-4.31) |
| WHO stage | |||||
| I & II | 1 | 1 | 1 | 1 | 1 |
| III | 2.40 (2.18-2.65) | 1.64 (1.48-1.82) | 1.61 (1.34-1.93) | 1.70 (1.34-2.16) | 1.53 (1.12-2.08) |
| IV | 4.06 (3.66-4.51) | 2.37 (2.09-2.70) | 2.42 (1.98-2.97) | 2.15 (1.69-2.75) | 2.14 (1.52-3.02) |
| Anaemia | |||||
| none | 1 | 1 | 1 | 1 | 1 |
| mild | 1.76 (1.64-1.89) | 1.37 (1.25-1.50) | 1.51 (1.31-1.75) | 1.37 (1.10-1.71) | 1.33 (1.00-1.76) |
| moderate | 3.10 (2.87-3.35) | 1.99 (1.82-2.17) | 2.40 (2.05-2.81) | 1.77 (1.41-2.22) | 1.57 (1.14-2.15) |
| severe | 4.72 (4.00-5.56) | 2.92 (2.60-3.28) | 3.89 (3.35-4.52) | 2.03 (1.46-2.84) | 1.66 (1.04-2.64) |
| TB at enrolment | 1.53 (1.40-1.65) | 0.82 (0.76-0.90) | 0.81 (0.73-0.91) | 0.83 (0.66-1.04) | 0.92 (0.72-1.18) |
| Weight (kg) | 0.96 (0.96-0.96) | 0.98 (0.97-0.98) | 0.97 (0.97-0.98) | 0.98 (0.98-0.99) | 0.99 (0.99-0.99) |
also adjusted for site of ART initiation;
mortality corrected via linkage to the National Population Register;
aHR: adjusted Hazard Ratio
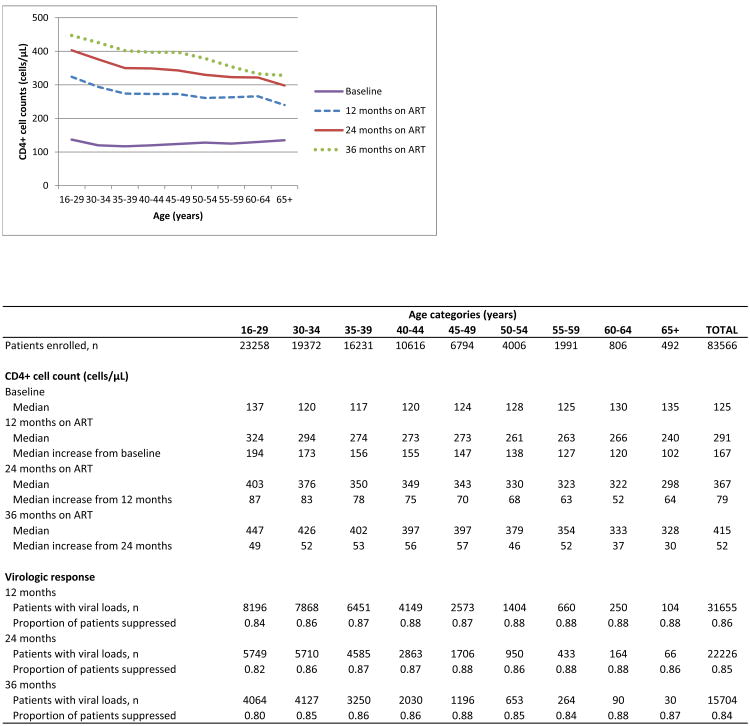
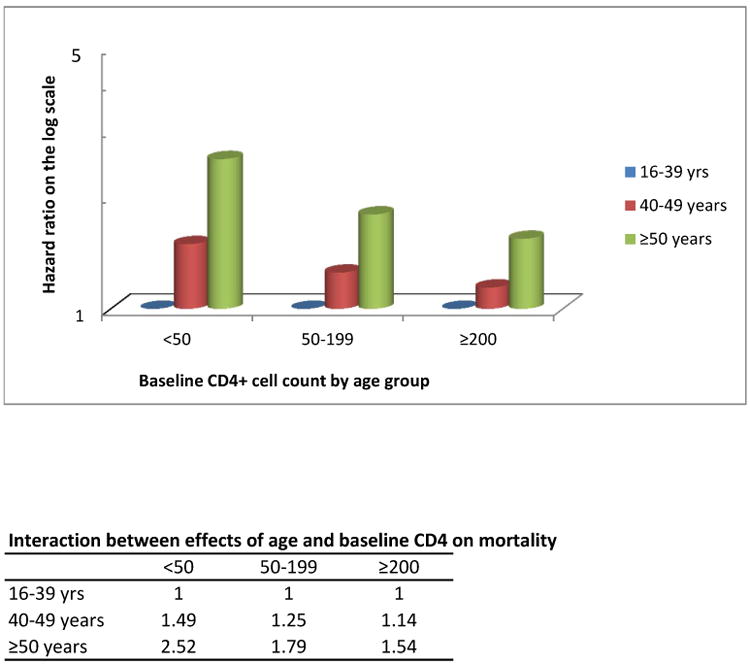
Although there was no difference in the baseline median CD4+ cell countsof the youngest and oldest age groups, immunologic responses on ART were clearly age-related, with smaller gains in CD4+ cell counts at older ages (Table 3). After 12 months on ART, the median CD4+ cell count increase from baseline was 167 cells/μL (IQR 92-159), ranging from 194 cells/μL (IQR 115-293) in the 16-29 year age group to 102 cells/μL (IQR 50-180) in those older than 65 years of age. The differences in immunologic responses observed over time appeared to be driven by responses in the first year on treatment (Figure 1). In subsequent years, immunologic response followed the same age pattern as in the first year. Although age increased the risk of mortality, this effect was modified by baseline immunologic status and was more pronounced at lower baseline CD4+ cell counts (Figure 2). Comparing patients ≥50 years with those 16-39 years old, there was a 1.5 fold higher hazard of death if they started ART at ≥200 cells/μL (aHR 1.54, 95% CI 1.33-1.78) and a 2.5 fold higher hazard if they started at <50 cells/μL (aHR 2.52, 95% CI 2.04-3.11) (Supplementary Table S4). Overall, 1 103/7 295 (15%) older patients started ART with CD4+ cell counts <50 cells/μL, ranging from 205/2 167 (9%) in Hlabisa to 150/608 (25%) in McCord.
Table 3.
Immunologic and virologic responses by age and duration on ART.
| Age categories (years) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16-29 | 30-34 | 35-39 | 40-44 | 45-49 | 50-54 | 55-59 | 60-64 | 65+ | TOTAL | |
| Patients enrolled, n | 23258 | 19372 | 16231 | 10616 | 6794 | 4006 | 1991 | 806 | 492 | 83566 |
| CD4+ cell count (cells/μL) | ||||||||||
| Baseline | ||||||||||
| Median (IQR) | 137 (62-201) | 120 (52-188) | 117 (51-186) | 120 (53-186) | 124 (59-190) | 128 (64-195) | 125 (60-190) | 130 (67-195) | 135 (75-208) | 125 (56-192) |
| 12 months on ART | ||||||||||
| Median (IQR) | 324 (223-449) | 294 (201-408) | 274 (189-384) | 273 (186-381) | 273 (187-384) | 261 (177-373) | 263 (177-366) | 266 (184-369) | 240 (167-336) | 291 (198-405) |
| Median increase from baseline | 194 (115-293) | 173 (100-263) | 156 (88-245) | 155 (85-241) | 147 (75-232) | 138 (72-222) | 127 (66-221) | 120 (57-219) | 102 (50-180) | 167 (92-159) |
| 24 months on ART | ||||||||||
| Median (IQR) | 403 (286-539) | 376 (269-503) | 350 (249-472) | 349 (247-474) | 343 (241-468) | 330 (227-451) | 323 (219-439) | 322 (214-441) | 298 (217-436) | 367 (258-497) |
| Median increase from 12 months | 87 (5-176) | 83 (13-159) | 78 (12-152) | 75 (9-149) | 70 (7-149) | 68 (5-135) | 63 (6-122) | 523 (-6-131) | 64 (4-170) | 79 (9-157) |
| 36 months on ART | ||||||||||
| Median (IQR) | 447 (313-591) | 426 (303-563) | 402 (290-528) | 397 (280-536) | 397 (286-544) | 379 (273-516) | 354 (245-477) | 333 (247-460) | 328 (260-448) | 415 (295-555) |
| Median increase from 24 months | 49 (-38-141) | 52 (-25-136) | 53 (-17-127) | 56 (-18-126) | 57 (-8-135) | 46 (-10-123) | 52 (-19-115) | 37 (-24-80) | 30 (-1-122) | 52 (-23-132) |
| Virologic response | ||||||||||
| 12 months | ||||||||||
| Patients with viral loads, n | 8196 | 7868 | 6451 | 4149 | 2573 | 1404 | 660 | 250 | 104 | 31655 |
| Proportion patients suppressed | 0.84 | 0.86 | 0.87 | 0.88 | 0.87 | 0.88 | 0.88 | 0.88 | 0.88 | 0.86 |
| 24 months | ||||||||||
| Patients with viral loads, n | 5749 | 5710 | 4585 | 2863 | 1706 | 950 | 433 | 164 | 66 | 22226 |
| Proportion patients suppressed | 0.82 | 0.86 | 0.87 | 0.87 | 0.88 | 0.86 | 0.88 | 0.88 | 0.86 | 0.85 |
| 36 months | ||||||||||
| Patients with viral loads, n | 4064 | 4127 | 3250 | 2030 | 1196 | 653 | 264 | 90 | 30 | 15704 |
| Proportion patients suppressed | 0.80 | 0.85 | 0.86 | 0.86 | 0.88 | 0.85 | 0.84 | 0.88 | 0.87 | 0.84 |
Figure 1. Median CD4 by age and duration on ART.
Figure 2. Hazard ratios of the interaction between effects of age and baseline CD4+ cell count on mortality.
Among patients with VL measures, virologic suppression was good across all ages ranging from 84% to 88% at 12 months on ART (Table 3). Good virologic suppression was maintained through to 36 months on treatment.
In crude and multivariable analysis patients all patients between 30 and 60 years of age at enrolment had a lower hazard of LTF compared with the youngest patients (aHR 0.82, 95% CI 0.74-0.91, 55-59 vs. 16-29 year old patients, Supplementary Table S5). The cumulative incidence of LTF at 12, 24 and 36 months on ART was 12.2% (95% CI 12.0-12.5), 18.9% (18.3-18.0) and 24.3% (23.9-24.5), with slightly lower estimates from competing risks analysis (Supplementary Table S1).
4 Discussion
In this study of 83566 ART-naive individuals starting treatment 2004-2013, we observed increasing proportions of patients ≥50 years old initiating ART in successive calendar years. The hazard of mortality increased with baseline age while LTF did not. Although baseline CD4+ cell counts were similar across all age groups, the effect of age on mortality was modified by baseline immunologic status and was more pronounced at lower CD4+ cell counts. Among older patients, 19% initiated ART at ≤50 cells/μL and comprised a high risk group. Early immunologic responses were diminished in older patients but virologic responses were good at all ages. At analysis closure, 14% of all patients alive and in care were aged ≥50 years old, suggesting the need for specific consideration in planning for future care.
In recent years there has been growing concern about the lack of data on HIV and ART in older adults in Africa (6, 7, 21) . Our study provides important new evidence that a substantial proportion of older individuals are initiating ART across South Africa, and that this proportion has increased each successive year of enrolment. Despite the absence of a programme targeting older individuals for HIV testing and treatment, nearly ten percent of all new patients were aged 50+ years, similar to findings from Malawi (22), Uganda (23) and nine countries in sub-Saharan Africa (24). This represents a substantial, and poorly understood, burden of care in years to come. Initiating ART in older individuals raises a number of important issues. People who are 50+ years are less likely to have ever tested for HIV than those aged 15-49 years and older people are less likely than younger ones to have used a condom during recent sex (25). Despite these factors, health care workers are less likely to consider an HIV diagnosis in an older compared with a young patient (26). In addition, compounding the body's natural ageing processes, older people experience higher rates of non-communicable diseases than younger individuals, with possible interactions between other chronic medications and ART (8). Health services need to target this neglected group for HIV testing and prevention. Health care workers require training to diagnose HIV in older individuals, initiate and manage them to ensure good outcomes on treatment.
In particular, our results suggest that older patients starting ART with CD4+ cell counts <50 cells/μL should be recognized as a high risk group. Our study confirms a dose response between increasing age at ART initiation and the hazard of mortality (9, 27) and the association between baseline CD4+ cell count and mortality is well-established. Poorer immunologic responses in older compared with younger patients in the first year on ART have been documented in West Africa (28). Our study extends these findings through to three years on treatment. In addition, to our knowledge this is the first study to report that baseline immunologic status modified the effect of age on mortality, allowing us to identify a group of patients requiring additional attention in ART programmes. While the risk of mortality increased with age, the effect of age was strongest at the lowest baseline CD4+ cell counts. In other words, among patients who were healthier the risk of mortality was not greatly increased by age at ART initiation. However among patients with severely compromised immune systems, age had a stronger effect on their mortality risk. In these cohorts, 15% of all older patients started treatment with CD4+ cell counts <50 cells/μL and in two cohorts, ≥20% were in this high risk group. At a programme level, older individuals starting ART at low CD4+ cell counts should be prioritised as a high risk category with specific clinical and policy considerations.
The increasing proportion of older patients starting ART over successive years of enrolment highlights the urgent need for more epidemiologic data on HIV in older adults. HIV prevalence is not generally measured in older individuals. Prevalence estimates are largely based on antenatal surveys limited to women of reproductive age and Demographic & Health Surveys (DHS) restricted to adults ≤50 years. Extending the age limit of such surveys, the 2012 South African household survey reported ±4% prevalence in individuals ≥60 years (29). In contrast, community-level rural surveys have reported prevalence between 60 and 70 years of age ranging from 10.3% to 19.8% in Limpopo (30) and 4.5% to 10% in KwaZulu Natal (31). While these are particularly severely affected areas with higher prevalence levels than national averages, it is also possible that older adults are under-represented in existing household surveys. Data are also needed on the duration of HIV infection in older individuals prior to ART initiation. Older people may have been infected a long time ago and may be a group of long-term survivors with particular features requiring research. In our study, older patients did not have more advanced HIV disease at ART initiation than younger patients. This finding suggests that at least some older patients may have been recently infected (30). There is an urgent need to extend epidemiologic measures beyond 50 years of age and to collect accurate data on HIV in older individuals to plan for their care.
The study is strengthened by large patient numbers including >7,000 patients older than 50 years at ART initiation and good mortality ascertainment through linkage to the National Population Register. The identification of a particular high risk group among older individuals provides important new clinical and policy information. A further strength is consistency of findings across cohorts, increasing confidence in the overall estimates. However, interpretation of these results is subject to several limitations. These data come from numerous ART programmes and there is a substantial amount of missing data such as baseline CD4+ cell counts, which we addressed by using multiple imputation. A further limitation is the lack of data on the cause of death. Previous studies have suggested that older adults may have higher rates of adherence to ART, which could impact on outcomes (36). However data on adherence were not routinely collected or included in these datasets and we were unable to explore this possible association. It is likely that our findings are generalisable to the national ART programme and to other settings in SSA, but further research is needed in different contexts.
Age is routinely reported as a demographic variable but its specific effect on HIV-related mortality has received surprisingly little attention in African ART programmes. Our study suggests that ten years into the national ART programme, it is time to address the needs of older adults with HIV in South Africa. Prevention and testing campaigns need to target older adults. Health care workers need training in diagnosing HIV in older individuals and in initiating and managing them on ART, to ensure good outcomes on treatment. The risks of co-morbidities and interactions between ART and co-medication in older patients need to be quantified. Policies for long-term care of older individuals with HIV are urgently needed.
Supplementary Material
Research in Context.
Evidence before the study
The rapid ageing of the world, together with the ageing of the HIV epidemic, pose major challenges to public health systems, particularly in developing countries. Ageing may occur faster with AIDS in Africa (1) and antiretroviral therapy (ART) may change the age composition of the HIV epidemic in sub-Saharan Africa (2). Despite the fact that South Africa has the largest ART program worldwide, few studies have explicitly assessed the effect of age on mortality on ART in South Africa (3-5). These have reported higher mortality among older patients compared with young adults on ART, for the first year on ART (5), through to 24 months(4) and beyond seven months on ART(3). Compared to younger patients, older patients had lower rates of loss to follow-up, poorer immunologic and better virologic responses to ART.
Added value of this study
Our study extended these findings through to three years on ART. In addition, our study provided new evidence that baseline immunologic status modified the effect of age on mortality on ART. Among patients who were healthier, the risk of mortality was not greatly increased by age at ART initiation. However, older patients initiating ART at ≤50 cells/μL were a particularly high risk group requiring specific clinical and policy considerations.
Implications of all available evidence
The available evidence confirms that a substantial proportion of older individuals are initiating ART across South Africa and that this proportion has increased each successive year of enrolment. Targeted programmes are required to increase voluntary counselling and testing in older individuals. Health care workers require training to diagnose HIV and to initiate ART earlier in older patients. In particular, patients ≥50 years of age enrolling on ART with CD4+ cell counts <50 cells/μL are a high risk group for whom treatment should be expedited. Policies for long-term care of older individuals with HIV are urgently needed.
References
1. Mills EJ, Rammohan A, Awofeso N. Ageing faster with AIDS in Africa. The Lancet 2011; 377(9772): 1131-3.
2. Hontelez JAC, de Vlas SJ, Baltussen R, et al. The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS 2012; 26 Supplement (S1): S19-S30.
3. Fatti G, Mothibi E, Meintjes G, Grimwood A. Antiretroviral treatment outcomes amongst older adults in a large multicentre cohort in South Africa. PLoS One 2014; 9(6): e100273.
4. Maskew M, Brennan A, MacPhail P, Sanne I, Fox MP. Poorer ART outcomes with increasing age at a large public sector HIV clinic in Johannesburg, South Africa. J IntAssoc Physicians AIDS Care (Chic Ill) 2012; 11(1): 57-65.
5. Mutevedzi PC, Lessells RJ, Rodger AJ, Newell M-L. Association of Age with Mortality and Virological and Immunological Response to Antiretroviral Therapy in Rural South African Adults.PLoS One 2011; 6(7): e21795.
Footnotes
Contributors: MC undertook the literature search, designed the study, undertook the data analysis, generated the tables and figures, interpreted the results, wrote all drafts and finalised the manuscript. LFJ & MS provided statistical support. FT, MM, RW, HP, JG & KS managed the cohort and transferred routinely-collected data. LM was senior author, commenting on the study design, analyses and manuscript. All authors reviewed the final manuscript, approved the version to be published and agree to be accountable for all aspects of work in ensuring that questions related to accuracy and integrity of any part are appropriate investigated and resolved.
Conflicts of interest: The authors declare that there are no conflicts of interest.
References
- 1.World Health Organization. Active ageing: a policy framework. Geneva: 2005. [Google Scholar]
- 2.National Research Council (US) Committee on Population. The National Academies Collection: Reports funded by National Institutes of Health. In: Cohen B, Menken J, editors. Aging in Sub-Saharan Africa: Recommendation for Furthering Research. Washington (DC): National Academies Press (US), National Academy of Sciences; 2006. [PubMed] [Google Scholar]
- 3.Joubert J, Bradshaw D. Population Ageing and Health Challenges in South Africa. In: Steyn K, Fourie J, Temple N, editors. Chronic Diseases of Lifestyle in South Africa: 1995-2005. Cape Town: South African Medical Research Council; 2006. [Google Scholar]
- 4.Hontelez JAC, Lurie MN, Newell ML, Bakker R, Tanser F, Barnighausen T, et al. Ageing with HIV in South Africa. AIDS. 2011;25:1665–73. doi: 10.1097/QAD.0b013e32834982ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.South African National AIDS Council. Progress Report on the National Strategic Plan for HIV, TB and STIs (2012-2016) Pretoria: South African National AIDS Council; 2014. [Google Scholar]
- 6.Negin J, Barnighausen T, Lundgren JD, Mills EJ. Aging with HIV in Africa: the challenges of living longer. Aids. 2012;26(Suppl 1):S1–5. doi: 10.1097/QAD.0b013e3283560f54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendavid E, Ford N, Mills EJ. HIV and Africa's elderly: the problems and possibilities. AIDS. 2012;26(SupplementS1):S85–S91. doi: 10.1097/QAD.0b013e3283558513. [DOI] [PubMed] [Google Scholar]
- 8.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338 doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 9.Mutevedzi PC, Lessells RJ, Rodger AJ, Newell ML. Association of Age with Mortality and Virological and Immunological Response to Antiretroviral Therapy in Rural South African Adults. PLoS One. 2011;6(7):e21795. doi: 10.1371/journal.pone.0021795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aboderin IA, Beard JR. Older people's health in sub-Saharan Africa. Lancet. 2014 doi: 10.1016/S0140-6736(14)61602-0. [DOI] [PubMed] [Google Scholar]
- 11.Balestre E, Eholie SP, Lokossue A, Sow PS, Charurat M, Minga A, et al. Effect of age on immunological response in the first year of antiretroviral therapy in HIV-1-infected adults in West Africa. AIDS. 2012;26(8):951–7. doi: 10.1097/QAD.0b013e3283528ad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eduardo E, Lamb MR, Kandula S, Howard A, Mugisha V, Kimanga D, et al. Characteristics and Outcomes among Older HIV-Positive Adults Enrolled in HIV Programs in Four Sub-Saharan African Countries. PLoS ONE. 2014;9(7):e103864. doi: 10.1371/journal.pone.0103864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greig J, Casas EC, O'Brien DP, Mills EJ, Ford N. Association between older age and adverse outcomes on antiretroviral therapy: a cohort analysis of programme data from nine countries. AIDS. 2012;26(SupplementS1):S31–S7. doi: 10.1097/QAD.0b013e3283558446. [DOI] [PubMed] [Google Scholar]
- 14.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24(4):563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 15.Brinkhof MWG, Spycher BD, Yiannoutsos C, Weigel R, Wood R, Messou E, et al. Adjusting Mortality for Loss to Follow-Up: Analysis of Five ART Programmes in Sub-Saharan Africa. PLoS One. 2010;5(11):e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornell M, Technau K, Fairall L, Wood R, Moultrie H, van Cutsem G, et al. Monitoring the South African National Antiretroviral Treatment Programme, 2003-2007: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99(9):653–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. Journal of Clinical Epidemiology. 2013 doi: 10.1016/j.jclinepi.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schomaker M, Gsponer T, Estill J, Fox M, Boulle A. Non-ignorable loss to follow-up: correcting mortality estimates based on additional outcome ascertainment. Stat Med. 2014;33(1):129–42. doi: 10.1002/sim.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin D. Multiple imputation after 18+ years. J Am Stat Assoc. 1006;19:473–89. [Google Scholar]
- 20.van Buuren S, Boshuizen HC, DI K. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Shetty P. Grey matter: ageing in developing countries. Lancet. 2012;379(9823):1285–7. doi: 10.1016/s0140-6736(12)60541-8. [DOI] [PubMed] [Google Scholar]
- 22.Negin J, van Lettow M, Semba M, Martiniuk A, Chan A, Cumming RG. Anti-retroviral treatment outcomes among older adults in Zomba district, Malawi. PLoS One. 2011;6(10):e26546. doi: 10.1371/journal.pone.0026546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakanda C, Birungi J, Mwesigwa R, Ford N, Cooper CL, Au-Yeung C, et al. Association of aging and survival in a large HIV-infected cohort on antiretroviral therapy. AIDS. 2011;25(5):701–5. doi: 10.1097/QAD.0b013e3283437ed7. [DOI] [PubMed] [Google Scholar]
- 24.Greig J, Casas EC, O'Brien DP, Mills EJ, Ford N. Association between older age and adverse outcomes on antiretroviral therapy: a cohort analysis of programme data from nine countries. AIDS. 2012;26(Suppl 1):S31–7. doi: 10.1097/QAD.0b013e3283558446. [DOI] [PubMed] [Google Scholar]
- 25.UNAIDS. The Gap report. Geneva: Joint United Nations Programme on AIDS; 2014. [Google Scholar]
- 26.Negin J, Nemser B, Cumming R, Lelerai E, Ben Amor Y, Pronyk P. HIV attitudes, awareness and testing among older adults in Africa. AIDS Behav. 2012;16(1):63–8. doi: 10.1007/s10461-011-9994-y. [DOI] [PubMed] [Google Scholar]
- 27.Maskew M, Brennan A, MacPhail P, Sanne I, Fox MP. Poorer ART outcomes with increasing age at a large public sector HIV clinic in Johannesburg, South Africa. J Int Assoc Physicians AIDS Care (Chic Ill) 2012;11(1):57–65. doi: 10.1177/1545109711421641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balestre E, Eholie SP, Lokossue A, Sow PS, Charurat M, Minga A, et al. Effect of age on immunological response in the first year of antiretroviral therapy in HIV-1-infected adults in West Africa. Aids. 2012;26(8):951–7. doi: 10.1097/QAD.0b013e3283528ad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simbayi LC, Shisana O, Rehle T, Onoya D, Jooste S, Zungu N, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: 2014. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Olive FX, Angotti N, Houle B, Klipstein-Grobusch K, Kabudula C, Menken J, et al. Prevalence of HIV among those 15 and older in rural South Africa. AIDS Care. 2013;25(9):1122–8. doi: 10.1080/09540121.2012.750710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallrauch C, Barnighausen T, Newell ML. HIV prevalence and incidence in people 50 years and older in rural South Africa. S Afr Med J. 2010;100(12):812–4. doi: 10.7196/samj.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braitstein P, Boulle A, Nash D, Brinkhof MW, Dabis F, Laurent C, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health (Larchmt) 2008;17(1):47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 33.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9(9):e1001304. doi: 10.1371/journal.pmed.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muula AS, Ngulube TJ, Siziya S, Makupe CM, Umar E, Prozesky HW, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight L, McGrath N, van Rooyen H, Humphries H, van Heerden A, Richter L. Characteristics of sexually experienced HIV testers aged 18 to 32 in rural South Africa: baseline results from a community-based trial, NIMH Project Accept (HPTN 043) BMC Public Health. 2014;14(1):1164. doi: 10.1186/1471-2458-14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghidei L, Simone MJ, Salow MJ, Zimmerman KM, Paquin AM, Skarf LM, et al. Aging, antiretrovirals, and adherence: a meta analysis of adherence among older HIV-infected individuals. Drugs & aging. 2013;30(10):809–19. doi: 10.1007/s40266-013-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.