Abstract
Optical Coherence Tomography (OCT) is a powerful clinical tool that measures near infrared light backscattered from the eye and other tissues. OCT is used for assessing changes in retinal structure, including layer thicknesses, detachments and the presence of drusen in patient populations. Our custom-built OCT system for the mouse eye quantitatively images all layers of the neural retinal, the RPE, Bruchs’ membrane and the choroid. Longitudinal assessment of the same retinal region reveals that the relative intensities of retinal layers are highly stable in healthy tissue, but show progressive increases in intensity in a model of retinal degeneration. The observed changes in OCT signal have been correlated with ultrastructural disruptions that were most dramatic in the inner segments and nuclei of the rods. These early changes in photoreceptor structure coincided with activation of retinal microglia, which migrated vertically from the inner to the outer retina to phagocytose photoreceptor cell bodies (Levine et al. 2014). We conclude that quantitative analysis of OCT light scattering signals may be a useful tool for early detection and subcellular localization of cell stress prior to cell death, and for assessing the progression of degenerative disease over time. Future efforts to develop sensitive approaches for monitoring microglial dynamics in vivo may likewise elucidate earlier signs of cellular stress during retinal degeneration.
XX.1 Phototransduction signaling and photoreceptor degeneration
Despite the prevalence of photoreceptor degeneration in the general population, we know little about how photoreceptors die. In contrast, we know more about the biochemistry, physiology and cell biology of rod photoreceptors than of any other retinal cell type. Mutations in proteins that help to transduce light into electrical signals (phototransduction proteins) often cause prolonged electrical signaling but only degeneration in certain instances. For example, prolonged rod signaling that arises from defects in rhodopsin deactivation causes Oguchi disease and can lead to light-dependent degeneration (Paskowitz et al. 2006). In contrast, loss of the protein complex responsible for G protein deactivation (RGS9-1, Gβ5-L, and R9AP) also greatly prolongs signaling and causes visual impairment, but does not lead to photoreceptor damage (Nishiguchi et al. 2004). Other RP-related mutations in phototransduction proteins do not cause defective signaling per se, but rather cause protein misfolding (Tzekov et al. 2011). Although the unfolded protein response (UPR) itself causes apoptosis, degeneration resulting from theses mutations can be exacerbated by visible light (Paskowitz et al. 2006). To better understand the interplay between electrical signaling and the cell biology of degenerating photoreceptors, it is first essential to monitor photoreceptors longitudinally in vivo without exposure to the high intensity visible light typically used during fundus imaging (Cideciyan et al. 2005).
OCT, which uses the backscattering of near-infrared light to visualize retinal structure, allows longitudinal assessment of the same retinal region over time without exposure to visible light that would activate phototransduction and bleach visual pigment. When OCT images are not subject to auto-gain adjustments and are aligned with landmarks like retinal vessels, the back-scattered light increases within photoreceptor-specific layers during light-dependent degeneration (Cideciyan et al. 2005; Zam et al. 2013). In mice, such changes in light scattering can precede typical measures of photoreceptor cell loss like outer nuclear layer thickness, and have been correlated with ultrastructural disruptions in the inner segments and cell bodies (Levine et al. 2014). Further development of quantitative OCT methods may prove to be a useful tool for early detection of cell stress prior to cell death.
XX.2 Quantitative OCT light scattering measurements and their ultrastructural correlates
We have recently constructed a Fourier-Domain OCT (Fd-OCT) system for imaging the mouse eye (Zam et al. 2013). Like most OCT systems, ours uses a broad bandwidth near-infrared light source for a reference beam and a highly sensitive CMOS camera as the detector of the spectrometer. The detector captures the spectral power density of the reference beam, which is modulated by the interference arising from the light backscattered with varying delays from reflecting elements in the retina. To derive an A-scan, which is the retinal scattering profile as a function of depth (z), the Fd-OCT system computes the inverse Fourier transform of the measured spectral density function, yielding an intensity profile I(z), proportional to the square root of the intensity Isample(z) of the light backscattered from the sample at each depth (z) in the retina: thus, , where Iref is the (constant) average intensity of the reference beam (Wojtkowski 2010; Oldenburg et al. 2013). B-scans, I(x, z), are compounded of successive A-scans, where x is the lateral position in the retina. Commercial OCT systems typically display OCT B-scan data on a logarithmic (decibel) scale:
where S(x, z) is proportional to the display pixel value. In contrast, all analysis of our images was performed on 16-bit linear intensity B-scan data.
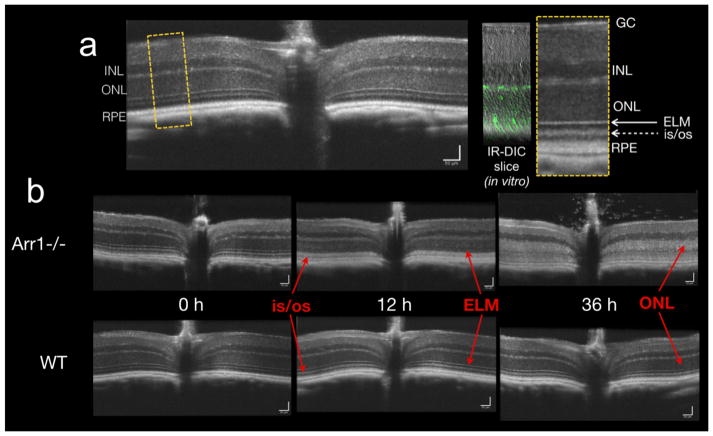
The average B-scan intensity value I(x,z) was measured from each retinal layer at precisely same eccentricity (x) over time in the same animal (Fig. XX.1a). In a mouse model of dim light damage (Arr1−/− mice; Xu et al. 1997; Chen et al. 1999) a profound increase in the intensity of the reflectance of the inner segment/outer segment border was apparent after 12 hours of light exposure. By 36 hrs, a 2-fold increase in intensity had spread to the outer nuclear layer, which corresponds to a 4-fold light scattering change within the retina itself (Fig. XX.1b). While these changes were consistently apparent in the B-scans across all animals examined, there were also small variations in absolute intensity across imaging sessions that arose from differences in alignment and other factors affecting image quality. The intensity of the INL reflectance, which was unaffected by light exposure, was used to normalize the light scattering changes in the photoreceptor layers, reducing the baseline intensity values for both WT and Arr1−/− strains (Levine et al. 2014).
Figure XX1. Increased OCT light scattering during light-induced photoreceptor degeneration.
a. B-scan of a WT (c57Bl/6J) mouse. Yellow dashed box is shown expanded on the right and compared to a retinal slice in which the cones express GFP (7m8-hLM-GFP) to help demark the photoreceptor layers. GC- ganglion cell layer; INL- inner nuclear layer; ONL – outer nuclear layer; ELM – external limiting membrane; is/os – inner segment/outer segment border; RPE – retinal pigment epithelium. b. Dark-reared Arr1−/− (Xu et al. 1997) and WT (c57Bl/6J) mice were imaged sequentially before and after the onset of 200 lux constant light. By 12 hrs, the ELM had disappeared and the is/os border showed increased scattering in the Arr1−/− mouse. By 36 hrs, the increased scattering had spread to the ONL and was correlated with increased chromatin condensation and related ultrastructural changes (Levine et al. 2014).
Rhodopsin mutant dogs have also shown acute increases in OCT light scattering with photoreceptor degeneration, which is dramatically accelerated by bright light (Cideciyan et al. 2005). The observed changes in light scattering were restricted to illuminated retinal regions and did not progressively enlarge over time, though the extent and time course of degeneration depended on light intensity. In this model, the changes in light scattering were evident within one hour and seemed to initiate at the inner segment/outer segment border and spread over time to the outer nuclear layer and beyond. A similar trend but different time scale was observed in the Arr1−/− OCT images and was correlated with ultrastructural changes within the photoreceptors themselves (Fig. XX.1b and Levine et al. 2014).
It is not known whether the slower progression of degeneration common in most human retinal degenerations can likewise be detected with longitudinal OCT intensity comparisons. One challenge for the future will be to test the limits of OCT imaging sensitivity in other animal models of degeneration that proceed with different rates and from different retinal loci. In adapting this approach to the clinic, it will also be important to further develop and distribute computational tools for image analysis that allow post-hoc image registration without intensity normalization, which most commercial platforms currently hard-wire into their imaging systems.
XX.3 Monitoring microglial dynamics during photoreceptor degeneration in vivo
In all forms of retinal damage and degeneration, activated phagocytic monocytes like microglia (resident in CNS tissue) and macrophages (infiltrated from the circulation) can exacerbate the loss of neural tissue (Streit et al. 2004). Microglia are the first responders to disease and injury in the retina, transforming from a highly dynamic, branched resting state to an amoeboid, activated state along a continuum of stages that may or may not be reversible and memoryless, depending on the severity and duration of the insult (Block et al. 2007; Langmann 2007). In Arr1−/− mice, microglia vertically migrate to the ONL and begin to engulf photoreceptor somata within 12 hrs of light exposure (Levine et al., 2014). Thus, visualizing microglial dynamics in intact retinal tissue could be a sensitive biological indicator for early signs of cell stress.
A common tool for imaging living microglia is a commercially available strain in which GFP has been knocked in to the fractalkine receptor locus (Cx3cr1GFP/GFP; Jackson Labs 005582). However, the loss of CX3CR1 expression in the knock-in mutant does reduce microglial dynamics (Liang et al. 2009). Moreover, homozygous Cx3cr1 knockout mice show photoreceptor degeneration and the accumulation of phagocytic monocytes in the subretinal space (Combadière et al. 2007). While heterozygous (Cx3cr1GFP/+) mice appear to have normal microglial behavior and are thus may be a convenient tool for answering certain questions about neuroinflammation in the retina, developing acute means for labeling microglia in vivo offers advantages for live tissue imaging in all species.
Viral transduction of microglia is a well-suited alternative for retinal studies because full access to the posterior eye can now be achieved by a single intravitreal injection (Dalkara et al. 2013). Microglial cells in the brain have been successfully infected with AAV2 or 5 using the promoters of F4/80 and CD11b, resulting in varying levels of expression (Cucchiarini et al. 2003). Low efficiency viral transductions would facilitate in vivo microglia imaging by making it more likely that a single microglia could be individually followed over an extended period (Cucchiarini et al. 2003). Indeed, using SLO and AO-SLO imaging methods in the mouse, it is now possible to follow the activation and migration of a microglial cells in vivo (Fig. XX.2a-c; Alt et al. 2014).
Figure XX2. Imaging retinal microglia.
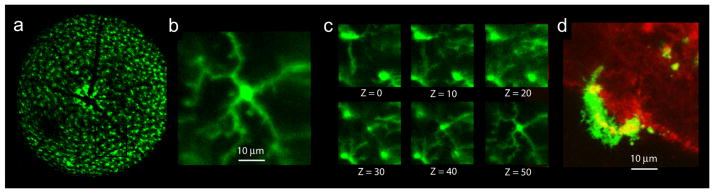
a. Wide-field SLO image of the retina of a live Cx3cr1GFP/+ mouse. b. AO-SLO of a single microglia cell from a. c. Six images of the AO-SLO z-stack from which b (Z = 50) was taken, spanning 50 μm of the IPL (~ 0.5 mm per Z-step). d. Ex vivo confocal image of an Arr1−/− Cx3cr1GFP/+ retinal explant: the microglia cell engulfed WGA-conjugated quantum dots (red), which accumulated within highly motile intracellular lysosomes (yellow).
Quantum dots, which are readily phagocytosed by microglia and macrophages, are another adaptable means for achieving cell-specific labeling (Jackson et al. 2007; Minami et al. 2012). In ex vivo retinal flatmounts, wheat germ agglutinin (WGA) conjugated quantum dots label rods, and can be become concentrated within the lysosomes of dynamic microglia (Fig. XX.2d). Thus, quantum dots tagged with specific cell-surface ligands may be a way to preferentially label active phagocytes targeting a specific cell type, providing a way to specifically detect regions of active phagocytosis, both ex vivo or in vivo.
XX.4 Conclusion
Applying ocular imaging approaches like OCT and AO-SLO to the mouse retina presents exciting opportunities for further developing methods like quantitative light scatter and microglial imaging, which could lead to earlier detection of cell stress and degeneration. Combined with the power of mouse genetics, viral transfection methods, and numerous available models of retinal degeneration, the mouse provides new avenues for studying the interplay between degeneration and inflammation, as well as the basic biology of normal retinal physiology and homeostasis across an individual’s lifetime.
References
- Alt C, Runnels JM, Mortensen LJ, et al. In vivo imaging of microglia turnover in the mouse retina after ionizing radiation and dexamethasone treatment. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.14-14254. IOVS-14-14254. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Chen J, Simon MI, Matthes MT, et al. Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness) Invest Ophthalmol Vis Sci. 1999;40:2978–2982. [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Aleman TS, et al. In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc Natl Acad Sci U S A. 2005;102:5233–5238. doi: 10.1073/pnas.0408892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadière C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiarini M, Ren X, Perides G, et al. Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors. Gene Ther. 2003;10:657–667. doi: 10.1038/sj.gt.3301925. [DOI] [PubMed] [Google Scholar]
- Dalkara D, Byrne LC, Klimczak RR, et al. In Vivo-Directed Evolution of a New Adeno-Associated VIrus for Therapeutic Outer Retinal Gene Delivery from the Vitreous. Sci Transl Med. 2013;5:189ra176. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- Jackson H, Muhammad O, Daneshvar H, et al. Quantum dots are phagocytized by macrophages and colocalize with experimental gliomas. Neurosurgery. 2007;60:524–530. doi: 10.1227/01.NEU.0000255334.95532.DD. [DOI] [PubMed] [Google Scholar]
- Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- Levine ES, Zam A, Zhang P, et al. Rapid light-induced activation of retinal microglia in mice lacking Arrestin–1. Vis Res. 2014 doi: 10.1016/j.visres.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KJ, Lee JE, Wang YD, et al. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci. 2009;50:4444–4451. doi: 10.1167/iovs.08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami SS, Sun B, Popat K, et al. Selective targeting of microglia by quantum dots. J Neuroinflammation. 2012;9:22. doi: 10.1186/1742-2094-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi KM, Sandberg MA, Kooijman AC, et al. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature. 2004;427:75–78. doi: 10.1038/nature02170. [DOI] [PubMed] [Google Scholar]
- Oldenburg AL, Chherti RK, Cooper JM, et al. Motility-, autocorrelation-, and polarized-sensitive optical coherence tomography discriminates cells and gold nanorods within 3D tissue cultures. Opt Lett. 2013;38:2923–2926. doi: 10.1364/OL.38.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskowitz DM, LaVail MM, Duncan JL. Light and inherited retinal degeneration. B J Ophthalmol. 2006;90:1060–1066. doi: 10.1136/bjo.2006.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WST. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzekov R, Stein L, Kaushal S. Protein Misfolding and Retinal Degeneration. Cold Spring Harb Perspect Biol. 2011;3:a007492. doi: 10.1101/cshperspect.a007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Neumann H. Role of microglia in neuronal degeneration and regeneration. Semin Immunopathol. 2009;31:513–525. doi: 10.1007/s00281-009-0180-5. [DOI] [PubMed] [Google Scholar]
- Wojtkowski M. High-speed optical coherence tomography: basics and applications. Appl Opt. 2010;49:D30–D61. doi: 10.1364/AO.49.000D30. [DOI] [PubMed] [Google Scholar]
- Xu J, Dodd RL, Makino CL, et al. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389:505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- Zam A, Dsouza R, Subhash HM, et al. Feasibility of correlation mapping optical coherence tomography (cmOCT) for anti-spoof sub-surface fingerprinting. J Biophotonics. 2013;6:663–667. doi: 10.1002/jbio.201200231. [DOI] [PubMed] [Google Scholar]