Abstract
BACKGROUND
Since the introduction of effective vaccines, the incidence of invasive Haemophilus influenzae type b (Hib) disease among children <5 years of age has decreased by 99% in the United States. In response to a limited vaccine supply that began in 2007, Hib booster doses were deferred for 18 months.
METHODS
We reviewed national passive and active surveillance (demographic and serotype) and vaccination status data for invasive H. influenzae disease in children aged <5 years before (1998–2007) and during (2008–2009) the vaccine shortage years to assess the impact of the vaccine deferral on Hib disease. We estimated the average annual number of Hib cases misclassified as unknown (not completed or missing) serotype.
RESULTS
From 1998 to 2007 and 2008 to 2009, the annual average incidence of Hib disease per 100 000 population was 0.2 and 0.18, respectively; no significant difference in incidence was found by age group, gender, or race. Among Hib cases in both time periods, most were unvaccinated or too young to have received Hib vaccine. During 2001 to 2009, there were <53 Hib cases per year, with an estimated 6 to 12 Hib cases misclassified as unknown serotype.
CONCLUSIONS
The booster deferral did not have a significant impact on the burden of invasive Hib disease in children <5 years of age. Continued surveillance and serotype data are important to monitor changes in Hib incidence, especially during vaccine deferrals. Hib booster deferral is a reasonable short-term approach to a Hib vaccine shortage.
Keywords: Haemophilus influenzae, Haemophilus influenzae type b, United States, epidemiology, children, vaccine-preventable diseases
In the United States, Haemophilus influenzae type b (Hib) was once the leading cause of bacterial meningitis and a common cause of other invasive diseases (eg, epiglottitis, pneumonia, and bacteremia) among children aged <5 years.1 With the introduction of the Hib vaccines between 1985 and 1990, the incidence of invasive Hib disease in children aged <5 years decreased by 99%.2–6
In December 2007, 1 manufacturer of 2 Hib conjugate vaccines voluntarily recalled certain lots because of questions concerning the sterility of equipment used during their manufacture.7 Production of both vaccines was suspended, temporarily limiting Hib vaccine supply in the United States.7 To ensure that all US children were able to complete the primary Hib vaccination series,* on December 18, 2007, the Centers for Disease Control and Prevention (CDC) recommended deferral of the Hib vaccine booster dose (administered at 12–15 months of age) for all children except those at increased risk for invasive Hib disease, including American Indian/Alaskan native children and children with certain immunosuppressive conditions.7 In July 2009, production of 2 Hib vaccines was increased by another manufacturer to address the limited vaccine supply; as a result, on July 26, 2009, administration of the Hib booster dose was reinstated for all children at their next medical visit.8 In August 2009, a new Hib vaccine was licensed for use as a booster dose.9 Subsequently, providers were recommended to contact all children with deferred Hib booster doses and offer catch-up vaccination in September 2009.9 The 2 recalled Hib vaccines returned to the market between January and August 2010.10,11
The prolonged Hib deferral raised concerns about possible increases in Hib transmission and disease in the United States. This article provides a review of surveillance and vaccination status data for H. influenzae, including Hib, in children aged <5 years during the 10 years before the vaccine shortage and during the 18-month shortage period to determine whether the incidence or epidemiology of Hib disease was affected.
METHODS
We used 2 data sources to document invasive Hib disease in children aged <5 years between January 1, 1998 and December 31, 2009. The first was the National Notifiable Diseases Surveillance System (NNDSS), a passive surveillance system that provides reports of notifiable diseases, including H. influenzae, from 52 jurisdictions (50 states, DC, and New York city); data are electronically transmitted from states to the CDC on a weekly basis.12,13 Between November 2008 and December 2009, NNDSS data were supplemented with active follow-up with state health departments. CDC personnel contacted state health departments that reported cases of H. influenzae in children aged <5 years within 1 week of receipt of the case report at the CDC to collect missing/unknown or incomplete data. States without any reported H. influenzae cases also were contacted periodically to confirm reporting of 0 cases. All 52 state health departments were contacted during this period.
The second data source was the Active Bacterial Core surveillance (ABCs) system, an active laboratory-and population-based surveillance system that provides monthly reports of H. influenzae cases from all or parts of 10 states in the United States.14 ABCs is supported by the CDC as part of its Emerging Infections Program network.14,15 The population under surveillance ranged from 26 514 662 in 1998 to 36 748 349 in 2009 (representing 9.8% of the US population in 1998 and 12.0% in 2009). Duplicate records were deleted from NNDSS and ABCs data sets and data were merged by birth date and county and state codes.
In both systems, a case was defined as isolation of H. influenzae from a normally sterile body site (eg, blood or cerebrospinal fluid) in a person aged <5 years. Illness outcome was based on patient status at the time of hospital discharge. Serotyping of H. influenzae was performed by using slide agglutination or polymerase chain reaction. All isolates from ABCs sites were sent to the CDC, where serotype was confirmed by using slide agglutination, Haemophilus quad identification plates or API Neisseria-Haemophilus strips, and polymerase chain reaction.16–18 The CDC result was used as the final serotype in the ABCs data set. For NNDSS serotype results confirmed through active calling, the confirmed serotype result was used in the merged data set; if the serotype result was missing or unknown, the ABCs serotype was used. For all other cases that were in both the NNDSS and ABCs data sets and had discordant serotype results, the serotype result from the ABCs data set was used in the merged data set. In this analysis, H. influenzae isolates were classified based on their serotype: serotype b (Hib), nontypeable (non-encapsulated), and non-b (serotypes a, c, d, e, and f).
Calculations of Hib incidence using data from the NNDSS are affected by unknown serotype data. Serotype data may be unknown if serotyping is not conducted, or data may be missing or misclassified during transmission from states to the CDC. Hib cases may be misreported as an unknown serotype, underestimating the true burden of disease. The proportion of Hib cases from among all H. influenzae cases in ABCs data was used to estimate the annual number of Hib cases misclassified as unknown serotype for the time period 2001 to 2009; these years were chosen because the number of reported Hib cases remained relatively stable at <50 cases per year. We multiplied the proportion of Hib cases from pooled ABCs data for 2001 to 2009 by the total number of cases with unknown serotype each year to calculate the annual estimated number of Hib cases misreported as unknown serotype. Owing to small case numbers, we were unable to calculate estimates using ABCs data by age group or individual year.
Data were analyzed by using SAS version 9.2 (SAS Institute, Inc, Cary, NC) and StatXact (Cytel, Inc, Cambridge, MA). Data from 1998 to 2007 and 2008 to 2009 were defined as the preshortage and shortage periods, respectively. Calculations of serotype discrepancies between NNDSS and ABCs data sets were limited to the time period of 2002 to 2009. Incidence rates were calculated by using US census data.19 Incidence rates are reported as cases per 100 000 population. Two-sided P values using Fisher’s exact test were calculated for differences in disease rates, vaccine status, and percent of jurisdictions with unknown serotype data between preshortage and shortage years. Confidence intervals around the rate differences were calculated by using methods described by Miettinen and Nurminen.20 A McNemar test for matched pairs was used for differences in number of cases with known serotype in 2009. The case-fatality ratio was calculated by using the proportion of cases with known outcomes as the denominator. Because vaccine data before 2002 in the merged NNDSS/ABCs data set were not complete, vaccination status of Hib cases during pre-shortage years was calculated only for 2002 to 2007.
RESULTS
H. influenzae Cases
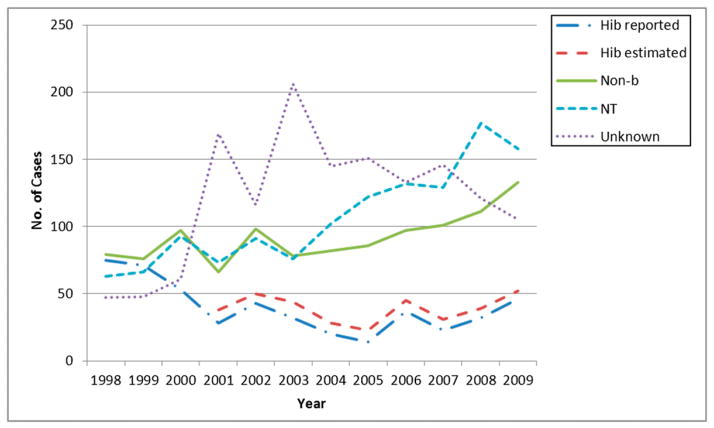
In the preshortage period (1998–2007), there were 3425 reported cases of H. influenzae in children aged <5 years, of which 396 (12%) were Hib, 947 (28%) were nontypeable (NT), 860 (25%) were non-b, and 1222 (36%) were H. influenzae with an unknown (not typed, missing, or not transmitted) serotype. A median of 35 Hib (range, 14–75), 92 NT (range, 63–132), 84 non-b (range, 66–101), and 139 unknown serotype (range, 47–206) cases occurred each year (Fig 1). In the shortage period (2008–2009), there were 883 reported cases of H. influenzae in children <5 years of age, of which 78 (9%) were Hib, 335 (38%) were NT, 244 (28%) were non-b, and 226 (26%) were H. influenzae with an unknown serotype. A median of 39 Hib (range, 32–46), 168 NT (range, 158–177), 122 non-b (range, 111–133), and 113 unknown serotype cases (range, 105–121) occurred each year (Fig 1).
FIGURE 1.
Number of H. influenzae cases aged <5 years, per year, by serotype, 1998–2009.
In the serotype discrepancy analysis for 2002 to 2009, 3143 H. influenzae cases were reported. Of these, 2602 cases (83%) were identified by NNDSS alone, 34 (1%) by ABCs alone, and 507 (16%) by both surveillance systems. Among these 507 cases, 157 (31%) had discrepant serotype results. Of these, 125 (80%) and 8 (5%) had an unknown serotype in NNDSS and ABCs, respectively. Four cases reported as Hib in NNDSS were reported as NT (n = 3) or unknown (n = 1) in ABCs; 8 cases reported as Hib in ABCs were reported as non-b (n = 2) or unknown (n = 6) in NNDSS.
At least 1 case of H. influenzae in children aged <5 years was reported in a mean of 43 jurisdictions per year in the preshortage period, in 46 jurisdictions during 2008, and in 45 jurisdictions in 2009. On average, 36% of jurisdictions had unknown serotype data for ≥50% of cases <5 years of age in the preshortage period. In 2008, 13 (29%) had unknown serotype data for ≥50% of cases. During 2009, when active calling of state health departments to solicit unknown serotype data occurred, 9 (20%) had unknown serotype data for ≥50% of reported cases. These differences were not statistically significant (P = .419). In 2009, serotype data were missing or unknown for 191 of 407 H. influenzae cases (47%) in children aged <5 years reported through NNDSS. Serotype results were identified for 124 of these cases (65%) through active calling. After active calling, the number of H. influenzae cases <5 years of age with a known serotype increased significantly from 216 (53%) to 340 (84%) (P < .001).
Hib Cases
In the preshortage and shortage periods, the incidence of Hib disease was highest among infants aged <1 year and lowest among children aged 2 to 4 years (Table 1). The incidence of Hib disease was highest among American Indian/Alaskan native children (Table 1). The case-fatality rate was higher during the shortage period as compared with the preshortage period (14% and 5%, respectively; P = .05). All deaths in the preshortage period occurred among children aged <1 year. During the shortage period, 4 deaths occurred among children aged <1 year, 2 deaths among children aged 1 year, and 4 deaths among children aged 2 to 4 years. There was no statistically significant difference in incidence rates of Hib disease by age groups, gender, or race between the preshortage and shortage periods (Table 1).
TABLE 1.
Demographic Characteristics of Hib Cases Aged <5 y, 1998–2009
| 1998–2007 (n= 396)
|
2008–2009 (n= 78)
|
P | |||
|---|---|---|---|---|---|
| N | Incidencea | N | Incidencea | ||
| Age, y | |||||
| <1 | 245 | 0.61 | 46 | 0.54 | .42 |
| 1 | 76 | 0.19 | 13 | 0.15 | .43 |
| 2–4 | 75 | 0.06 | 19 | 0.08 | .50 |
| <5 | 396 | 0.20 | 78 | 0.18 | .50 |
| Male gender | 210 | 0.21 | 44 | 0.20 | .90 |
| Raceb | |||||
| White | 245 | 0.16 | 46 | 0.14 | .52 |
| Black | 34 | 0.11 | 13 | 0.20 | .06 |
| AI/AN | 39 | 1.40 | 5 | 0.80 | .23 |
| Asian Pacific | 10 | 0.11 | 0 | 0 | .13 |
| Outcome | |||||
| Died (CFRc) | 21 (5.1%) | 10 (14.2%) | .05 | ||
AI/AN, American Indian/Alaskan native.
Annual average, per 100 000 persons.
Other or unknown race (n = 68 for 1998–2007 and 14 for 2008–2009).
Case-fatality ratio, among cases with known outcome.
By using a multiplier of 5.8%, we estimated that the average annual number of Hib cases misclassified as unknown serotype ranged from 6 to 12 cases between 2001 and 2009. Combining the reported number of Hib cases with the estimated number of missed Hib cases per year, a median of 39 (range, 23–52) Hib cases occurred per year between 2001 and 2009 (Fig 1). Estimated incidence rates between 2001 and 2009 ranged from 0.11 to 0.26, in comparison with reported incidence rates, which ranged from 0.07 to 0.22.
Vaccination Status Among Hib Cases
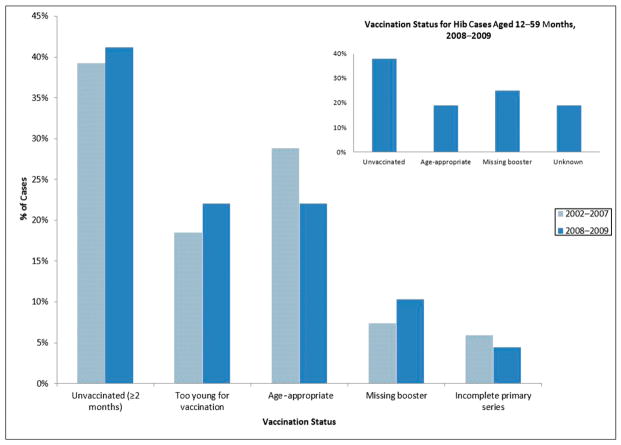
From 2002 to 2007, 169 cases of Hib occurred in children aged <5 years. Among these cases, 34 (20%) had unknown vaccination status. Of the 135 cases with known vaccine status, most were either eligible but unvaccinated (had not received any doses of Hib vaccine) or were too young (aged <2 months) to have received any Hib vaccine doses. In 18 cases (13%), children were behind schedule because of an incomplete primary series or a missing booster dose (Fig 2). Among Hib cases in children aged ≥2 months, 71 (65%) were behind schedule or unvaccinated.
FIGURE 2.
Vaccine status for Hib cases aged <5 years, 2002–2009, among those with known vaccine status. No statistically significant difference existed between vaccination status across the different time periods.
From 2008 to 2009, 78 cases of Hib occurred in children aged <5 years. Among these cases, 10 (13%) had unknown vaccine status. Of the 68 cases with known vaccine status, most were unvaccinated or were too young to have received any Hib vaccine doses. Ten cases (15%) were behind schedule owing to an incomplete primary series or a missing booster dose (Fig 2). Among Hib cases in children aged ≥2 months (eligible for Hib vaccine), 38 (72%) were behind schedule or unvaccinated. Based on their age (12–59 months), 32 children should have had their booster dose deferred. Of these, 6 (19%) had an unknown vaccine status, 6 (19%) were age-appropriately vaccinated, and 12 (38%) were unvaccinated. Eight (25%) had completed the primary series but were missing the booster dose; based on age at onset of disease, 4 of these cases could have received a booster dose before the shortage, and 4 would have been deferred a booster dose (Fig 2). There was no statistical difference between the vaccine status of Hib cases between the preshortage and shortage periods.
During the shortage period, the median Hib incidence rate among all jurisdictions was 0.12 (range, 0–1.79); 5 states had an incidence rate ≥0.85. Minnesota was the only state that experienced an increase in Hib cases in children who were <5 years of age during the shortage,21 with an incidence rate of 0.83. Excluding the 6 Minnesota cases from the 2008–2009 national data did not significantly change the national incidence rates or case-fatality rates of Hib disease, vaccine status of Hib cases, or estimates of Hib cases misclassified as unknown serotype.
DISCUSSION
Based on our review of national Hib reporting, the Hib vaccine shortage did not significantly alter the national incidence or epidemiology of invasive Hib disease in children aged <5 years. Rates of Hib disease overall and by age group, gender, and race did not differ during the vaccine shortage as compared with the 10 years before the shortage, and there was no significant difference in vaccination status among Hib cases before or during the shortage. The case-fatality rate was higher during the shortage period; however, the difference is not explained by a change in disease incidence or vaccination status among cases. If the deferral resulted in a decreased “cushion of protection” from herd immunity, we might have expected increased mortality in children <1 year old, the highest-risk age group; instead, most deaths during the shortage occurred in children aged ≥1 year. In addition, the case-fatality rate (CFR) among reported Hib cases after the shortage (CDC 2010 data) was 13%, which is comparable with the CFR during the shortage.
Few data exist on the impact of prolonged Hib vaccine shortages on rates of Hib disease. Experience from the United Kingdom suggests that rates of invasive Hib disease increase after prolonged periods without booster doses. Seven years after the introduction of a 3-dose primary Hib series without a booster dose, rates of Hib disease increased in vaccinated children in the United Kingdom. A sustained and dramatic decrease in Hib disease occurred after a booster dose was recommended.22,23 A mathematical model of Hib disease in the United States during a vaccine shortage predicts Hib incidence would not change significantly with temporary shortages, but it would likely begin increasing 3 years after the start of a shortage.24 Our findings are consistent with this model.
High vaccination coverage (93%)†25 at the beginning of the shortage26 likely provided a cushion of protection against rapid increases in Hib disease. Hib vaccination leads to decreases in oropharyngeal colonization among both vaccinated and unvaccinated children,27–29 reducing the risk of disease transmission. Although some states had declines in coverage with the Hib vaccine primary series during the shortage, the cohort of unprotected children remained small.30 Although the number of Hib cases increased above baseline in Minnesota during the booster deferral, a subsequent carriage study among 1631 children aged <5 years found no Hib carriage,31 suggesting no increase in disease transmission. These data suggest that the increase in Hib cases was likely not a direct result of the vaccine shortage.
The vaccination status of Hib cases from 1998 to 2009 highlights the continued risk of invasive disease among unvaccinated and under vaccinated children. Before and during the vaccine shortage, more than half of Hib cases in children aged ≥2 months (eligible for Hib vaccine) were either in unvaccinated children or in those behind schedule. Hib vaccine efficacy is high, ranging from 93% to 100% after the 3-dose primary series,32–35 and invasive Hib disease after full vaccination with the primary series and booster dose (vaccine failure) is rare.35–37 Between 1996 and 2001, vaccine failure surveillance in Israel, Australia, and 10 countries in Europe identified 330 cases of invasive Hib disease in children who had completed a 3-dose primary series and only 34 cases in children who had received a 3-dose primary series and a booster dose,37 illustrating the importance of full vaccination in preventing Hib disease.
Although rare, Hib disease still occurs despite high vaccination coverage and low carriage prevalence in children, suggesting continued circulation and transmission of Hib,31 albeit at very low levels. Reaching the 2010 Healthy People Objective of 0 cases of Hib disease in children aged <5 years may not be realistic.38 Instead, efforts should focus on maintaining current low rates of Hib disease through high vaccination coverage and appropriate chemoprophylaxis for contacts of cases. This focus is reflected in the 2020 Healthy People Objectives, which has set an Hib incidence rate of 0.27 cases per 100 000 children aged <5 years as the new goal.39
Continued H. influenzae surveillance is important so that Hib cases are identified and appropriate chemoprophylaxis measures can be taken. Incomplete serotype data at the national level hamper surveillance efforts, however. Serotype data were missing for 47% of H. influenzae cases <5 years of age reported by NNDSS in 2009; after active calling of health departments to solicit missing information, 84% of cases had known serotype results. Data transmission difficulties likely explain a substantial proportion of the missing serotype data at the national level; however, active calling of health departments by the CDC to solicit data on an ongoing basis is not a practical solution. Electronic surveillance systems should be assessed and modified to improve data transmission. Health departments should continue to focus on ensuring that all isolates from cases of invasive H. influenzae disease, especially those in children <5 years of age, are serotyped by using slide agglutination or genotyped by using polymerase chain reaction.40 Our estimates of Hib cases missed because of incomplete serotype information suggest we are likely underestimating the true burden of Hib disease, but not to a large degree, because the annual number of Hib cases, including the estimated number of cases missed, has remained <53 since 2001.
CONCLUSIONS
These findings provide evidence that the incidence and epidemiology of invasive Hib disease in children aged <5 years was not significantly impacted by the vaccine shortage, and a booster dose deferral is a reasonable approach to Hib vaccine shortages in the short-term. Prolonged shortages, however, may have a larger impact on Hib disease and require alternative strategies.
WHAT’S KNOWN ON THIS SUBJECT
Since the introduction of effective vaccines in the United States, the incidence of invasive Haemophilus influenzae type b (Hib) disease in children aged <5 years has decreased by 99%. In 2007, in response to limited vaccine supply, Hib booster doses were deferred for 18 months.
WHAT THIS STUDY ADDS
This review found no significant change in the incidence of invasive Hib disease in the United States during the booster dose deferral period, suggesting that booster dose deferral is a reasonable approach to Hib vaccine shortages in the short-term.
Acknowledgments
FUNDING: No external funding.
We thank the local and state health departments, the Emerging Infections Program staff, and the CDC ABCs program staff for all their contributions, especially those who contributed during active solicitation for missing data in 2009. We also thank Brian D. Plikaytis and Elizabeth Zell of the Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC for their contributions.
ABBREVIATIONS
- ABCs
Active Bacterial Core surveillance
- CDC
Centers for Disease Control and Prevention
- Hib
Haemophilus influenzae type b
- NNDSS
National Notifiable Diseases Surveillance System
- NT
nontypeable
Footnotes
The recommended Hib primary vaccination series consists of 3 doses given at 2, 4, and 6 months of age or 2 doses given at 2 and 4 months of age, depending on the vaccine type used.
Hib vaccine coverage measurements before 2009 measured 3 or more doses of a Hib-containing vaccine, not taking into account the type of vaccine received. These measurements likely underestimated primary series coverage and overestimated primary series plus booster coverage.25
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Wenger JD, Hightower AW, Facklam RR, Gaventa S, Broome CV The Bacterial Meningitis Study Group. Bacterial meningitis in the United States, 1986: report of a multi-state surveillance study. J Infect Dis. 1990;162(6):1316–1323. doi: 10.1093/infdis/162.6.1316. [DOI] [PubMed] [Google Scholar]
- 2.Bisgard KM, Kao A, Leake J, Strebel PM, Perkins BA, Wharton M. Haemophilus influenzae invasive disease in the United States, 1994–1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis. 1998;4(2):229–237. doi: 10.3201/eid0402.980210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams WG, Deaver KA, Cochi SL, et al. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA. 1993;269(2):221–226. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1987–1995. MMWR Morb Mortal Wkly Rep. 1996;45(42):901–906. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Progress toward eliminating Haemophilus influenzae type b disease among infants and children—United States, 1987–1997. MMWR Morb Mortal Wkly Rep. 1998;47(46):993–998. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children—United States, 1998–2000. MMWR Morb Mortal Wkly Rep. 2002;51(11):234–237. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Interim recommendations for the use of Haemophilus influenzae type b (Hib) conjugate vaccines related to the recall of certain lots of Hib-containing vaccines (PedvaxHIB and Comvax) MMWR Morb Mortal Wkly Rep. 2007;56(50):1318–1320. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Updated recommendations for use of Haemophilus influenzae type b (Hib) vaccine: reinstatement of the booster dose at ages 12–15 months. MMWR Morb Mortal Wkly Rep. 2009;58(24):673–674. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Licensure of a Haemophilus influenzae type b (Hib) vaccine (Hiberix) and updated recommendations for use of Hib vaccine. MMWR Morb Mortal Wkly Rep. 2009;58(36):1008–1009. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. [Accessed July 18, 2011];Immunization Works! 2010 February 2010 Issue. Available at: www.cdc.gov/vaccines/news/newsltrs/imwrks/2010/201002.htm.
- 11.Food and Drug Administration. [Accessed July 18, 2011];Biologic Product Shortages. 2011 Available at: www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Shortages/default.htm.
- 12.Centers for Disease Control and Prevention. [Accessed November 14, 2011];National Notifiable Diseases Surveillance System. Available at: www.cdc.gov/osels/ph_surveillance/nndss/nndsshis.htm.
- 13.Centers for Disease Control and Prevention. [Accessed November 14, 2011];National Electronic Telecommunications System for Surveillance. Available at: www.cdc.gov/osels/ph_surveillance/nndss/netss.htm.
- 14.Centers for Disease Control and Prevention. [Accessed January 12, 2011];Active Bacterial Core surveillance (ABCs) Available at: www.cdc.gov/abcs/overview/index.html.
- 15.Schuchat A, Hilger T, Zell E, et al. Active Bacterial Core Surveillance Team of the Emerging Infections Program Network. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis. 2001;7(1):92–99. doi: 10.3201/eid0701.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Mair R, Hatcher C, et al. Detection of bacterial pathogens in Mongolia meningitis surveillance with a new real-time PCR assay to detect Haemophilus influenzae. Int J Med Microbiol. 2011;301(4):303–309. doi: 10.1016/j.ijmm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Pittman M. Variation and type specificity in the bacterial species Hemophilus influenza. J Exp Med. 1931;53(4):471–492. doi: 10.1084/jem.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenza. 2. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 19. [Accessed August 10, 2010];CDC Wonder: Census Population Information. Available at: http://wonder.cdc.gov/population.html.
- 20.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4(2):213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Invasive Haemophilus influenzae Type B disease in five young children—Minnesota, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(3):58–60. [PubMed] [Google Scholar]
- 22.Ladhani S, Slack MP, Heys M, White J, Ramsay ME. Fall in Haemophilus influenzae serotype b (Hib) disease following implementation of a booster campaign. Arch Dis Child. 2008;93(8):665–669. doi: 10.1136/adc.2007.126888. [DOI] [PubMed] [Google Scholar]
- 23.Trotter CL, Ramsay ME, Slack MP. Rising incidence of Haemophilus influenzae type b disease in England and Wales indicates a need for a second catch-up vaccination campaign. Commun Dis Public Health. 2003;6(1):55–58. [PubMed] [Google Scholar]
- 24.Jackson ML, Rose CE, Cohn A, et al. Modeling insights into Haemophilus influenzae type b disease, transmission, and vaccine programs. Emerg Infect Dis. 2012;18(1):13–20. doi: 10.3201/eid1801.110336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Changes in measurement of Haemophilus influenzae serotype b (Hib) vaccination coverage—National Immunization Survey, United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(33):1069–1072. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) [Accessed November 24, 2010];Estimated vaccination coverage with individual vaccines and selected vaccination series among children 19–35 months of age by state and local area, July 2006–June 2007. Available at: www.cdc.gov/vaccines/stats-surv/nis/data/tables_0607.htm.
- 27.Mohle-Boetani JC, Ajello G, Breneman E, et al. Carriage of Haemophilus influenzae type b in children after widespread vaccination with conjugate Haemophilus influenzae type b vaccines. Pediatr Infect Dis J. 1993;12(7):589–593. doi: 10.1097/00006454-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Takala AK, Eskola J, Leinonen M, et al. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis. 1991;164(5):982–986. doi: 10.1093/infdis/164.5.982. [DOI] [PubMed] [Google Scholar]
- 29.Barbour ML. Conjugate vaccines and the carriage of Haemophilus influenzae type b. Emerg Infect Dis. 1996;2(3):176–182. doi: 10.3201/eid0203.960303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santibanez TA, Shefer A, Briere EC, Cohn AC, Groom AV. Effects of a nationwide Hib vaccine shortage on vaccination coverage in the United States. Vaccine. 2012;30(5):941–947. doi: 10.1016/j.vaccine.2011.11.075. [DOI] [PubMed] [Google Scholar]
- 31.Lowther SA, Shinoda N, Juni BA, et al. Hib Survey Team. Haemophilus influenzae type b infection, vaccination, and H. influenzae carriage in children in Minnesota, 2008–2009. Epidemiol Infect. 2012;140(3):566–574. doi: 10.1017/S0950268811000793. [DOI] [PubMed] [Google Scholar]
- 32.Recommendations for use of Haemophilus b conjugate vaccines and a combined diphtheria, tetanus, pertussis, and Haemophilus b vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1993;42(RR-13):1–15. [PubMed] [Google Scholar]
- 33.Haemophilus b conjugate vaccines for prevention of Haemophilus influenzae type b disease among infants and children two months of age and older: recommendations of the ACIP. MMWR Recomm Rep. 1991;40(RR-1):1–7. [PubMed] [Google Scholar]
- 34.Heath PT. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr Infect Dis J. 1998;17(suppl 9):S117–S122. doi: 10.1097/00006454-199809001-00005. [DOI] [PubMed] [Google Scholar]
- 35.Heath PT, Booy R, Azzopardi HJ, et al. Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom. JAMA. 2000;284(18):2334–2340. doi: 10.1001/jama.284.18.2334. [DOI] [PubMed] [Google Scholar]
- 36.Ladhani S, Borrow R, Heath PT, Ramsay ME, Booy R. Low serum serotype-specific pneumococcal antibody concentrations in young children with Haemophilus influenzae serotype b (Hib) vaccine failure. Vaccine. 2010;28 (28):4440–4444. doi: 10.1016/j.vaccine.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 37.Ladhani S, Heath PT, Slack MP, et al. Participants of the European Union Invasive Bacterial Infections Surveillance Network. Haemophilus influenzae serotype b conjugate vaccine failure in twelve countries with established national childhood immunization programmes. Clin Microbiol Infect. 2010;16(7):948–954. doi: 10.1111/j.1469-0691.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 38.Santosham M. Can Haemophilus influenzae type b disease be eliminated from the United States? J Pediatr. 2000;137(3):295–298. doi: 10.1067/mpd.2000.109020. [DOI] [PubMed] [Google Scholar]
- 39. [Accessed December 5, 2011];Healthy People 2020 topics and objectives. Available at: www.healthypeople.gov/2020/topicsobjectives2020/default.aspx.
- 40.Dolan JM, Satterfield DA, Hatcher CP, et al. Real-time PCR assays for the detection of H. influenzae serotypes a, b, and f and sequencing of the capsule biosynthesis operons of serotypes c, d, and e. Proceedings of the 110th Annual American Society for Microbiology; May 23–May 27, 2010; San Diego, CA. [Google Scholar]