Abstract
Objective
People with Parkinson disease (PD) frequently develop dementia, which is associated with neocortical deposition of alpha-synuclein (α-syn) in Lewy bodies and Lewy neurites. In addition, neuronal loss and deposition of aggregated α-syn also occur in multiple subcortical nuclei that project to neocortical, limbic, and basal ganglia regions. Therefore, we quantified regional deficits in innervation from these PD-affected subcortical nuclei, by measuring the neurotransmitters and neurotransmitter transporter proteins originating from projections of dopaminergic neurons in substantia nigra pars compacta, serotonergic neurons in dorsal raphé nuclei, noradrenergic neurons in locus coeruleus, and cholinergic neurons in nucleus basalis of Meynert.
Methods
High-performance liquid chromatography and novel enzyme-linked immunosorbent assays were performed to quantify dopaminergic, serotonergic, noradrenergic, and cholinergic innervation in postmortem brain tissue. Eight brain regions from 15 PD participants (with dementia and Braak stage 6 α-syn deposition) and six age-matched controls were tested.
Results
PD participants compared to controls had widespread reductions of dopamine transporter in caudate, amygdala, hippocampus, inferior parietal lobule (IPL), precuneus, and visual association cortex (VAC) that exceeded loss of dopamine, which was only significantly reduced in caudate and amygdala. In contrast, PD participants had comparable deficits of both serotonin and serotonin transporter in caudate, middle frontal gyrus, IPL, and VAC. PD participants also had significantly reduced norepinephrine levels for all eight brain regions tested. Vesicular acetylcholine transporter levels were only quantifiable in caudate and hippocampus and did not differ between PD and control groups.
Interpretation
These results demonstrate widespread deficits in dopaminergic, serotonergic, and noradrenergic innervation of neocortical, limbic, and basal ganglia regions in advanced PD with dementia.
Introduction
The defining histopathologic feature of Parkinson disease (PD) is the accumulation of misfolded alpha-synuclein (α-syn) protein in Lewy bodies and Lewy neurites.1 Postmortem examinations of PD brains indicate stage-dependent deposition of aggregated α-syn and topographical neuronal loss in multiple subcortical nuclei2 including substantia nigra, nucleus basalis of Meynert (cholinergic),3 locus coeruleus (noradrenergic),4 and dorsal raphe nuclei (serotonergic).5,6 Neocortical accumulation of α-syn accompanies the development of dementia that commonly occurs in PD and likely contributes to impaired function of cortical neurons.7–9 However, the affected subcortical nuclei project rostrally to striatal, limbic, and neocortical regions; and the loss of innervation from these nuclei may also contribute to impaired executive, visuospatial, attentional, and memory function in PD.
Cognitive and psychiatric changes in PD are linked in a variety of ways to alterations in multiple neurotransmitter systems.10–14 Previous studies have measured levels of individual neurotransmitters or transporter proteins in a limited number of regions such as caudate, putamen, and neocortex.15–17 However, few studies have examined the degree to which deficits in multiple neurotransmitter systems occur across neocortical and limbic regions.
The aim of the current study was to determine the extent of regional deficits in dopaminergic, serotonergic, noradrenergic, and cholinergic innervation in postmortem tissue from those that had advanced PD and dementia and neurologically normal controls. We combined measures of neurotransmitters and corresponding transporter proteins in basal ganglia, limbic, and neocortical regions. We used high-performance liquid chromatography (HPLC) to measure neurotransmitter levels and developed novel sandwich enzyme-linked immunosorbent assays (ELISAs) to measure relevant transporters. The results indicate widespread loss of dopaminergic, serotonergic, and noradrenergic innervation in advanced PD.
Materials and Methods
Standard protocol approvals and patient consents
The Human Research Protection Office at Washington University in Saint Louis approved this study. Written informed consent to perform a brain autopsy was obtained from all participants. After death, the immediate next-of-kin were contacted and confirmed consent for brain removal and retention for research purposes.
Study participants
The cohort included 15 PD participants with dementia and six healthy age-matched neurologically normal control participants. All PD participants and one control participant were recruited through the Movement Disorders Center (MDC), and five control participants were recruited through the Knight Alzheimer Disease Research Center (ADRC); both centers are located at Washington University in Saint Louis. PD participants had a clear response to levodopa (l-DOPA). Postmortem brain tissue was collected between April 2008 and May 2013. Inclusion criteria for the study were (1) PD participants who had a clinical diagnosis of idiopathic PD based on the United Kingdom Parkinson Disease Society Brain Bank diagnostic criteria18 and either Clinical Dementia Rating (CDR global score) ≥1 or a clinical diagnosis of dementia; and (2) control participants with no family history of PD and no dementia (CDR ≤ 0.5). Exclusion criteria for all participants were (1) any neurological diagnosis other than PD; (2) psychiatric disorders other than depression or anxiety; and (3) unwillingness to consent to brain autopsy.
Motor assessments
MDC clinicians evaluated motor symptoms using the Unified Parkinson Disease Rating Scale motor subscale III (UPDRS-III). Evaluations were performed on anti-parkinsonian medications.
Dementia ratings
Dementia was rated using the CDR scale by experienced raters. The CDR included assessment of whether cognitive dysfunction was sufficiently severe to impair activities of daily living. PD participants with CDR ≥ 1, in accordance with criteria for dementia in PD,19 were included in the study. PD participants were included in the study regardless of the time of onset of cognitive decline or dementia (as elicited by history) relative to the onset of motor symptoms.
Tissue dissection
The protocols to retrieve, dissect, preserve, perform histology and immunohistochemistry, and diagnose with established neuropathologic diagnostic criteria are routinely done in the Betty Martz Laboratory of the Knight ADRC.20 The brain regions were dissected from frozen 1 cm coronal slices and included the body of the caudate nucleus; gray matter from anterior cingulate gyrus (ACG), middle frontal gyrus (MFG), inferior parietal lobule (IPL), precuneus, and visual association cortex (VAC); hippocampus/entorhinal cortex; and amygdala.21 Detailed procedures of tissue handling and dissection are described further in Data S1.
Extraction and analysis of neurotransmitters by HPLC
Neurotransmitters dopamine (DA), serotonin (5HT), and norepinephrine (NE) were extracted and analyzed by HPLC with electrochemical detection using a method modified from Xia et al.22 described in Data S1.
Extraction and analysis of neurotransmitter transporter proteins by ELISA
The extraction method for transporter proteins was adapted from previously published methods23 and is described in Data S1. Capture and detection antibodies used for dopamine transporter (DAT), serotonin transporter (SERT), and vesicular acetyl choline transporter (VAChT) sandwich ELISAs were screened from commercially available antibodies (Table S1). DAT recombinant protein from Novus Biologicals, LLC, Littleton, CO (H00006531-P01) was used as a standard for DAT ELISA. SERT and VAChT ELISAs used recombinant SERT and VAChT that were expressed as his-tag proteins in HEK293FT cells and purified by Nickel affinity chromatography based on previous methods,24 described in more detail in Data S1. The sandwich ELISA protocols were optimized for each neurotransmitter transporter protein and also are described in detail in Data S1.
Statistical analysis
Data were analyzed using GraphPad Prism software, version 4 (Graph Pad Software, Inc., La Jolla, CA) with details described in Data S1. Due to unequal group sizes and non-normal distribution of data points, nonparametric Mann–Whitney U tests were applied to compare groups for HPLC and ELISA data. All tests were two-tailed and corrected for multiple comparisons with the Holm–Bonferroni method25 using a significance level of 0.05.
Results
Demographics and clinical information of participants
Table1 summarizes the demographics and clinical information of the study participants. Age at death did not significantly differ between control and PD participants (Table1). The male/female ratio was higher in PD compared to controls (Table1). Detailed clinical and pathological information for each participant is included in Table S2.
Table 1.
Demographics and clinical information of the study participants1
| Controls | PD with dementia | P | |
|---|---|---|---|
| Demographics | |||
| Participants, N | 6 | 15 | NA |
| Age at death, y | 84 (70–100) | 79 (71–93) | 0.512 |
| Male/female, N | 2/4 | 12/3 | 0.043 |
| Clinical characteristics of PD participants with dementia | |||
| Age at PD diagnosis, y | NA | 63 (54–82) | NA |
| Duration of motor impairment, y | NA | 14 (8–27) | NA |
| UPDRS-III score (OFF medications) | NA | 44 (35–73.5) | NA |
| LEDD, mg | NA | 800 (0–1350) | NA |
| SSRI medications, N | NA | 10/15 | NA |
| Other antidepressant medications4, N | NA | 2/15 | NA |
| AChE inhibitor medications5, N | NA | 8/15 | NA |
| Neuroleptic medications6, N | NA | 14/15 | NA |
NA, not applicable; UPDRS, Unified Parkinson Disease Rating Scale; LEDD, levodopa equivalent daily dose; SSRI, selective serotonin reuptake inhibitors; AChE, acetylcholinesterase.
Values are medians (range) unless otherwise indicated.
Mann–Whitney U test.
Chi-square test.
Mirtazepine and venlafaxine.
Donepezil, rivastigmine, and galantamine.
Quetiapine and clozapine.
Assay performance characteristics for HPLC and ELISA assays
The HPLC assay lower limits of quantification (LLOQs) were 0.025 ng/mL for both DA and NE, and 0.10 ng/mL for 5HT, corresponding to 0.125 ng/g wet wt for DA and NE, and 0.5 ng/g wet wt for 5HT. The percent coefficients of variation (%CVs) for DA, 5HT, and NE assays are reported in Table S3.
Specificity of the ELISA antibodies used for the quantification of DAT, SERT, and VAChT were confirmed by immunoprecipitation (IP) and western blotting (WB) (Fig. S1). Representative standard curves for each ELISA are shown in Fig. S2. The ELISA LLOQs for DAT, SERT, and VAChT were 0.1, 0.016, and 0.3 ng/mL in the ELISA well, respectively, which corresponded to 3.3, 0.5, and 9.9 ng/mL in the extracts, and 33, 5, and 99 ng/g wet wt tissue. Since all group comparisons were performed for an individual brain region within the same plate and no comparisons were performed between plates, intraplate %CVs were the most relevant measures of assay consistency, which are included in Table S3.
Neurotransmitter and transporter measures
DA and DAT
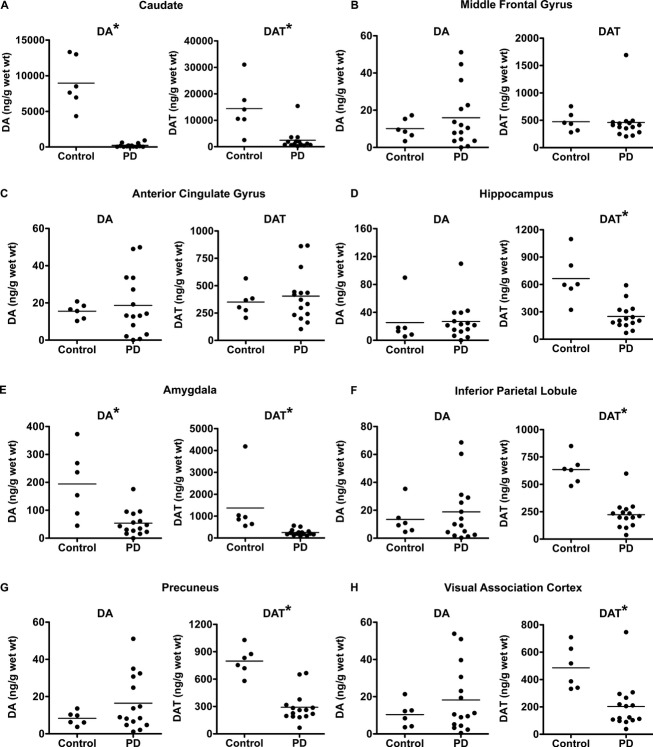
PD participants had significantly lower DA levels compared to control participants for caudate and amygdala (Fig.1, Table2). DA levels for control and PD participants in MFG, ACG, hippocampus, IPL, precuneus, and VAC did not significantly differ (Fig.1). Table2 lists levels of DA in PD expressed as a percentage of controls and their corresponding P values for all brain regions.
Figure 1.
Comparison of DA and DAT levels between control and PD with dementia participants. DA and DAT levels in control and PD with dementia for caudate (A), middle frontal gyrus (B), anterior cingulate gyrus (C), hippocampus (D), amygdala (E), inferior parietal lobule (F), precuneus (G), and visual association cortex (H). Statistically significant group differences (P < 0.05) are indicated by an asterisk. DA, dopamine; DAT, dopamine transporter; PD, Parkinson disease.
Table 2.
Levels of neurotransmitters and transporters in PD with dementia as a percentage of controls in all brain regions
| DA | DAT | 5HT | SERT | NE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| %C | P | %C | P | %C | P | %C | P | %C | P | |
| Caudate | 2.5 | 0.001 | 17 | 0.003 | 31.6 | 0.003 | 38.5 | 0.007 | 7.3 | 0.001 |
| MFG | 158 | 0.79 | 97.3 | 0.5 | 19.7 | 0.001 | 19 | 0.005 | 9.65 | 0.001 |
| ACG | 120 | 0.9 | 115.4 | 0.8 | 52.5 | 0.011 | 68.3 | 0.17 | 3.9 | 0.002 |
| Hippocampus | 106 | 0.46 | 37.5 | 0.002 | 67.4 | 0.2 | 102.8 | 0.97 | 27.3 | 0.007 |
| Amygdala | 27.8 | 0.001 | 18.1 | 0.001 | 60.5 | 0.06 | 74.6 | 0.11 | 10.92 | 0.002 |
| IPL | 140.3 | 0.84 | 35 | 0.001 | 16.8 | 0.001 | 32.13 | 0.001 | 4.2 | 0.001 |
| Precuneus | 198.3 | 0.41 | 36.5 | 0.001 | 68.6 | 0.63 | 66.4 | 0.213 | 17.6 | 0.001 |
| VAC | 176 | 0.67 | 41.8 | 0.003 | 11.8 | 0.003 | 9.9 | 0.001 | 15.8 | 0.001 |
Shaded boxes represent measures that were significantly different between control and PD with dementia at P < 0.05, adjusted for multiple comparisons performed for each measure. DA, dopamine; DAT, dopamine transporter; 5HT, serotonin; SERT, serotonin transporter; NE, norepinephrine; C, control; MFG, middle frontal gyrus; ACG, anterior cingulate gyrus; IPL, inferior parietal lobule; VAC, visual association cortex.
PD participants had widespread significant reductions in DAT levels compared to control participants for caudate, hippocampus, amygdala, IPL, precuneus, and VAC (Fig.1, Table2). DAT levels in MFG and ACG did not significantly differ between control and PD participants (Fig.1, Table2).
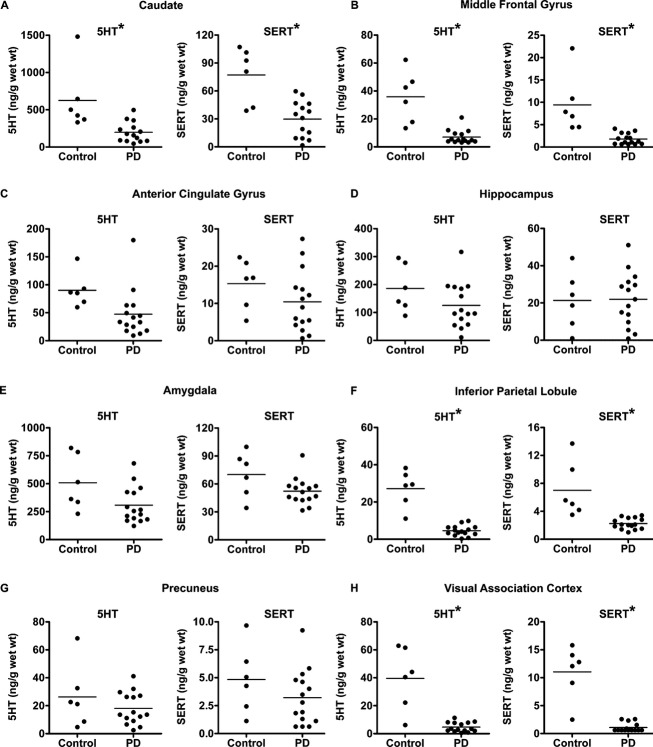
5HT and SERT
PD participants had significantly lower 5HT levels compared to control participants for four of the eight brain regions tested, which include caudate, MFG, IPL, and VAC (Fig.2, Table2), whereas 5HT levels did not differ between control and PD participants for ACG, hippocampus, amygdala, and precuneus (Fig.2, Table2).
Figure 2.
Comparison of 5HT and SERT levels between control and PD with dementia participants. 5HT and SERT levels in control and PD with dementia for caudate (A), middle frontal gyrus (B), anterior cingulate gyrus (C), hippocampus (D), amygdala (E), inferior parietal lobule (F), precuneus (G), and visual association cortex (H). Statistically significant group differences (P < 0.05) are indicated by an asterisk. 5HT, serotonin; SERT, serotonin transporter; PD, Parkinson disease.
Similar to 5HT, PD participants also had significantly lower SERT levels compared to control participants for the same brain regions that include caudate, MFG, IPL, and VAC (Fig.2, Table2), but not for ACG, hippocampus, amygdala, and precuneus.
NE
PD participants had significantly lower NE levels compared to control participants for all eight brain regions tested, which include caudate, MFG, ACG, hippocampus, amygdala, IPL, precuneus, and VAC (Fig.3, Table2).
Figure 3.
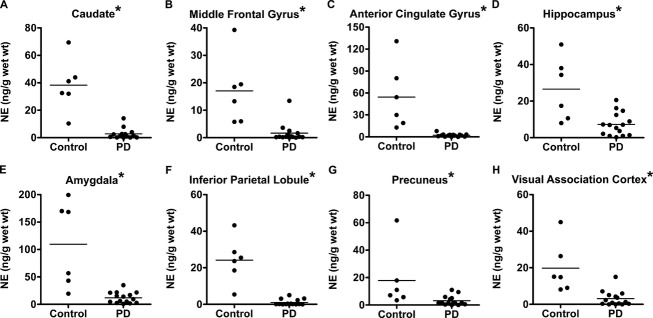
Comparison of NE levels between control and PD with dementia participants. NE levels in control and PD with dementia for caudate (A), middle frontal gyrus (B), anterior cingulate gyrus (C), hippocampus (D), amygdala (E), inferior parietal lobule (F), precuneus (G), and visual association cortex (H). Group differences were statistically significant for all of the brain regions tested (P < 0.05), as indicated by an asterisk. NE, norepinephrine; PD, Parkinson disease.
VAChT
VAChT levels were below the LLOQ for samples from MFG, ACG, amygdala, IPL, precuneus, and VAC. VAChT levels were only quantifiable and did not significantly differ between PD and control participants for caudate and hippocampus (Fig. S3).
Neurotransmitter/transporter ratios
DA/DAT ratio was higher in caudate compared to other brain regions in controls, whereas caudate DA/DAT was dramatically lower in PD and similar to other regions in PD (Table S4). 5HT/SERT ratios were similar across all regions for controls and PD.
Relationship between neurochemical measures, clinical and pathological features
We grouped PD participants (n = 15) according to different pathological subtypes; α-syn only (n = 6); α-syn with amyloid β (Aβ) deposition (Braak amyloid stages B–C) (n = 7); and α-syn with Aβ plus at least moderate neocortical tau deposition (Braak tau stages 5–6) (n = 2).26 Since the sample size for the α-syn with Aβ plus tau group was too low to allow statistical testing, we compared the α-syn only and α-syn with Aβ deposition groups and did not find any significant group differences in any of the neurotransmitter or transporter measures. Furthermore, we did not find any significant correlations between neurochemical measures and CDR, Mini Mental State Examination (MMSE), or UPDRS-III scores within PD participants, when corrected for multiple comparisons.
Discussion
Postmortem analysis of multiple brain regions in advanced PD with dementia compared to age-matched neurologically normal controls revealed widespread reductions in neurotransmitter levels of DA, 5HT, and NE, along with reductions in their corresponding transporter proteins, DAT and SERT. PD participants had reduced dopaminergic innervation in six brain regions as reflected by significantly reduced DAT levels in caudate, hippocampus, amygdala, IPL, precuneus, and VAC. Reduced DAT levels were accompanied by reduced DA in only two regions (caudate and amygdala), which likely reflects exogenous l-DOPA administration. PD participants had reduced serotonergic innervation in four brain regions (caudate, MFG, IPL, and VAC). In contrast to the dopaminergic system, regional reductions of 5HT matched that of SERT. Finally, PD participants had marked reductions in noradrenergic innervation, reflected by reduced NE levels in all brain regions tested including caudate, MFG, ACG, hippocampus, amygdala, IPL, precuneus, and VAC. In contrast to the reductions in dopaminergic, serotonergic, and noradrenergic systems, we did not observe PD-related reductions in VAChT levels, but we were only able to quantify VAChT in caudate and hippocampus. The observed deficits in dopaminergic, serotonergic, and noradrenergic innervation of multiple brain regions provide insights into PD-related brain dysfunction, particularly in the context of PD with dementia.
Measurements of DAT revealed more widespread dopaminergic deficits compared to DA. Relative preservation of DA levels in regions where DAT losses occur likely reflects exogenous l-DOPA administration that increases DA concentration in brain regions with residual DOPA decarboxylase activity yet does not affect DAT on residual dopaminergic presynaptic terminals. Similarly, exogenous l-DOPA could lead to higher than normal levels of DA in some cortical regions that could potentially relate to drug-induced cognitive side effects.27 The differences in DA/DAT ratios among different brain regions in controls likely reflect regional differences in DOPA decarboxylase and DA storage capacities for intact DAT-containing presynaptic terminals. Alternatively, nonselective DA uptake by SERT or NET also could influence DA/DAT ratios.28 Higher levels of DA synthesis and storage compared to DAT are likely to cause the significantly higher DA/DAT ratio measured in caudate compared to cortical regions in controls (caudate, 0.63; cortical regionsmedian, 0.02). The lower caudate DA/DAT ratio (0.08) in PD patients indicates a relative loss in DA synthesis and storage capacity for the remaining DAT-containing terminals.
In contrast, regional reductions of 5HT matched reductions of SERT in PD and the relative invariance of 5HT/SERT ratios across regions in both controls and PD suggest that exogenous drug administration does not influence these ratios. PD-related degeneration of 5HT neurons equally affects local 5HT concentration and presynaptic terminal membranous SERT, a major difference between the 5HT/SERT system and the DA/DAT system in PD.
Dramatic reductions of NE levels in all brain regions reflects extensive loss of innervation from locus coeruleus neurons, which may further contribute to cortical neuron dysfunction related to local synucleinopathy in advanced PD. The loss of NE secondary to locus coeruleus lesions in animal models alters cerebral oxidative metabolism and blood flow,29 which fits with the observed excessive oxidative metabolism in mildly affected, never-medicated PD patients and could indicate uncoupling of oxidative phosphorylation.30 Altered oxidative metabolism as well as other consequences of noradrenergic deficits, including altered neuroinflammatory pathways,31–33 may contribute to attention, cognitive, or mood dysfunction in PD.
Our observed neurotransmitter and transporter deficits in caudate agree with a number of previous studies. The reduced caudate levels of DAT for PD participants in our study agree with previously reported reductions in striatal DAT by autoradiography,34 immunohistochemistry and WB,35 positron emission tomography (PET),15 and single photon-emission computed tomography (SPECT) imaging.36 The magnitude of DA and DAT reductions in caudate (2.5% and 17.5% of controls, respectively) is greater in our study compared to two other studies, which observed reductions in DA and DAT markers in caudate ranging from 9% to 30% of controls, and more pronounced reductions (2–3% of controls) in putamen.16,37 The reduced caudate SERT levels in our study agree with previous measurements of caudate 5HT,38 and with previous measurements of caudate SERT levels by WB analysis,16 PET,39 and SPECT imaging.40 Reduced caudate NE was observed in one prior study,41 while another study did not detect changes in caudate NE.37 Our VAChT measurements are consistent with findings of in vivo PET imaging in neurologically normal individuals, where the density of VAChT is higher in the striatum and hippocampus compared to neocortical regions.42,43
A few previous studies reported neurotransmitter changes in cortical regions although they examined a smaller number of regions compared to our study. Our findings confirm the absence of significant DA loss in frontal and cingulate cortex for PD participants on l-DOPA despite reductions of DA in caudate.38 Similar changes in the levels of 5HT were observed in frontal cortex, but unlike our study, significant reductions of 5HT were observed in cingulate cortex.38 Interestingly, the same group also observed widespread deficits in NE levels in frontal, cingulate, hippocampus, and entorhinal cortex, although the relative differences between control and PD was less pronounced than in our study. The significant reductions in NE in frontal cortex observed by Goldstein et al.41 also agree with our study. Modest discrepancies in findings between our study and these other studies may be related to differences in disease duration, severity of disease, cognitive status, drug exposure, or analysis methods.
Novel aspects of our study include the analysis of a large number of brain regions, and the simultaneous measurement of multiple neurotransmitters with their corresponding transporters in a homogenous population of end-stage PD. Such concurrent measurements have not been reported previously, with the exception of two studies that measured only striatal neurotransmitters and transporters for just dopaminergic and serotonergic systems.16,37 Unlike PET44 or SPECT45 imaging radioligands that bind to multiple members of the neurotransmitter transporter family, the DAT, SERT, and VAChT ELISAs developed in this study are highly specific based on the epitopes recognized by the capture and detection antibodies. These measures were further validated using IP and WB. The results from the novel ELISAs agree with previous findings of transporter reductions, suggesting consistency and reliability of the developed assays. Moreover, ELISAs offer an advantage in sensitivity and throughput compared to immunohistochemical or WB methods.16,35,46,47 Furthermore, the higher sensitivity of the ELISAs enabled quantification of DAT and SERT in multiple cortical regions where levels are significantly lower than caudate.
This study has several limitations. The modest sample size and homogeneously severe cognitive impairment precluded analysis of whether the neurotransmitter or transporter differences relate to specific cognitive phenotypes associated with PD. The number of males and females was not equal, particularly in the PD group. The higher percentage of males within the PD group reflects to some extent the higher prevalence of PD in males,48,49 and also may be explained by sex differences in participation in the study or in the death rates among the PD participants. The widespread and dramatic differences between PD and control participants are unlikely to be explained by differences in sex ratios between the two groups, but the possibility exists that sex may have small influences on baseline levels which only can be excluded with further studies of larger sample sizes. The small sample sizes (n = 2–3) obtained when groups were subdivided based on sex prevented within group or between group comparisons to investigate this further using nonparametric statistics. Some measurements demonstrate significant dispersion not only in PD participants, where variability in disease manifestation may contribute, but also in control participants. In addition to biologic variability, additional contributing factors specific to the nature of this study include variability related to postmortem intervals, dissection of postmortem tissue, and drug exposure. The lack of an ELISA for quantification of NET limited our ability to further assess and confirm the loss of noradrenergic innervation throughout the brain regions examined. VAChT ELISA was not sufficiently sensitive to measure VAChT levels in cortical regions, and thus we were not able to determine whether degeneration of nucleus basalis cholinergic neurons results in altered cholinergic innervation of these brain regions.
Our results demonstrate widespread deficits in dopaminergic, serotonergic, and noradrenergic innervation of neocortical, limbic, and basal ganglia regions in advanced PD with dementia. Our data do not prove whether these findings reflect the presence or absence of dementia. Future enrollment of additional PD participants with mild and no cognitive impairment will enable further characterization of the range of neurotransmitter and transporter deficits in PD, and determine whether levels are correlated with specific cognitive phenotypes. The novel DAT and SERT ELISAs could be further extended to other disorders, such as schizophrenia, Tourette's syndrome, attention deficit hyperactivity disorder, or depression, where dysregulation of DAT or SERT are implicated.50–53 Furthermore, combining postmortem measurements of neurotransmitters and their corresponding transporters with in vivo PET imaging would be helpful to further define specific brain regions and neurotransmitter systems affected in PD.
Acknowledgments
Support for this work was provided by a grant from the Michael J. Fox Foundation, National Institutes of Health grants NS075321, NS41509, NS058714, and NS48924 from the National Institute of Neurological Disorders and Stroke; P50AG005681 and P01AG003991 from the National Institute on Aging; UL1 TR000448 from the National Institutes of Health National Center for Advancing Translational Sciences; the American Parkinson Disease Association (APDA) Advanced Research Center for Parkinson Disease at Washington University in St. Louis; the Greater St. Louis Chapter of the APDA; and the Barnes Jewish Hospital Foundation (Elliot Stein Family Fund and Parkinson Disease Research Fund). We are grateful for the technical support of the Betty Martz Laboratory for Neurodegenerative Research at Washington University in Saint Louis.
Author Contributions
Study concept and organization: Drs. Kotzbauer, Perlmutter, Campbell, and Cairns; execution: Drs. Cairns, Buddhala, Loftin, and Kuley; acquisition of data: Drs. Campbell, Buddhala, Loftin, and Kuley; analysis and interpretation of data: all authors; drafting of the manuscript: Drs. Buddhala and Kotzbauer; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: Drs. Campbell, Kotzbauer, and Buddhala; obtained funding: Drs. Kotzbauer and Perlmutter. Drs. Kotzbauer and Campbell had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting Information
Materials and methods.
Figure S1. Assessment of ELISA antibody specificity.
Figure S2. Representative standard curves for the transporter ELISA assays.
Figure S3. Comparison of VAChT levels between control and PD with dementia participants.
Table S1. Capture and detection antibodies used in the ELISA assays.
Table S2. Clinical and pathological information of study participants.
Table S3. Assay performance characteristics of HPLC and ELISA assays.
Table S4. Ratios of neurotransmitters to transporters between control and PD with dementia.
References
- Spillantini MG, Crowther RA, Jakes R, et al. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, et al. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011;221:564–573. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesi M, Soldani P, Giorgi FS, et al. The role of the locus coeruleus in the development of Parkinson's disease. Neurosci Biobehav Rev. 2000;24:655–668. doi: 10.1016/s0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Guttman M, Boileau I, Warsh J, et al. Brain serotonin transporter binding in non-depressed patients with Parkinson's disease. Eur J Neurol. 2007;14:523–528. doi: 10.1111/j.1468-1331.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson's disease. J Neural Transm. 2004;111:1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O’Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 2011;134(Pt 5):1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Hurtig HI, Trojanowski JQ, Galvin J, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54:1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- Bosboom JL, Stoffers D, Wolters E. The role of acetylcholine and dopamine in dementia and psychosis in Parkinson's disease. J Neural Transm Suppl. 2003;65:185–195. doi: 10.1007/978-3-7091-0643-3_11. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov Disord. 2014;29:634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P, Fox SH. The serotonergic system in motor and non-motor manifestations of Parkinson's disease. Exp Brain Res. 2013;230:463–476. doi: 10.1007/s00221-013-3621-2. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Kejonen K, Jakala P, et al. Reduction of noradrenaline impairs attention and dopamine depletion slows responses in Parkinson's disease. Eur J Neurosci. 1998;10:1429–1435. doi: 10.1046/j.1460-9568.1998.00145.x. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, et al. Cognitive deficits and striato-frontal dopamine release in Parkinson's disease. Brain. 2008;131(Pt 5):1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Kazumata K, Dhawan V, Chaly T, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med. 1998;39:1521–1530. [PubMed] [Google Scholar]
- Kish SJ, Tong J, Hornykiewicz O, et al. Preferential loss of serotonin markers in caudate versus putamen in Parkinson's disease. Brain. 2008;131(Pt 1):120–131. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- Shannak K, Rajput A, Rozdilsky B, et al. Noradrenaline, dopamine and serotonin levels and metabolism in the human hypothalamus: observations in Parkinson's disease and normal subjects. Brain Res. 1994;639:33–41. doi: 10.1016/0006-8993(94)91761-2. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. ; quiz 837. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Taylor-Reinwald L, Morris JC Alzheimer's Disease Neuroimaging I. Autopsy consent, brain collection, and standardized neuropathologic assessment of ADNI participants: the essential role of the neuropathology core. Alzheimers Dement. 2010;6:274–279. doi: 10.1016/j.jalz.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, Huijzen CV. The human central nervous system: a synopsis and atlas. 3rd rev. ed. Berlin, New York: Springer; 1988. [Google Scholar]
- Xia Y, Cheng S, He J, et al. Effects of subchronic exposure to benzo[a]pyrene (B[a]P) on learning and memory, and neurotransmitters in male Sprague–Dawley rat. Neurotoxicology. 2011;32:188–198. doi: 10.1016/j.neuro.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Worsley JN, Moszczynska A, Falardeau P, et al. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol Psychiatry. 2000;5:664–672. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- Engel LA, Jing Z, O’Brien DE, et al. Catalytic function of PLA2G6 is impaired by mutations associated with infantile neuroaxonal dystrophy but not dystonia-parkinsonism. PLoS One. 2010;5:e12897. doi: 10.1371/journal.pone.0012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Kotzbauer PT, Cairns NJ, Campbell MC, et al. Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch Neurol. 2012;69:1326–1331. doi: 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, et al. Dopaminergic modulation of high-level cognition in Parkinson's disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125(Pt 3):584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harik SI, LaManna JC, Light AI, Rosenthal M. Cerebral norepinephrine: influence on cortical oxidative metabolism in situ. Science. 1979;206:69–71. doi: 10.1126/science.482927. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Videen TO, Markham J, et al. Cerebral mitochondrial metabolism in early Parkinson's disease. J Cereb Blood Flow Metab. 2008;28:1754–1760. doi: 10.1038/jcbfm.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Galea E, Gavriluyk V, et al. Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation: implications for Alzheimer's disease. J Neurosci. 2002;22:2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Zeppenfeld D, McConnell E, et al. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37:2496–2512. doi: 10.1007/s11064-012-0818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukawa K, McGeer EG, McGeer PL. Autoradiographic study on dopamine uptake sites and their correlation with dopamine levels and their striata from patients with Parkinson disease, Alzheimer disease, and neurologically normal controls. Mol Chem Neuropathol. 1993;18:133–144. doi: 10.1007/BF03160027. [DOI] [PubMed] [Google Scholar]
- Miller GW, Staley JK, Heilman CJ, et al. Immunochemical analysis of dopamine transporter protein in Parkinson's disease. Ann Neurol. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Booij J, Tissingh G, Boer GJ, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;62:133–140. doi: 10.1136/jnnp.62.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Levey AI, Rajput A, et al. Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson's disease. Neurology. 1996;47:718–726. doi: 10.1212/wnl.47.3.718. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, et al. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson's disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Kerenyi L, Ricaurte GA, Schretlen DJ, et al. Positron emission tomography of striatal serotonin transporters in Parkinson disease. Arch Neurol. 2003;60:1223–1229. doi: 10.1001/archneur.60.9.1223. [DOI] [PubMed] [Google Scholar]
- Haapaniemi TH, Ahonen A, Torniainen P, et al. [123I]beta-CIT SPECT demonstrates decreased brain dopamine and serotonin transporter levels in untreated parkinsonian patients. Mov Disord. 2001;16:124–130. doi: 10.1002/1531-8257(200101)16:1<124::aid-mds1007>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, et al. Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol. 2011;18:703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl DE, Minoshima S, Fessler JA, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease, and Parkinson's disease. Ann Neurol. 1996;40:399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- Petrou M, Frey KA, Kilbourn MR, et al. In vivo imaging of human cholinergic nerve terminals with (-)-5-(18)F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med. 2014;55:396–404. doi: 10.2967/jnumed.113.124792. [DOI] [PubMed] [Google Scholar]
- Remy P, Doder M, Lees A, et al. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128(Pt 6):1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Berding G, Brucke T, Odin P, et al. [[123I]beta-CIT SPECT imaging of dopamine and serotonin transporters in Parkinson's disease and multiple system atrophy. Nuklearmedizin. 2003;42:31–38. [PubMed] [Google Scholar]
- Gaspar P, Gray F. Dementia in idiopathic Parkinson's disease. A neuropathological study of 32 cases. Acta Neuropathol. 1984;64:43–52. doi: 10.1007/BF00695605. [DOI] [PubMed] [Google Scholar]
- Siegal D, Erickson J, Varoqui H, et al. Brain vesicular acetylcholine transporter in human users of drugs of abuse. Synapse. 2004;52:223–232. doi: 10.1002/syn.20020. [DOI] [PubMed] [Google Scholar]
- Lubomski M, Louise Rushworth R, Lee W, et al. Sex differences in Parkinson's disease. J Clin Neurosci. 2014;21:1503–1506. doi: 10.1016/j.jocn.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Smith KM, Dahodwala N. Sex differences in Parkinson's disease and other movement disorders. Exp Neurol. 2014;259:44–56. doi: 10.1016/j.expneurol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zheng MQ, Gerdes JM. Development of effective PET and SPECT imaging agents for the serotonin transporter: has a twenty-year journey reached its destination? Curr Top Med Chem. 2010;10:1499–1526. doi: 10.2174/156802610793176792. [DOI] [PubMed] [Google Scholar]
- Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson's disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox Res. 2006;10:167–179. doi: 10.1007/BF03033354. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- Runyon SP, Carroll FI. Dopamine transporter ligands: recent developments and therapeutic potential. Curr Top Med Chem. 2006;6:1825–1843. doi: 10.2174/156802606778249775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and methods.
Figure S1. Assessment of ELISA antibody specificity.
Figure S2. Representative standard curves for the transporter ELISA assays.
Figure S3. Comparison of VAChT levels between control and PD with dementia participants.
Table S1. Capture and detection antibodies used in the ELISA assays.
Table S2. Clinical and pathological information of study participants.
Table S3. Assay performance characteristics of HPLC and ELISA assays.
Table S4. Ratios of neurotransmitters to transporters between control and PD with dementia.