Abstract
Objective
To investigate anti-neurofascin 155 (NF155) antibody-positive chronic inflammatory demyelinating polyneuropathy (CIDP).
Methods
Sera from 50 consecutive CIDP patients diagnosed in our clinic, 32 patients with multiple sclerosis, 40 patients with other neuropathies including 26 with Guillain–Barré syndrome (GBS)/Fisher syndrome, and 30 healthy controls were measured for anti-NF antibodies by flow cytometry using HEK293 cell lines stably expressing human NF155 or NF186. Four additional CIDP patients with anti-NF155 antibodies referred from other clinics were enrolled for clinical characterization.
Results
The positivity rate for anti-NF155 antibodies in CIDP patients was 18% (9/50), who all showed a predominance of IgG4 subclass. No other subjects were positive, except one GBS patient harboring IgG1 anti-NF155 antibodies. No anti-NF155 antibody carriers had anti-NF186 antibodies. Anti-NF155 antibody-positive CIDP patients had a significantly younger onset age, higher frequency of drop foot, gait disturbance, tremor and distal acquired demyelinating symmetric phenotype, greater cervical root diameter on magnetic resonance imaging neurography, higher cerebrospinal fluid protein levels, and longer distal and F-wave latencies than anti-NF155 antibody-negative patients. Marked symmetric hypertrophy of cervical and lumbosacral roots/plexuses was present in all anti-NF155 antibody-positive CIDP patients examined by neurography. Biopsied sural nerves from two patients with anti-NF155 antibodies demonstrated subperineurial edema and occasional paranodal demyelination, but no vasculitis, inflammatory cell infiltrates, or onion bulbs. Among anti-NF155 antibody-positive patients, treatment responders more frequently had daily oral corticosteroids and/or immunosuppressants in addition to intravenous immunoglobulins than nonresponders did.
Interpretation
Anti-NF155 antibodies occur in a subset of CIDP patients with distal-dominant involvement and symmetric nerve hypertrophy.
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) presents with various features in addition to demyelination, including muscle atrophy, sensory ataxia, tremor, asymmetrical or focal involvement, and nerve hypertrophy. However, biomarkers for these features remain ill defined. T-cell- and macrophage-mediated demyelination is assumed to play a major role in CIDP although some patients harbor autoantibodies against proteins at the nodes of Ranvier.1 Antibodies against contactin-1, a paranodal axolemmal protein, were detected in 6% of CIDP patients, who commonly showed advanced age, predominant motor involvement, aggressive symptom onset, and early axonal involvement.2 Another autoantigen, neurofascin (NF), comprised two major isoforms: axonal NF186, which interacts with neuronal cell adhesion molecules to cluster sodium channels at the nodes, and glial NF155, expressed at the paranodal loops of oligodendrocytes in the central nervous system3 and in Schwann cells of the peripheral nervous system (PNS),4 which interacts with axonal contactin-1 and contactin-associated protein (Caspr) to form a septal barrier excluding the nodal complex from the internodes.5 Contradictory results were reported for the presence of anti-NF186 antibodies in CIDP. One study showed a 12% positivity rate,6 and another 0%.7 Measurement of anti-NF155 antibodies by enzyme-linked immunosorbent assay in two studies revealed low positivity rates (2.5%7 and 3.8%8) to human NF155 although 22% positivity to rat NF155 was reported.9 We previously reported that patients with combined central and peripheral demyelination (CCPD) frequently harbored anti-rat NF155 antibodies while some CIDP patients were also positive.10 In the present study, we developed a more specific antibody assay using human NF155 and found anti-NF155 antibodies occurred in a subset of CIDP patients with distal-dominant involvement and symmetric nerve hypertrophy.
Subjects and Methods
Subjects
Fifty-five consecutive CIDP patients, who met the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) definite electrodiagnostic criteria for CIDP, were diagnosed at Kyushu University Hospital between 2004 and 2014. Among them, 50 patients were enrolled in the present study, after excluding five patients whose sera were unavailable. Sera from 32 patients with multiple sclerosis (MS) according to the revised McDonald criteria,11 26 patients with Guillain–Barré syndrome (GBS)12 or Fisher syndrome (FS),13 seven patients with vasculitic neuropathy, three patients with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) syndrome,14 three patients with hereditary motor and sensory neuropathy including two with the duplication of the peripheral myelin protein 22 gene, one patient with anti-myelin-associated glycoprotein antibody-positive neuropathy, and 30 healthy controls (HCs) were used for anti-NF155 and -NF186 antibody measurement. To characterize the clinical features, four additional anti-NF155 antibody-positive CIDP patients referred from other clinics were included. CIDP patients were classified into subtypes according to the EFNS/PNS CIDP guidelines15: distal acquired demyelinating symmetric (DADS) neuropathy was diagnosed by the presence of disproportionately prolonged motor distal latencies (DL) resulting in a terminal latency index (TLI) ≤0.25 in at least two nerves16; and responders to treatment were defined as patients whose Hughes functional scale scores17 at the last visit were decreased by ≥1 grade compared with those at peak of illness. This study was approved by the Kyushu University Hospital Ethics Committee.
Flow cytometric assay for anti-NF155 and anti-NF186 antibodies
Flow cytometry (FCM) detected IgG binding to cells expressing recombinant human NF155 or NF186 to exclude bias among evaluators. Generation of transformed cell lines stably expressing NF155 or NF186 is described in Data S1. NF155-turbo green fluorescent protein (turbo GFP)-transfected and naive HEK293 cells were evenly mixed and resuspended in Dulbecco's modified Eagle's medium containing 1% fetal bovine serum and 1 mmol/L ethylenediaminetetraacetic acid (EDTA) (FCM buffer) at a concentration of 1.0 × 106 cells/mL, and rotated at 4°C for 60 min. Serum samples (2.5 μL) were mixed with 47.5 μL of cell-containing solution (1:20 dilution). After incubation at 4°C for 60 min, cells were washed and bound IgG was detected with Alexa 647-labeled anti-human IgG antibodies (Life Technologies, Carlsbad, CA), diluted 1:500 with FCM buffer. After incubation at 4°C for 60 min, cells were washed and resuspended in 100 μL phosphate-buffered saline (PBS) containing 5 mmol/L EDTA and analyzed by MACSQuant Analyzer (Miltenyi Biotec, Bergisch Gladbach, Germany). The mean fluorescence intensity (MFI) of cell-associated turbo GFP and Alexa 647 was measured for each sample. Cells expressing NF155-turbo GFP and naive cells were easily separable according to the MFI of turbo GFP (Fig.1A). The MFI of cell-associated Alexa 647 was measured to detect human IgG bound to NF155, using cells without NF155 expression as a negative control. Positive and negative patients were clearly separable, and MFI ratio and delta MFI values were proportionately decreased on sequential dilution of serum from an anti-NF155 antibody-positive patient (Fig.1B). For each serum sample, the MFI ratio was calculated by dividing Alexa 647 MFI of NF155-transfected cells by Alexa 647 MFI of NF155-untransfected cells, and delta MFI was calculated by subtracting Alexa-647 MFI of NF155-untransfected cells from Alexa 647 MFI of NF155-transfected cells. Cutoff points for MFI ratio and delta MFI were set at 10 and 100, respectively, based on preliminary experiments. Antibodies against human recombinant NF186 protein were also measured using the same method. In anti-NF155 antibody-positive patients, IgG subclass profiles were examined using phycoerythrin (PE)-conjugated mouse anti-human IgG1, IgG2, IgG3, and IgG4 antibodies (Beckman Coulter Inc., Brea, CA) at 1:500 dilution. Conventional cell-based assays for anti-NF155 and anti-NF186 antibodies are described in Data S1.
Figure 1.
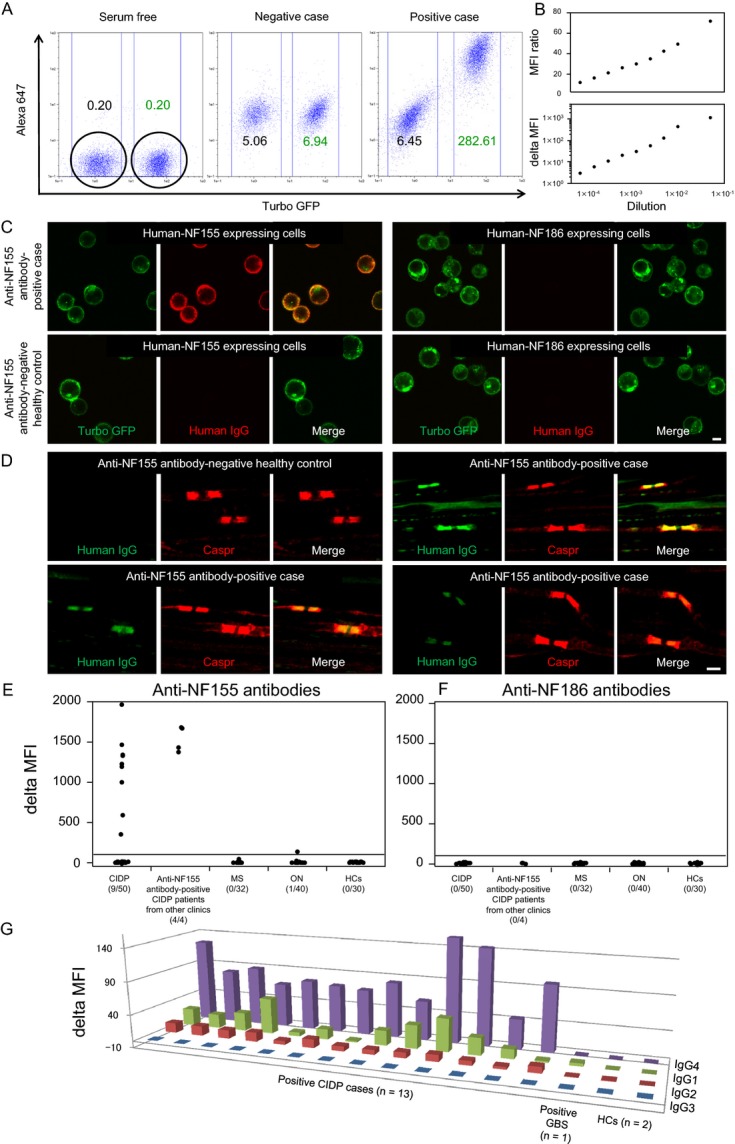
Anti-NF1155 and anti-NF186 antibody assays by flow cytometry and cell- and tissue-based immunohistochemistry. (A) Representative flow cytometry assay results showing serum-free (left), negative (middle), and positive (right) conditions. The MFI of cell-associated turbo GFP and Alexa 647 was measured for each sample. Cells expressing NF155-turbo GFP and naive cells are easily separable according to the MFI of turbo GFP. The MFI of cell-associated Alexa 647 was measured to detect human IgG bound to NF155, using cells without NF155 expression as a negative control. Note that positive and negative patients are clearly separable. For each serum sample, the MFI ratio was calculated by (Alexa 647 MFI of NF155-transfected cells/Alexa 647 MFI of NF155-untransfected cells), and delta MFI was calculated by (Alexa 647 MFI of NF155-transfected cells − Alexa-647 MFI of NF155-untransfected cells). Cutoff points for MFI ratio and delta MFI were set at 10 and 100, respectively, based on preliminary experiments. Antibodies against human recombinant NF186 protein were also measured using the same method. (B) Calibration curves using serum from a representative CIDP patient with anti-NF155 antibodies show proportionate decreases in MFI ratio and delta MFI values, by serum serial dilution (1:20, 1:100, 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400, and 1:12,800). (C) Immunohistochemistry of cells expressing human NF155-turbo GFP or NF186-turbo GFP shows that serum from a representative CIDP patient with anti-NF155 antibodies reacts with human NF155-expressing cells, but not with human NF186-expressing cells. Scale bar = 10 μm. (D) Double immunostaining of mouse teased sciatic nerve fibers with anti-Caspr antibody and sera from anti-NF155 antibody-positive CIDP patients or anti-NF155 antibody-negative healthy control. Similar paranodal staining patterns were observed for anti-Caspr antibody and patients’ sera. Scale bar = 2 μm. (E) Delta MFI for anti-NF155 antibodies. (F) Delta MFI for anti-NF186 antibodies. Numbers of positive samples/number of examined samples are expressed in parentheses. (G) Delta MFI for each IgG subclass in anti-NF155 antibodies. IgG4 antibodies are predominant in all 13 anti-NF155 antibody-positive CIDP patients, while IgG1 and IgG2 anti-NF155 antibodies are also present to a lesser extent. One GBS patient with anti-NF155 antibodies harbored IgG1 alone. CIDP, chronic inflammatory demyelinating polyneuropathy; GBS, Guillain–Barré syndrome; GFP, green fluorescence protein; HCs, healthy controls; MFI, mean fluorescence intensity; MS, multiple sclerosis; NF, neurofascin; ON, other neuropathies.
Immunostaining of mouse teased sciatic nerve fibers
Sciatic nerves were dissected from 10-week-old C57BL/6 mice at our animal facility and fixed for 10 min in freshly prepared PBS containing 4% paraformaldehyde. After washing with PBS, fixed nerves were teased and transferred to glass slides, permeabilized with 2% Triton X-100 in PBS for 30 min, blocked in 10% goat serum and 1% Triton X-100 in PBS for 60 min, then incubated at 4°C in blocking solution containing rabbit anti-Caspr antibodies (diluted 1:500) (Abcam, Cambridge, U.K.) and sera from anti-NF155 antibody-positive CIDP patients or HCs (diluted 1:20). After 2 days, teased nerve fibers were washed three times in PBS for 30 min and incubated for 60 min with Alexa 488-labeled anti-human IgG and Alexa 647-labeled anti-rabbit IgG (Life Technologies), diluted 1:500. Finally, teased nerve fibers were washed three times in PBS for 30 min, mounted with PermaFluor (Thermo Scientific, Waltham, MA) and examined by confocal microscopy (A1; Nikon, Tokyo, Japan), using 488- and 638-nm lasers for excitation.
Neuroimaging
Magnetic resonance imaging (MRI) neurography with three-dimensional nerve-SHeath signal increased with INKed rest-tissue rapid acquisition with relaxation Enhancement Imaging (3D SHINKEI)18 of cervical roots and brachial plexuses, and lumbosacral roots and plexuses were acquired using a 3.0-T whole-body clinical imager (Achieva; Philips Healthcare, Best, the Netherlands) in all CIDP cases visiting Kyushu University Hospital after 2012 when 3D SHINKEI imaging became available, including seven anti-NF155 antibody-positive and 20 anti-NF155 antibody-negative patients. Details of 3D SHINKEI parameters are described in Data S1. Data analyses were performed by one neuroradiologist (A.H.), blinded to the diagnosis. The diameter of the cervical nerve root was defined as the maximum vertical length of the root. The largest root diameters among bilateral C5–C8 roots were compared between CIDP patients with and without anti-NF155 antibodies.
Electrophysiology
Nerve conduction studies were performed using conventional procedures as described previously.19 Motor nerve conduction studies including F-wave analyses were performed in the median, ulnar, tibial, and peroneal nerves. Sensory nerve conduction studies were performed in median, ulnar, and sural nerves. Bilateral values were used when available. Base-to-peak amplitudes were measured for compound muscle action potentials (CMAP) and sensory nerve action potentials (SNAP). TLI was calculated using the formula: distal conduction distance (mm)/forearm conduction velocity (m/sec)/DL (msec).
Pathological studies of biopsied sural nerves
Pathology of biopsied sural nerve specimens from two anti-NF155 antibody-positive CIDP patients was studied. Duration from onset to time of biopsy was 9 months in a 39-year-old female and 18 years in a 40-year-old male. SNAP of the sural nerve at the time of biopsy were absent in both patients. Nerves were assessed using standard Epon-embedded sections stained with toluidine blue, as described previously.20 Detailed methods are described in Data S1.
Statistics
For comparisons between two groups, qualitative variables were analyzed using the Fisher exact test. Continuous variables that followed a parametric distribution were analyzed by Student's t-test, and nonparametric variables were analyzed by Mann–Whitney U test. Correlations were calculated with Pearson's coefficient. The threshold for significance was set at P < 0.05.
Results
Frequency of anti-NF155 and anti-NF186 antibodies
Positivity rates for anti-NF155 antibodies by FCM among patients with CIDP, MS, other neuropathies, or HCs were 18% (9/50), 0% (0/32), 2.5% (1/40), and 0% (0/30), respectively, (Fig.1E and S1A). Positive samples were also confirmed positive by conventional cell-based immunohistochemistry assays (Fig.1C). Anti-NF155 antibody-positive CIDP patient sera bound to the paranodal regions of mouse teased sciatic nerve fibers, with a staining pattern similar to anti-Caspr antibody (Fig.1D). When CIDP patients were classified into subtypes,15,16 DADS neuropathy had the highest positivity rate (3/5, 60.0%), while 6/36 (16.7%) typical CIDP patients were positive (Table S1). By contrast, no patients with multifocal acquired demyelinating sensory and motor neuropathy (MADSAM), focal, pure motor, or pure sensory types were positive for anti-NF155 antibodies. No subjects examined were positive by anti-NF186 antibody assay, except for one MS patient with weak positivity only observed by the MFI ratio (Fig.1F and S1B).
IgG subclass of anti-NF155 antibodies
In all 13 anti-NF155 antibody-positive CIDP patients, including those from other clinics, IgG4 antibodies were predominant, while lower levels of IgG1 and IgG2 anti-NF155 antibodies were also present (Fig.1G and S1C). One GBS patient with anti-NF155 antibodies harbored low IgG1 levels.
Clinical features
Age at onset was lower in anti-NF155 antibody-positive CIDP patients than in anti-NF155 antibody-negative patients (P < 0.0001) (Table1). DADS phenotype, drop foot, gait disturbance, and tremor were more frequently observed in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (P = 0.0014, 0.0242, 0.0484, and 0.0300, respectively). Cerebrospinal fluid (CSF) protein levels were markedly higher in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (P < 0.0001). The frequency of brain MRI lesions (suggestive of inflammatory demyelination) was threefold greater in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients. When only CIDP patients subjected to MRI neurography in our department were analyzed, similar trends were observed for anti-NF155 antibody status (Table S2).
Table 1.
Demographic features of CIDP patients with and without anti-NF155 antibodies1
| All patients | NF155 antibody-negative CIDP | NF155 antibody-positive CIDP | P-value | |
|---|---|---|---|---|
| Demographics | N = 54 | N = 41 | N = 13 | |
| Sex ratio (male:female) | 38:16 | 30:11 | 8:5 | NS |
| Age at onset (age range) (years)2 | 42.4 ± 18.4 (13–76) | 47.9 ± 17.0 (13–76) | 25.2 ± 10.7 (13–50) | <0.0001 |
| Age at examination (years) | 44.5 ± 19.3 | 50.3 ± 17.6 | 26.2 ± 11.9 | <0.0001 |
| Follow-up period (months) | 70.1 ± 94.2 | 70.5 ± 89.3 | 69.1 ± 112.2 | NS |
| Clinical phenotype | n/N (%) | n/N (%) | n/N (%) | |
| Typical | 37/54 (68.5) | 30/41 (73.2) | 7/13 (53.8) | NS |
| DADS | 8/54 (14.8) | 2/41 (4.9) | 6/13 (46.2) | 0.0014 |
| MADSAM | 4/54 (7.4) | 4/41 (9.8) | 0/13 (0.0) | NS |
| Focal | 2/54 (3.7) | 2/41 (4.9) | 0/13 (0.0) | NS |
| Pure sensory | 2/54 (3.7) | 2/41 (4.9) | 0/13 (0.0) | NS |
| Pure motor | 1/54 (1.9) | 1/41 (2.4) | 0/13 (0.0) | NS |
| Hughes functional scale score | N = 54 | N = 41 | N = 13 | |
| At the peak of illness | 2.31 ± 0.91 | 2.22 ± 0.88 | 2.62 ± 0.96 | NS |
| At the last visit | 1.63 ± 0.90 | 1.54 ± 0.90 | 1.92 ± 0.86 | NS |
| Mode of onset | n/N (%) | n/N (%) | n/N (%) | |
| Acute | 0/54 (0.0) | 0/41 (0.0) | 0/13 (0.0) | NS |
| Subacute | 5/54 (9.3) | 4/41 (9.8) | 1/13 (7.7) | NS |
| Chronic | 49/54 (90.7) | 37/41 (90.2) | 12/13 (92.3) | NS |
| Symptoms and signs | n/N (%) | n/N (%) | n/N (%) | |
| Visual disturbance | 6/54 (11.1) | 3/41 (7.3) | 3/13 (23.1) | NS |
| Facial sensory disturbance | 10/54 (18.5) | 7/41 (17.1) | 3/13 (23.1) | NS |
| Facial palsy | 4/54 (7.4) | 2/41 (4.9) | 2/13 (15.4) | NS |
| Limb weakness | 52/54 (96.3) | 39/41 (95.1) | 13/13 (100) | NS |
| Muscle atrophy (UE) | 22/54 (40.7) | 19/41 (46.3) | 3/13 (23.1) | NS |
| Muscle atrophy (LE) | 24/54 (44.4) | 16/41 (39.0) | 8/13 (61.5) | NS |
| Drop foot | 22/54 (40.7) | 13/41 (31.7) | 9/13 (69.2) | 0.0242 |
| Gait disturbance | 43/54 (79.6) | 30/41 (73.2) | 13/13 (100) | 0.0484 |
| Cerebellar ataxia | 6/54 (11.1) | 4/41 (9.8) | 2/13 (15.4) | NS |
| Tremor | 15/54 (27.8) | 8/41 (19.5) | 7/13 (53.8) | 0.0300 |
| Disturbance of superficial sensation | 40/54 (74.1) | 32/41 (78.0) | 8/13 (61.5) | NS |
| Disturbance of deep sensation | 48/54 (88.9) | 35/41 (85.4) | 13/13 (100) | NS |
| Blood and CSF tests | n/N (%) | n/N (%) | n/N (%) | |
| Monoclonal protein | 3/49 (6.1) | 3/36 (8.3) | 0/13 (0.0) | NS |
| ANA ≥1:160 | 4/54 (7.4) | 2/41 (4.9) | 2/13 (15.4) | NS |
| CSF protein amounts (mg/dL) | 157.1 ± 132.9 | 103.8 ± 75.8 | 317.0 ± 141.1 | <0.0001 |
| CSF cell counts (/μL) | 3.2 ± 5.1 | 2.7 ± 5.5 | 4.9 ± 3.1 | NS |
| CSF albuminocytologic dissociation | 39/52 (75.0) | 32/39 (82.1) | 7/13 (53.8) | 0.0644 |
| Inflammatory demyelination on MRI3 | n/N (%) | n/N (%) | n/N (%) | |
| Brain lesions | 6/40 (15.0) | 3/31 (9.7) | 3/9 (33.3) | NS |
| Spinal cord lesions | 3/31 (9.7) | 3/24 (12.5) | 0/7 (0.0) | NS |
ANA, antinuclear antibodies; CIDP, chronic inflammatory demyelinating polyneuropathy; DADS, distal acquired demyelinating symmetric neuropathy; LE, lower extremities; MADSAM, multifocal acquired demyelinating sensory and motor neuropathy; n, number of positive patients; N, number of patients collated; NF, neurofascin; NS, not significant; UE, upper extremities; CSF, cerebrospinal fluid; CNS, central nervous system.
All continuous values are shown as mean ± SD.
In several CIDP patients with CNS lesions suggestive of demyelination, the ages at onset of peripheral neuropathy were used.
Conventional brain and spinal cord MRI studies were performed as described previously29 using a 1.5- or 3.0-T whole-body clinical imager (Achieva; Philips Healthcare).
MRI neurography findings
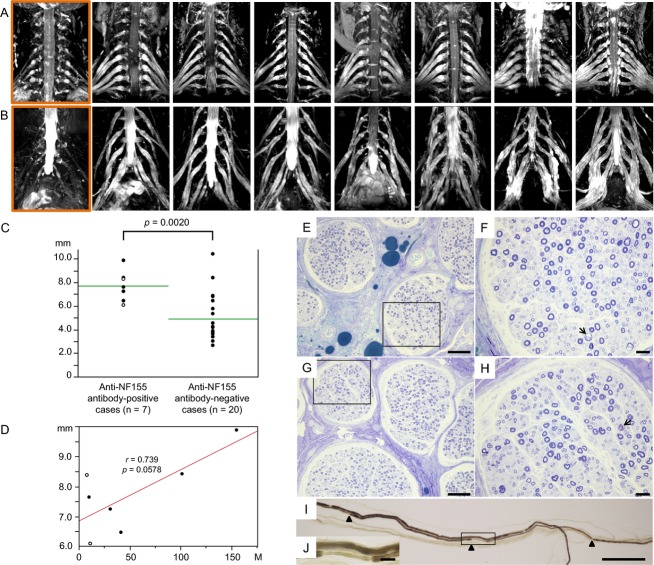
The cervical and lumbosacral roots/plexuses of all seven anti-NF155 antibody-positive CIDP patients showed marked symmetric hypertrophy (Fig.2A and B). Measurement of the largest root diameters among bilateral C5–C8 roots were significantly greater in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (7.7 ± 1.3 mm vs. 4.9 ± 2.0 mm, P = 0.0020, Fig.2C). The frequency of the largest nerve roots with a diameter >6.0 mm was more common in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (100% vs. 25%, P = 0.0009). The root diameters showed a trend toward positive correlation with disease duration in anti-NF155 antibody-positive CIDP patients (r = 0.739, P = 0.0578, Fig.2D), but not in anti-NF155 antibody-negative patients (data not shown).
Figure 2.
Cervical and lumbosacral neurography and pathology of sural nerve specimens from anti-NF155 antibody-positive CIDP patients. (A) Images of cervical roots and brachial plexuses of seven anti-NF155 antibody-positive CIDP patients and one representative anti-NF155 antibody-negative CIDP patient (the left end in a rectangle). (B) Images of lumbosacral roots and plexuses in the same patients as (A). Marked nerve hypertrophy was observed in all anti-NF155 antibody-positive CIDP patients. (C) Comparison of the largest cervical root diameters between CIDP patients with and without anti-NF155 antibodies. A significantly higher frequency of largest nerve roots >6.0 mm was observed in anti-NF155 antibody-positive CIDP patients than in anti-NF155 antibody-negative CIDP patients (100% vs. 25%, P = 0.0009). Open circles indicate patients with DADS. (D) Relationship between cervical root diameter and disease duration in anti-NF155 antibody-positive CIDP patients. Open circles indicate patients with DADS. (E) Toluidine blue staining of the sural nerve from a 39-year-old female with a disease duration of 9 months. (F) Higher magnification of the rectangular field in (E). (G) Toluidine blue staining of the sural nerve from a 40-year-old male with a disease duration of 18 years. (H) Higher magnification of the rectangular field in (G). Subperineurial edema is present in the absence of vasculitis, infiltration of inflammatory cells, or onion bulb formation. Demyelinated fibers (arrow) and naked axons are infrequently present in both patients, while myelinated fiber loss is more evident in the patient with the longer disease duration, but is still not severe. (I) Teased nerve fiber specimens from a 40-year-old male with longer disease duration. Paranodal demyelination is partial. Arrowheads indicate nodes of Ranvier. (J) Higher magnification of the rectangular field in (I). Scale bars: E and G = 100 μm; F and H = 20 μm; I = 200 μm; J = 20 μm. CIDP, chronic inflammatory demyelinating polyneuropathy; DADS, distal acquired demyelinating symmetric neuropathy; NF, neurofascin.
Nerve conduction study findings
Significantly longer F-wave latencies of the median, ulnar, and tibial nerves (P = 0.0033, <0.0001, and 0.0109, respectively) and DL of the ulnar and tibial nerves (P = 0.0009 and 0.0001, respectively) were observed in anti-NF155 antibody-positive CIDP patients than in anti-NF155 antibody-negative CIDP patients (Table2). Although DL in the median nerve did not differ significantly between the two groups, the frequency of median nerves with >50% DL prolongation above the upper normal limit (an EFNS/PNS electrodiagnostic criterion) was higher in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (81.0% vs. 43.7%, P = 0.0029). CMAP amplitudes and TLIs of the tibial nerve were lower in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (P = 0.0011 and <0.0001, respectively). Motor conduction velocity did not differ significantly between groups. Sensory conduction velocity (SCV) of the median and ulnar nerves was significantly decreased in anti-NF155 antibody-positive patients compared with anti-NF155 antibody-negative patients (P = 0.0038 and <0.0001, respectively), and unevoked SNAP frequencies in the median and ulnar nerves were higher in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (81.0% vs. 42.9%, P = 0.0026 and 75.0% vs. 38.2%, P = 0.049, respectively). These significant differences remained after eliminating patients with MADSAM and focal phenotypes (data not shown). Abnormalities in DL of the median, ulnar, and tibial nerves, in F-wave latency of the ulnar and tibial nerves, in CMAP amplitudes of the tibial nerve, and in SCV of the median, ulnar, and sural nerves were significantly higher in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (Table S3).
Table 2.
Nerve conduction study findings in CIDP patients with and without anti-NF155 antibodies1
| All CIDP patients | NF155 antibody-negative CIDP | NF155 antibody-positive CIDP | P-value | |
|---|---|---|---|---|
| Median nerve | N = 92 | N = 71 | N = 21 | |
| Distal latency (msec) | 6.9 ± 3.0 (92/92) | 6.7 ± 3.3 (71/71) | 7.7 ± 1.4 (21/21) | NS |
| Terminal latency index | 0.36 ± 0.18 | 0.37 ± 0.19 | 0.32 ± 0.16 | NS |
| MCV (m/sec) | 35.0 ± 12.2 | 35.7 ± 12.1 | 32.7 ± 12.4 | NS |
| CMAP amplitude (mV) | 4.7 ± 3.3 | 4.7 ± 3.7 | 4.7 ± 1.8 | NS |
| F-wave latency (msec) | 45.3 ± 13.6 (62/91) | 42.4 ± 11.4 (46/70) | 53.7 ± 16.3 (16/21) | 0.0033 |
| SCV (m/sec) | 43.8 ± 9.7 (44/91) | 45.1 ± 8.8 (40/70) | 30.8 ± 10.1 (4/21) | 0.0038 |
| SNAP amplitude (μV) | 5.1 ± 3.7 | 5.3 ± 3.72 | 3.2 ± 2.92 | NS |
| Ulnar nerve | N = 88 | N = 68 | N = 20 | |
| Distal latency (msec) | 4.9 ± 1.8 (88/88) | 4.6 ± 1.8 (68/68) | 6.0 ± 1.1 (20/20) | 0.0009 |
| Terminal latency index | 0.46 ± 0.19 | 0.48 ± 0.193 | 0.42 ± 0.16 | NS |
| MCV (m/sec) | 37.6 ± 13.5 | 39.0 ± 13.0 | 32.9 ± 14.3 | 0.0758 |
| CMAP amplitude (mV) | 4.2 ± 2.7 | 4.1 ± 2.9 | 4.3 ± 2.0 | NS |
| F-wave latency (msec) | 44.5 ± 17.0 (56/88) | 37.8 ± 8.4 (40/68) | 61.4 ± 21.2 (16/20) | <0.0001 |
| SCV (m/sec) | 43.2 ± 9.0 (47/88) | 44.9 ± 7.5 (42/68) | 28.8 ± 7.6 (5/20) | <0.0001 |
| SNAP amplitude (μV) | 3.7 ± 3.0 | 4.0 ± 3.12 | 1.2 ± 0.972 | 0.0529 |
| Tibial nerve | N = 92 | N = 71 | N = 21 | |
| Distal latency (msec) | 8.0 ± 4.2 (75/92) | 7.0 ± 3.7 (60/71) | 12.2 ± 3.8 (15/21) | 0.0001 |
| Terminal latency index | 0.49 ± 0.18 | 0.53 ± 0.17 | 0.31 ± 0.11 | <0.0001 |
| MCV (m/sec) | 32.6 ± 9.8 | 33.7 ± 9.6 | 28.5 ± 9.4 | 0.0741 |
| CMAP amplitude (mV) | 4.0 ± 4.4 | 4.8 ± 4.5 | 0.78 ± 1.8 | 0.0011 |
| F-wave latency (msec) | 64.3 ± 14.6 (44/92) | 62.3 ± 14.1 (39/71) | 79.7 ± 9.5 (5/21) | 0.0109 |
| Sural nerve | N = 93 | N = 71 | N = 22 | |
| SCV (m/sec) | 44.5 ± 6.1 (52/93) | 45.2 ± 6.4 (41/71) | 41.9 ± 3.9 (11/22) | NS |
| SNAP amplitude (μV) | 7.0 ± 5.8 | 6.6 ± 5.8 | 8.4 ± 5.9 | NS |
CIDP, chronic inflammatory demyelinating polyneuropathy; CMAP, compound muscle action potentials; MCV, motor conduction velocity; N, number of examined nerves; NF, neurofascin; SCV, sensory conduction velocity; SNAP, sensory nerve action potentials; NS, not significant.
All continuous values are shown as mean ± SD, with number of evoked nerves/number of examined nerves in parentheses. Normal values of distal latencies: median, 3.49 ± 0.34 msec; ulnar, 2.59 ± 0.39 msec; tibial 3.96 ± 1.00 msec. Normal values of MCV: median, 57.7 ± 4.9 m/sec; ulnar, 58.7 ± 5.1 m/sec; tibial, 48.5 ± 3.6 m/sec. Normal values of CMAP amplitudes: median, 7.0 ± 3.0 mV; ulnar, 5.7 ± 2.0 mV; tibial 5.8 ± 1.9 mV. Normal values of F-wave latencies: median, 26.2 ± 2.2 msec; ulnar, 27.6 ± 2.2 msec; tibial 47.7 ± 5.0 msec. Upper limit of normal (ULN) of distal latencies: median, 4.2 msec; ulnar, 3.4 msec; tibial 6.0 msec. Lower limit of normal (LLN) of MCV: median, 48 m/sec; ulnar, 49 m/sec; tibial, 41 m/sec. LLN of CMAP amplitudes: median, 3.5 mV; ulnar, 2.8 mV; tibial, 2.9 mV. ULN of F-wave latencies: median, 31 msec; ulnar, 32 msec; tibial, 58 msec. LLN of SCV: median, 44 m/sec; ulnar, 44 m/sec; sural, 45 m/sec.
The unevoked SNAP frequencies in the median and ulnar nerves were significantly higher in anti-NF155 antibody-positive patients than in anti-NF155 antibody-negative patients (81.0% vs. 42.9%, P = 0.0026, 75.0% vs. 38.2%, P = 0.049, respectively).
One outlier was omitted.
Pathological findings of biopsied sural nerves
Subperineurial edema was evident and demyelinated fibers and naked axons were occasionally present in both specimens examined (Fig.2E–J). Myelinated fiber loss was more evident in the patient with longer disease duration (4930/mm2) compared with shorter disease duration (6420/mm2), although fiber loss was not severe (control = 7710 ± 1130/mm2). Both specimens showed no vasculitis, infiltration of inflammatory cells, or onion bulb formation. The teased nerve fiber study demonstrated the presence of paranodal demyelination in 3/50 myelinated fibers in both patients.
Differences in adopted immunotherapies between treatment responders and nonresponders in anti-NF155 antibody-positive CIDP patients
Improvements in neurological findings of anti-NF155 antibody-positive CIDP patients after IVIg, intravenous corticosteroid pulse therapy, oral corticosteroids, and plasmapheresis were observed in 4/13 (30.8%), 3/8 (37.5%), 5/8 (62.5%), and 4/6 (66.7%) patients, respectively. Among 13 anti-NF155 antibody-positive CIDP patients, eight patients were treatment responders, while five were nonresponders. Comparison of treatment modalities between responders and nonresponders showed that the number of treatment modalities was greater in responders than in nonresponders (P = 0.0118) (Table3). IVIg was administered to all patients regardless of treatment response, while corticosteroid therapy was used significantly more often in responders than in nonresponders. Daily oral corticosteroids and/or immunosuppressants were also administered to responders more frequently than nonresponders (P = 0.0047).
Table 3.
Comparison of treatment modality between responders and nonresponders in anti-NF155 antibody-positive CIDP patients
| Total patients (N = 13) | Responders (N = 8) | Nonresponders (N = 5) | P-value | |
|---|---|---|---|---|
| Number of applied immunotherapies1(median, IQR) | 2 (2, 3.5) | 3 (2.25, 4) | 2 (1, 2) | 0.0118 |
| n/N (%) | n/N (%) | n/N (%) | ||
| IVIg | 13/13 (100) | 8/8 (100) | 5/5 (100) | NS |
| Corticosteroids | 10/13 (76.9) | 8/8 (100) | 2/5 (40.0) | 0.0350 |
| Oral corticosteroids | 8/13 (61.5) | 7/8 (87.5) | 1/5 (20.0) | 0.0319 |
| Intravenous corticosteroids | 8/13 (61.5) | 7/8 (87.5) | 1/5 (20.0) | 0.0319 |
| Plasmapheresis | 6/13 (46.2) | 5/8 (62.5) | 1/5 (20.0) | NS |
| Other immunosuppressants | 4/13 (30.8) | 4/8 (50.0) | 0/5 (0.0) | NS |
| Corticosteroids and plasmapheresis | 5/13 (38.5) | 5/8 (62.5) | 0/5 (0.0) | 0.0754 |
| Daily immunosuppressants and/or corticosteroids at the last visit | 7/13 (53.8) | 7/8 (87.5) | 0/5 (0.0) | 0.0047 |
NF, neurofascin; CIDP, chronic inflammatory demyelinating polyneuropathy; IVIg, intravenous immunoglobulin; IQR, interquartile range; n, number of involved patients; N, number of patients collated; NS, not significant.
Applied immunotherapies among IVIg, corticosteroids, plasmapheresis, and other immunosuppressants (azathioprine or cyclosporine) were counted from 0 to 4.
Discussion
The present study demonstrated anti-human NF155 antibodies were present in 18% of CIDP patients tested by specific cell-based FCM assays using human NF155 and that IgG4 anti-NF155 antibodies were associated with younger onset age, higher frequencies of drop foot, gait disturbance, tremor and DADS phenotype, greater spinal root diameter on MRI neurography, higher CSF protein levels, and more pronounced prolongation of distal and F-wave latencies in CIDP patients.
By using human recombinant NF155 and cell-based FCM assays, the specificity of our cell-based anti-NF155 antibody assay to CIDP was 98.9% based on a total IgG assay and 100% based on IgG4 subclass assay. Nonetheless, the positivity rate of anti-NF155 antibodies among CIDP patients in this study was much higher than in two previous studies (2.5%7 and 3.8% by ELISA8) using human recombinant NF155 as an antigen. Although another study9 reported similar positivity rates to ours, this study performed ELISA using the extracellular domain of rat recombinant NF155 derived from NS0 murine myeloma cells, which may cause nonspecific binding as reported previously.7 The discrepancy between our study and two previous studies using the same human NF155 may be attributable in part to differences in inclusion criteria and races studied. We included only electrodiagnostic definite CIDP patients, who were classified to the definite CIDP category, whereas one article did not describe the diagnostic criteria used,7 and another adopted EFNS/PNS diagnostic criteria but did not state whether probable or possible CIDP cases were enrolled.8
A previous study described the features of four anti-NF155 antibody-positive patients only, where four cases presented with severe motor and sensory involvement, three cases with predominantly distal involvement, and three cases with disabling tremor.8 In the current study, anti-NF155 antibody was not detected in any patients with MADSAM or focal CIDP. We revealed, for the first time, statistically significant differences in clinical features between anti-NF155 antibody-positive and -negative cases. Anti-NF155 antibody-positive cases had a younger age at disease onset, in contrast to older age at onset in anti-contactin-1 antibody-positive CIDP preferentially affecting axons in the early course.2 Predominantly distal motor and sensory involvement and disabling tremor were significantly more common in anti-NF155 antibody-positive cases than in antibody-negative cases, in accord with a previous small case series.8 However, proximal parts of limbs can also be affected as shown in our and previous studies, suggesting the emergence of anti-NF155 antibodies are not confined to the DADS phenotype but might also be present in the typical CIDP phenotype. We previously reported the occurrence of anti-NF155 antibodies in CCPD patients.10 In the present study of CIDP patients, brain MRI lesions suggestive of demyelination were more common in anti-NF155 antibody-positive CIDP patients than in antibody-negative patients, suggesting an association of anti-NF155 antibodies with brain white matter involvement. Further large-scale studies are required to clarify this point.
A distinguishing feature of anti-NF155 antibody-positive CIDP is marked symmetric nerve hypertrophy on MRI. Nerve hypertrophy is regarded as a cardinal feature of CIDP by both pathological and neuroimaging studies21,22; however, relevant immunological biomarkers for hypertrophy are undefined. Here, the presence of anti-NF155 antibodies was associated with diffuse symmetric but not focal nerve hypertrophy. We examined all CIDP patients who regularly visited Kyushu University Hospital from 2012 when 3D SHINKEI imaging became available. Although CIDP cases examined before the introduction of 3D SHINKEI imaging and four anti-NF155 antibody-positive CIDP cases referred from other clinic were not examined for MRI neurography, we consider it relevant that all anti-NF155 antibody-positive cases examined by MRI neurography in our institute uniformly showed hypertrophy of nerve roots and proximal nerve segments. As root diameters in anti-NF155 antibody-positive cases showed a trend toward a positive correlation with disease duration, the presence of anti-NF155 antibodies may predict progressive nerve hypertrophy during the late course of disease.
Marked prolongation of distal and F-wave latencies in anti-NF155 antibody-positive CIDP patients suggested preferential involvement of nerve terminals and spinal roots. More frequent and severe involvement of median and ulnar nerves compared with sural nerves in sensory nerve conduction studies indicated that distal parts of nerves might be more vulnerable in anti-NF155 antibody-positive patients, because median and ulnar sensory conduction studies involve nerve terminals, while those of the sural nerve involve the intermediated nerve segment.23 Extremely high CSF protein levels in anti-NF155 antibody-positive patients also indicated preferential involvement of spinal roots. Preferential sites of involvement in anti-NF155 antibody-positive CIDP are compatible with the observation that autoantibody-mediated neuropathies preferentially affect the distal nerve terminals and nerve roots where the blood–nerve barrier is anatomically deficient or leaky.24,25
Sera from anti-NF155 antibody-positive CIDP patients bound specifically to paranodal regions of peripheral nerves, suggesting the paranodes are primary targets. However, anti-NF155 antibodies observed in this study were mainly IgG4, which lacks complement binding and activating capabilities. Biopsied sural nerve specimens from two anti-NF155 antibody-positive CIDP patients showed no inflammatory features, although SNAP were not evoked in either patient. Collectively, these observations suggest the primary role of IgG4 anti-NF155 antibodies may be blockade of interactions between NF155 and Caspr/contactin-1, leading to conduction failure. This might be consistent with the finding that myelinating glia-specific ablation of NF155 induces a loss of septae-like transverse bands at the paranodal axoglial junction and decreased conduction velocities in peripheral nerves even though the thickness of myelin at the internodes was intact.26 We assume that blockade of NF155 and Caspr/contactin-1 interactions may lead to features similar to those of myelinating-glia-specific NF155 null mice.
Because of a lack of histological examination of proximal nerve segments, the mechanism of marked symmetric spinal root and plexus hypertrophy in anti-NF155 antibody-positive CIDP cases remains to be elucidated. In anti-NF155 antibody-positive CIDP patients, nerve edema caused by severe disruption of the blood–nerve barrier at spinal roots, indicated by a marked increase in CSF protein levels, might be one mechanism underlying symmetric spinal root/plexus hypertrophy. Even the sural nerve showed subperineurial edema, supporting this mechanism. Alternatively, onion bulb formation might be partly responsible for nerve hypertrophy as shown in biopsied CIDP brachial plexus,21 although we did not observe such features in the biopsied sural nerves.
Anti-NF155 antibodies were previously found in a fraction of CIDP patients’ refractory to IVIg.8 Our results also suggest that IVIg alone is not sufficient to improve the disabilities of such patients. However, our study had some limitations concerning the evaluation of treatment efficacy. First, the number of anti-NF155 antibody-positive patients was small. Second, we used the Hughes score, which may not be adequate for evaluating upper limb involvement. However, all our anti-NF155 antibody-positive cases had gait disturbance as a cardinal feature. Thus, the Hughes score might be useful for evaluating overall effectiveness of treatments. Third, various treatment regimens were applied because the study was retrospective. With these limitations in mind, corticosteroids combined with IVIg might be more beneficial than IVIg alone, because corticosteroids were used more often in treatment responders than in nonresponders. Corticosteroids are widely used to treat diseases related to disease-specific IgG4 autoantibodies, such as pemphigus27 and thrombotic thrombocytopenic purpura.28 Of note, treatment responders were more frequently administered daily oral corticosteroids and/or immunosuppressants compared with nonresponders, suggesting the necessity of long-term immunosuppression for sustained improvement. Future prospective studies to clarify adequate treatment modalities in anti-NF155 antibody-positive CIDP are required.
In conclusion, anti-NF155 antibodies measured by high-specificity assays defined a distinct subset of CIDP patients presenting with younger onset age, tremor, extremely high CSF protein levels, symmetric spinal root and plexus hypertrophy, and marked prolongation of distal and F-wave latencies, and therefore might be a biomarker for a unique subset of CIDP.
Acknowledgments
This study was partly supported by Health and Labour Sciences Research Grants on Intractable Diseases (H24-Nanchitou-Ippan-055 and H26-Itaku-Nan-Ippan-069) from the Ministry of Health, Labour, and Welfare, Japan, KAKENHI (24591265) and a “Glial Assembly” Grant-in-Aid for Scientific Research on Innovative Areas (FY2013-2017) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Center for Clinical and Translational Research of Kyushu University Hospital. We thank M. Satake (Department of Neurology, Hamanomachi Hospital), K. Sonoda (Department of Neurology, Kagoshima Medical Association Hospital), T. Mukaino and K. Takase (Department of Neurology, Aso Iizuka Hospital), W. Shiraishi, Y. Iwanaga, and A. Yamamoto (Department of Neurology, Japan Community Health Care Organization Kyushu Hospital), and H. Kishikawa (Faculty of Medicine, Kyushu University) for their cooperation.
Author Contributions
H. O., R. Y., N. K., D. M., T. M., H. M., and J. K. designed this study. H. O. and N. O. conducted the experiments. H. O., R. Y., A. H., N. O., M. K., H. S., S. K., H. K., F. T., Y. F., and K. I. collected the data. H. O., R. Y., A. H., N. O., S. K., T. M., H. M., and J. K. analyzed the data. H. O., R. Y., D. M., and J. K. wrote this article.
Conflict of Interest
R. Y.: grants/grants pending, the Ministry of Education, Culture, Sports, Science and Technology, Japan, Bayer Schering Pharma, Biogen Idec, Novartis Pharma, and Mitsubishi Tanabe Pharma. D. M.: grants/grants pending, the Ministry of Health, Labour and Welfare, Japan. S. K.: grants/grants pending, Sumitomo Dainippon Pharma, Teijin, Novartis, Astellas Pharma, Glaxo Smith Kline, Sanofi, Nihon Pharmaceutical, Otsuka Pharmaceutical, Japan Blood Products Organization, Eisai, Cosmic Corporation, and Genzyme Japan; speaking fees, Nihon Pharmaceutical, Sumitomo Dainippon Pharma, Teijin, Kyowa Kirin, Japan Blood Products Organization, Glaxo Smith Kline, Otsuka Pharmaceutical, Eisai, and Pfizer. T. M.: grants/grants pending, Bayer Schering Pharma, Biogen Idec, Novartis Pharma, and Mitsubishi Tanabe Pharma. H. M.: consultancy, Novartis Pharma; speaking fees, Astellas Pharma and Japan Blood Products Organization. J. K.: consultancy, Biogen Idec Japan; grants/grants pending, the Ministry of Health, Labour and Welfare, Japan, the Science and Technology Agency and the Ministry of Education, Culture, Sports, Science, and Technology, Japan, Japan Blood Products Organization, and Takeda Pharmaceutical Ltd.; speaking fees, Bayer Healthcare, Mitsubishi Tanabe Pharma, and Otsuka Pharmaceutical; travel expenses, Bayer Healthcare.
Supporting Information
Supplementary methods.
Table S1. Frequency of anti-NF155 antibodies among patients with various neurological diseases and healthy controls.
Table S2. Demographic features of CIDP patients with and without anti-NF155 antibodies evaluated by 3D SHINKEI sequencea.
Table S3. Frequencies of abnormal recordings in CIDP patients with and without anti-NF155 antibodies.
Figure S1. MFI ratios for anti-NF155 and anti-NF186 antibodies.
References
- Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011;7:507–517. doi: 10.1038/nrneurol.2011.121. [DOI] [PubMed] [Google Scholar]
- Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2013;73:370–380. doi: 10.1002/ana.23794. [DOI] [PubMed] [Google Scholar]
- Tait S, Gunn-Moore F, Collinson JM, et al. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J Cell Biol. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Pedraza L, Huang JK, Colman DR. Organizing principles of the axoglial apparatus. Neuron. 2001;30:335–344. doi: 10.1016/s0896-6273(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Odaka M, Yuki N. Nodal proteins are target antigens in Guillain-Barré syndrome. J Peripher Nerv Syst. 2012;17:62–71. doi: 10.1111/j.1529-8027.2012.00372.x. [DOI] [PubMed] [Google Scholar]
- Ng JKM, Malotka J, Kawakami N, et al. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology. 2012;79:2241–2248. doi: 10.1212/WNL.0b013e31827689ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querol L, Nogales-Gadea G, Rojas-Garcia R, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology. 2014;82:879–886. doi: 10.1212/WNL.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Nguyen T, Yuki N, et al. Antibodies to neurofascin exacerbate adoptive transfer experimental autoimmune neuritis. J Neuroimmunol. 2014;277:13–17. doi: 10.1016/j.jneuroim.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Kawamura N, Yamasaki R, Yonekawa T, et al. Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology. 2013;81:714–722. doi: 10.1212/WNL.0b013e3182a1aa9c. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(suppl):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- Odaka M, Yuki N, Hirata K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry. 2001;70:50–55. doi: 10.1136/jnnp.70.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dispenzieri A. POEMS syndrome: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:214–223. doi: 10.1002/ajh.23644. [DOI] [PubMed] [Google Scholar]
- European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society – first revision. J Peripher Nerv Syst. 2010;15:1–9. doi: 10.1111/j.1529-8027.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- Larue S, Bombelli F, Viala K, et al. Non-anti-MAG DADS neuropathy as a variant of CIDP: clinical, electrophysiological, laboratory features and response to treatment in 10 cases. Eur J Neurol. 2011;18:899–905. doi: 10.1111/j.1468-1331.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- Hughes RAC, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial of prednisolone in acute polyneuropathy. Lancet. 1978;2:750–753. doi: 10.1016/s0140-6736(78)92644-2. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Takahara T, Kwee TC, et al. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse. Magn Reson Med Sci. 2013;12:111–119. doi: 10.2463/mrms.2012-0063. [DOI] [PubMed] [Google Scholar]
- Osoegawa M, Ochi H, Yamada T, et al. High incidence of subclinical peripheral neuropathy in myelitis with hyperIgEaemia and mite antigen-specific IgE (atopic myelitis): an electrophysiological study. Intern Med. 2002;41:684–691. doi: 10.2169/internalmedicine.41.684. [DOI] [PubMed] [Google Scholar]
- Kaji R, Oka N, Tsuji T, et al. Pathological findings at the site of conduction block in multifocal motor neuropathy. Ann Neurol. 1993;33:152–158. doi: 10.1002/ana.410330204. [DOI] [PubMed] [Google Scholar]
- Duggins AJ, McLeod JG, Pollard JD, et al. Spinal root and plexus hypertrophy in chronic inflammatory demyelinating polyneuropathy. Brain. 1999;122:1383–1390. doi: 10.1093/brain/122.7.1383. [DOI] [PubMed] [Google Scholar]
- Tazawa K, Matsuda M, Yoshida T, et al. Spinal nerve root hypertrophy on MRI: clinical significance in the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy. Intern Med. 2008;47:2019–2024. doi: 10.2169/internalmedicine.47.1272. [DOI] [PubMed] [Google Scholar]
- Kuwabara S. The blood-nerve barrier and sensory nerve conduction. Clin Neurophysiol. 2007;118:1901–1902. doi: 10.1016/j.clinph.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol. 1990;5:265–311. [PubMed] [Google Scholar]
- Mizisin AP, Weerasuriya A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol. 2011;121:291–312. doi: 10.1007/s00401-010-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AM, Thaxton C, Pribisko AL, et al. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res. 2009;87:1773–1793. doi: 10.1002/jnr.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertl M, Jedlickova H, Karpati S, et al. Pemphigus. S2 Guideline for diagnosis and treatment – guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol. 2015;29:405–414. doi: 10.1111/jdv.12772. [DOI] [PubMed] [Google Scholar]
- Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323–335. doi: 10.1111/j.1365-2141.2012.09167.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Matsushita T, Osoegawa M, et al. Heterogeneity and continuum of multiple sclerosis in Japanese according to magnetic resonance imaging findings. J Neurol Sci. 2008;266:115–125. doi: 10.1016/j.jns.2007.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods.
Table S1. Frequency of anti-NF155 antibodies among patients with various neurological diseases and healthy controls.
Table S2. Demographic features of CIDP patients with and without anti-NF155 antibodies evaluated by 3D SHINKEI sequencea.
Table S3. Frequencies of abnormal recordings in CIDP patients with and without anti-NF155 antibodies.
Figure S1. MFI ratios for anti-NF155 and anti-NF186 antibodies.