Abstract
Objective
We tested whether declining motor function accelerates with age in older African Americans.
Methods
Eleven motor performances were assessed annually in 513 older African Americans.
Results
During follow-up of 5 years, linear mixed-effect models showed that motor function declined by about 0.03 units/yr (Estimate, −0.026, p<0.001); about 4% more rapidly for each additional year of age at baseline. A proportional hazard model showed that both baseline motor function level and its rate of change were independent predictors of death and incident disability (all p’s <0.001). These models showed that the additional annual amount of motor decline in 85 year old persons at baseline versus 65 year old persons was associated with a 1.5-fold higher rate of death and a 3-fold higher rate of developing Katz disability.
Conclusions
The rate of declining motor function accelerates with increasing age and its rate of decline predicts adverse health outcomes in older African Americans.
Keywords: Motor Decline, African American, Aging, Mortality, Disability
1 INTRODUCTION
Late-life motor impairment is a common concomitant of aging and increasingly recognized as a barrier to the maintenance of independence and well-being in old age.1–3 Many studies have shown that the level of motor function is associated with adverse health outcomes including the risk of death,4, 5 incident disability,1 cognitive decline and risk of Alzheimer’s disease.6 However, loss of motor function, such as reduced strength or slowed walking, is progressive in many older adults.7, 8
Nonetheless, there are few studies which have employed repeated objective motor performances in older African Americans to determine if the rate of change in motor function increases with age or have examined the extent to which the rate of progressive motor decline contributes to survival or incident disability in this population. Such data are crucial for efforts to decrease the personal and societal burden of late-life motor impairment in older African Americans and for efforts to eliminate health disparities.
We used clinical data collected from more than 500 community-dwelling older African Americans participating in the Minority Aging Research Study (MARS).9 Subjects underwent structured testing at baseline and at annual follow-up for up to 10 years. A global motor score was employed to summarize 11 motor performances as previously described.10
We examined its annual rate of change via a series of linear mixed-effect models which included terms for age, sex, and education and their interaction with the rate of change of global motor scores. Next, we examined whether baseline health status or frequency of physical activities might affect the rate of change in motor function. In further analyses, we examined a proportional hazard model which included separate terms for baseline level and the rate of change in global motor scores to determine whether they are independently associated with the risk of adverse health outcomes. Finally, we calculated the contribution of the rate of change in global motor scores to risk of death and incident disability.
2 METHODS
2.1 Participants
Participants included self-identified African Americans from an epidemiologic cohort study of risk factors for cognitive decline called the Minority Aging Research Study (MARS). 9 The cohort consists of non-institutionalized seniors over the age of 65 who agreed to annual clinical evaluations and cognitive testing. The cohort was recruited from various community-based organizations, churches, and senior subsidized housing facilities in and around the Chicago metropolitan area and was approved by the Institutional Review Board of Rush University Medical Center.
Since its inception in August, 2004, 932 subjects have been recruited into MARS, including 283 recruited from the Clinical Core of Rush Alzheimer’s Disease Center using identical recruitment procedures by the same staff.11 Due to participant burden, motor testing was only recently introduced in the MARS subjects from the Clinical Core. The longitudinal analyses which were examined in this work required us to include only persons who had completed a baseline motor assessment and had one or more follow-up assessments and is illustrated in Figure 1. On average, participant’s had 5 follow-up assessments (mean 5.1, SD=2.74; range 2–11).
Figure 1.
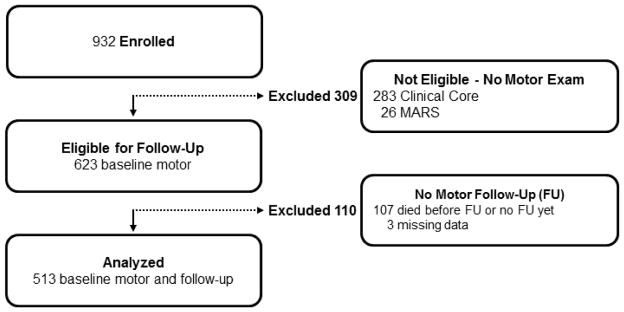
MARS Participants Included and Excluded from Current Study
2.2 Clinical Diagnoses
Clinical diagnoses were made using a multi-step process, as previously described.12 Cognitive function testing included 19 performance tests summarized into a composite measure of global cognition as described previously.13 Participants were then evaluated by an experienced physician who diagnosed dementia,14 stroke,15 or Parkinson’s disease (PD)16 or other neurological and psychiatric disorders based on published criteria.
2.3 Assessment of Motor Function
A uniform structured clinical evaluation is performed each year that includes medical history, neurologic examination, and neuropsychological performance tests.
Eleven motor performances employed by other investigators were assessed. (1) Grip and (2) pinch strength were measured bilaterally using the Jamar® hydraulic hand and pinch dynamometers (Lafayette Instruments, Lafayette) to assess manual strength. Upper extremity dexterity was based on (3) the number of pegs that could be placed into holes in a pegboard (Purdue Pegboard) in thirty seconds. Two trials were recorded for each hand. The four trials were averaged to provide a Purdue Pegboard score. In addition, (4) participants tapped an electronic tapper (Western Psychological Services, Los Angeles, CA) with their index finger as quickly as possible for ten seconds. Two trials were performed for each hand. The four trials were averaged together to yield a tapping score. To evaluate gait, we asked people to walk eight feet and turn 360° and measured the (5, 6) time and (7, 8) number of steps taken on each task. (9) To assess balance, we asked people to stand on each leg for ten seconds. (10) Persons were asked to stand on their toes for ten seconds. (11) We also asked people to walk an eight foot line heel to toe and counted the number of steps off line.
These measures were scaled and averaged to obtain a summary global motor score as previously described.10, 17 Composite measures of manual strength (2 tests), manual dexterity (2 tests), and gait (4 tests), were formed in a similar manner. We did not form a composite balance measure because the balance tests, unlike the other motor tests, were sometimes not attempted.10, 18 This composite global motor score has been previously reported to be associated with risk of mortality, incident disability and dementia.10 In addition, using this global motor score, we have found a wide range of degenerative brain neuropathologies are associated with declining motor function proximate to death.19, 20,21
2.4 Assessment of Other Covariates
Demographics: Sex was recorded at the baseline interview. Age in years was computed from self-reported date of birth, and date of the clinical examination at which the strength measures were first collected. Education (reported highest grade or years of education) was obtained at the time of the baseline cognitive testing. Body Mass Index (BMI): Weight and height were measured and recorded at each visit and BMI was calculated as weight in kilograms divided by height in meters squared. Chronic Health Conditions: As previously described,22 the number of 3 self-reported vascular risk factors (i.e. hypertension, diabetes mellitus, and smoking), and the number of 4 self-reported vascular diseases (i.e., myocardial infarction, congestive heart failure, claudication and stroke) were used in these analyses.22 Physical Activity: Physical activity was assessed using questions adapted from the 1985 National Health Interview Survey. Activities included walking for exercise, gardening or yard work and calisthenics. The number of activities performed was used in these analyses.23 Disability: Basic activities of daily living (ADLs) were assessed using a modified version of the Katz scale which assesses six activities: feeding, bathing, dressing, toileting, transferring, and walking across a small room. For these analyses, participants who reported needing help with or an inability to perform one or more tasks were classified as being disabled.24 Mortality: Study staff are in regular contact with participants. If we learn that a participant has died, we obtain the date of death from the death certificate, if possible. When an autopsy is obtained, date of death is known promptly. In cases where this was not possible, additional mechanisms for determining vital status included attempts with telephone calls to family contacts or National Death Index.
2.5 Statistical Analyses
We first examined the bivariate associations of global motor scores with demographic variables (age; sex; education) and other covariates via Spearman’s rank correlation (rho).
We next used linear mixed-effect models25 to assess both the level and the annual rate of change in global motor scores and its association with age, sex and education. The core model included a term for Time in years since as well as terms for 3 demographic variables at baseline and a term for their interaction with time since baseline. The term for Time indicates the average annual rate of change in global motor score for a typical participant (a 73 year old female with 15 years of education); the term for age indicates the average difference in global motor score at baseline associated with a year difference in age; and the interaction of age with time indicates the effect of a 1 year difference in age on the annual rate of change in global motor scores. Then we examined whether the interaction of age and the rate of change in motor scores was affected by several covariates that might affect motor function.
Finally, to determine the clinical significance of motor decline, we constructed Cox proportional hazards models to determine the association of the rate of motor decline and several adverse health outcomes. These models controlled for age, sex, education, and baseline global motor function. Models were examined graphically and analytically and assumptions were judged to be adequately met. A priori level of statistical significance was 0.05. Programming was done in SAS version 9.3 (SAS Institute Inc, Cary, NC).26
3 RESULTS
3.1 Descriptive Properties of Global Motor Score
There were 513 participants included in these analyses. Their age at baseline ranged from 58 to 94 with an average age of 73.4 (SD=6.12 years) with interquartile range of 7.58 years. Additional clinical characteristics of these participants at baseline are in Table 1.
Table 1.
Clinical Characteristics Participants at Baseline
| Variable | Mean (SD) or N (%) |
|---|---|
| Age (yrs) | 73.4 (6.12) |
| Sex (N,% female) | 377 (73.5) |
| Education (yrs) | 15.0 (3.53) |
| Mini-mental status exam (0–30) | 27.8 (2.30) |
| BMI (all) | 30.3 (6.34) |
| BMI male | 28.5 (5.23) |
| <20 | 3 (2.3%) |
| 20–<25 | 35 (31.5%) |
| 25–<30 | 42 (27.8%) |
| ≥30 | 53 (22.8%) |
| BMI female | 30.5 (6.61) |
| <20 | 6 (1.6%) |
| 20–<25 | 76 (20.2%) |
| 25–<30 | 115 (30.6%) |
| ≥30 | 179 (47.6%) |
| Global motor score | 1.00 (0.16) |
| Grip strength (kg) | 26.6 (9.52) |
| Pinch strength (kg) | 6.8 (2.41) |
| Finger taps for 10s | 55.2 (8.82) |
| Purdue pegboard (# pegs/30s) | 11.2 (2.22) |
| 8 foot walk speed (m/s) | 0.56 (1.06) |
| Steps for 8 foot walk | 6.5 (1.54) |
| Time to complete 360° turn (s) | 5.1 (2.13) |
| Steps for 360° turn | 8.2 (2.40) |
| Leg stand duration (s) | 6.6 (3.20) |
| Toe stand duration (s) | 8.5 (2.80) |
| Errors on tandem walk | 2.0 (2.98) |
| ADL Disability (≥1 ADL) | 19 (3.7%) |
| Vascular risk factors (0–3) | 1.5 (0.85) |
| Hypertension | 386 (75.2%) |
| Diabetes | 134 (26.1%) |
| Smoking (past or current) | 256 (49.9%) |
| Past | 224 (43.7) |
| Current | 32 (6.2%) |
| Vascular diseases (0–4) | 0.21 (0.48) |
| Stroke | 22 (4.3%) |
| Myocardial infarction | 35 (8.3%) |
| Congestive heart failure | 21 (4.1%) |
| Claudication | 28 (5.5%) |
| Physical activities (0–3) | 1.2 (0.95) |
Baseline global motor scores ranged from 0.57 to 1.33 with higher values indicating better function. Global motor scores were approximately normally distributed (mean, 1.00; SD, 0.16). Global motor score was associated with age (Rho= −0.48, p<0.001), education (Rho=0.23, p<0.001), BMI (Rho= −0.13, p=0.003), physical activities (Rho=0.25, p<0.001), vascular diseases (Rho −0.23, p<0.001) and vascular risk factors (Rho= −0.12, p=0.006). Global motor scores were similar in men (mean 1.01 (SD= 0.16) and women (mean 0.99; SD, 0.17, t[511] = −0.99, p=0.321).
3.2 Level and Annual Rate of Change in Global Motor Score in Older African American Adults
To document change in motor function and examine the associations of demographic variables with both the estimated level of motor function at baseline and its rate of change over time during the study, we employed a linear mixed-effect model which included 7 terms. The results for each of the 7 terms are presented in Table 2 (Model 1). This model derives a person-specific slope measure to summarize the annual rate of change in global motor scores (Term 1). This model includes an additional 6 terms. Three of these terms (Terms 2, 4 and 6) refer to the cross sectional associations of age, sex and education with the level of motor function at study baseline. The other 3 interaction terms refer to whether the rate of change of global motor scores varied with age, sex or education (Terms 3, 5 and 7). Table 2 (Model 1) shows the results for each of the 7 terms included in this model and are summarized below.
Table 2.
Annual Rate of Change in Motor Measures in Older African American Adults*
| Motor Measures | |||||
|---|---|---|---|---|---|
| Term | Model 1 Global Motor Score |
Model 2 Gait |
Model 3 Strength |
Model 4 Dexterity |
|
| 1 | Annual Change in motor measure | −0.026 (0.001,<0.001) | −0.024 (0.002,<0.001) | −0.025 (0.002,<0.001) | −0.010 (0.001,<0.001) |
| 2 | Age | −0.013 (0.001,<0.001) | −0.010 (0.001,<0.001) | −0.015 (0.002,<0.001) | −0.008 (0.001,<0.001) |
| 3 | Age X Annual Change in motor measure | −0.001 (0.0002,<0.001) | −0.001 (0.0003,<0.001) | −0.001 (0.0004,0.051) | −0.001 (0.0002,<0.001) |
| 4 | Sex (male) | 0.030 (0.014, 0.032) | 0.033 (0.016, 0.042) | 0.011 (0.027,0.698) | 0.015 (0.012, 0.187) |
| 5 | Sex X Annual Change in motor measure | 0.002 (0.002, 0.387) | 0.003 (0.003, 0.253) | 0.002 (0.004,0.586) | −0.003 (0.002, 0.156) |
| 6 | Education | 0.007 (0.002,<0.001) | 0.007 (0.002, 0.002) | 0.006 (0.004,0.103) | 0.006 (0.002,<0.001) |
| 7 | Education X Annual Change in motor measure | 0.000 (0.0003, 0.881) | 0.0002 (0.0004,0.637) | 0.0003 (0.001,0.680) | −0.001 (0.0003, 0.006) |
Each column (Model 1–4) in this table shows the results for a single linear mixed effects model examining a different motor measure. Each of the 4 models included the same 7 terms. Term 1 summarizes the annual rate of change in motor function. Three terms (gray) examined the cross-sectional associations of age, sex and education with the level of motor function at baseline. Three additional terms (white) examined whether the annual rate of change in motor function varied with age, sex or education. Each row shows the results for same term to facilitate comparison between the different motor measures examined in these analyses. Each cell shows the Estimate (Standard Error, p Value) for a single term in the model. The model coefficients are interpreted with respect to a female participant 73 years old at baseline, with 15 years of education.
Term1. Annual Rate of Change in Global Motor Score
The first term of this model summarizes the mean annual rate of change in the global motor score for an average participant, a 73 year old female with 15 years of education. During a mean follow-up of 5 years (mean = 5.1; SD = 3.28 years), since the sign for this term is negative, global motor score declined by about 0.03 unit/year.
Figure 2 illustrates the heterogeneity of declining motor function for a 25% random sample of the participants included in these analyses. Each line in the figure shows the person-specific change in the rate of global motor score during the study.
Figure 2. Person-specific paths of annual rate of change in global motor scores.
The figure is organized according to the age of the participant at each evaluation; the length of each line relative to the x-axis indicates the total years of observation for that individual. For visual clarity, the line segments shown in this figure correspond to a 25% random sample of the group. Each line segment is a person-specific estimated path obtained from a linear random-effects model which included terms for time in study; age, sex, and education; and their interactions with time. The line segment for each individual begins at the baseline age and continues until the age at the last motor assessment. The Y axis shows the scale for global motor scores and the X axis shows age.
Term2. Age and Level of Global Motor Score
At baseline, age was inversely related to the level of global motor score. Thus, for each additional year above the mean of 73 years, the level of global motor score was lower by 0.013 units/yr. Thus, during up to 10 years of follow-up, the estimated annual rate of decline is twice what would be expected based solely on the cross-sectional effect of age estimated at baseline. This can be derived by comparing the Estimate of Age: 0.013 (Term 2) versus Estimate of Change in global motor score: 0.026 (Term 1).
Term 3. Age and Annual Change in Global Motor Score
The third term summarized whether baseline age was associated with the annual rate of change of global motor score during the study. The estimate for this term was negative and significant suggesting that the rate of decline was more rapid in individuals who were older at study entry.
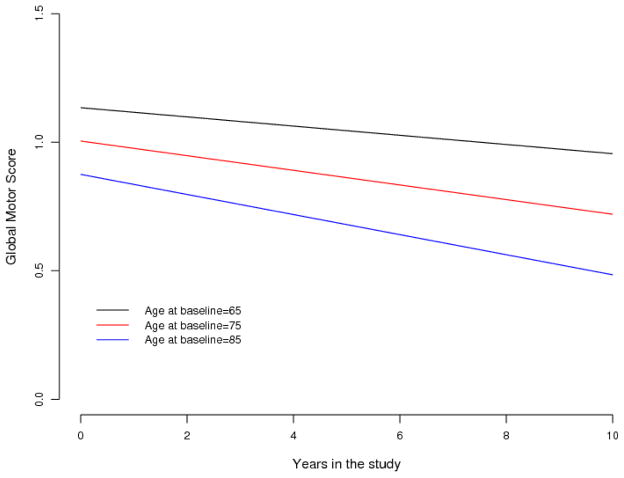
Figure 3 illustrates the effect of age on the trajectory of declining global motor scores for 3 average participants age 65, 75 and 85 years old, respectively, at study entry. Global motor scores declined by about 3.8% more rapidly per year (about 0.001 unit/yr) for each year above 73 years old at baseline (Table 2, Model 1-Term 3). Thus, the rate of decline of global motor scores accelerates with increasing baseline age and is almost twice as fast in individuals 85 years old at study entry versus individuals 65 years old.
Figure 3. Annual Rate of Change in Global Motor Scores Accelerates With Increasing Age at Baseline in Older African American Adults.
This figure illustrates how the annual rate of change in global motor scores varies with baseline age by showing mean trajectories of declining global motor scores during the study for 3 groups of MARS participants, females with 15 years of education and with different ages at baseline.
Terms 4–7. Sex and Education and Level and Annual Change in Global Motor Score
The level of global motor score at baseline was higher in males as the estimate for this term was positive (Terms 4). Similarly, the level of global motor score at baseline was higher for each additional year of education above 15 years (Term 6). Although motor function may differ between men and women across the lifespan, the rate of change in global motor scores did not vary between men and women in this sample (Term 5). The rate of change in global motor scores also did not vary with education (Term 7).
Secondary Analyses
The rate of declining global motor scores might be more rapid in participants with motor disorders. In sensitivity analyses, the associations of age with declining global motor scores were unchanged when we excluded cases with a history of PD (N=6) or stroke (N=22) at baseline (results not shown).
Chronic health conditions and lifestyle could account for a more rapid rate of declining global motor scores. The annual rate of change in global motor scores did not vary with chronic health conditions (Table 3, Terms A2 and B2), physical activity (Table 3, Terms C2) or BMI (Table 3, Terms D3 and D4). Chronic health conditions and physical inactivity could account for the more rapid rate of declining global motor scores with increasing age. The association of age and the rate of change in global motor scores was unchanged when we controlled for 3 chronic vascular risk factors and 4 diseases, BMI and self-reported physical activity (Table 3, Term 3).
Table 3.
Chronic Health Conditions and the Annual Rate of Change in Global Motor Scores in Older African American Adults
| Term | Model 1A | Model 1B | Model 1C | Model 1D | |
|---|---|---|---|---|---|
| 1 | Change in global motor score/year | −0.027(0.001, p<0.001) | −0.024 (0.002,<0.0000) | −.0027 (0.002,<0.001) | −0.027 (0.002,<0.001) |
| 2 | Age | −0.013 (0.001,<0.001) | −0.013 (0.001,<0.001) | −0.013 (0.001,<0.001) | −0.014 (0.001,<0.001) |
| 3 | Change in global motor score/year X age | −0.001 (0.0002,<0.001) | −0.001 (0.0002,<0.001) | −0.001 (0.0002,<0.001) | −0.001 (0.0002,<0.001) |
| 4 | Sex | 0.031 (0.013,0.021) | 0.034 (0.014,0.014) | 0.028 (0.013,0.034) | 0.013 (0.013,0.333) |
| 5 | Change in global motor score/year X sex | 0.002 (0.002,0.387) | 0.003 (0.002,0.269) | 0.002 (0.002,0.393) | 0.001 (0.002,0.669) |
| 6 | Education | 0.006 (0.002,<0.001) | 0.006 (0.002,<0.001) | 0.006 (0.002,0.001) | 0.005 (0.002,0.005) |
| 7 | Change in global motor score/year X education | 0.0001 (0.0003,0.788) | −0.0001 (0.0003,0.840) | 0.0000 (0.0003,0.924) | −0.0001 (0.0003,0.715) |
| A1 | Vascular Diseases | −0.065 (0.0126,<0.001) | |||
| A2 | Change in global motor score/year X vascular diseases | 0.001 (0.002,0.565) | |||
| B1 | Vascular risk factors | −0.023 (0.007,0.002) | |||
| B2 | Change in global motor score/year X vascular risk factors | −0.002 (0.001,0.102) | |||
| C1 | Physical activity | 0.045 (0.006,<0.001) | |||
| C2 | Change in global motor score/year X physical activity | 0.0002 (0.001,0.825) | |||
| D1 | BMI | −0.006 (0.001,<0.001) | |||
| D2 | BMI X BMI | −0.0002 (0.0001,0.089) | |||
| D3 | Change in global motor score/year X BMI | −0.005 (0.0002,0.013) | |||
| D4 | Change in global motor score/year X BMI x BMI | 0.0000 (0.0000,0.482) |
Each of the 4 columns is based on a separate linear mixed effect model. The model coefficients are interpreted with respect to a female participant 73 years old at baseline, with 15 years of education. Age was centered at 73 years and BMI at 30. Each cell shows the Estimate (Standard Error, p Value) for a different term included in the model. All 4 models included the same 7 terms (highlighted in gray) shown in Model 1 in Table 2. In contrast to the models in Table 2, each of the 4 models in this table added additional terms (highlighted in blue) to the basic model in order to examine whether chronic health conditions (Model 1A and 1B), physical activity (Model 1C) and body composition (Model 1D) accounted for either the level or rate of change of global motor scores in older African American adults.
3.3 Clinical Significance of the Annual Rate of Change in Global Motor Score in Older African American Adults
To determine the clinical significance of the annual rate of change in global motor scores, we constructed Cox proportional hazards models which adjusted for age, sex, education and baseline level of global motor score, to examine the risk of adverse health outcomes.
There were 66 of 513 (12.9%) persons who died during the study and 87 of 494 (17.6%) who developed Katz disability during follow-up. Baseline level of global motor score and its annual rate of change were independent predictors of death and disability (Table 4).
Table 4.
Association of Change in Global Motor Score and Adverse Health Outcomes+
| Model Term | Risk of Death | Risk of Disability |
|---|---|---|
| Age | 1.02 (0.97,1.07) | 1.06 (1.01, 1.10) |
| Sex | 1.52 (0.91, 2.53) | 0.40 (0.22, 0.71) |
| Education | 1.00 (0.92, 1.07) | 1.02 (0.96,1.09) |
| Baseline Global Motor Score | 0.56 (0.42,0.75) | 0.39 (0.30,0.51) |
| Change in Global Motor Score/year | 0.55 (0.42,0.72) | 0.37 (0.27,0.51) |
Hazard Ratios (95% Confidence Interval) for each of the terms in 2 separate Cox proportional hazard models showing the association of level of baseline and the annual rate of change in global motor score with survival and incident disability.
Next we used these models to calculate the increased risk of death and disability associated with increasing age. We compared the hazard rates of two groups of female participants with 15 years of education and average motor function at baseline, one group being age 65 at baseline and one group being age 85. In these 2 groups, the mean increase in average annual decline in global motor scores in the older group was associated with an 1.5-fold increased rate of death and a 3.1-fold increased rate of developing Katz disability.
3.4 Annual Rate of Change in the Components of Global Motor Score in Older Adults
Global motor score is constructed from several different motor performances which might decline at different rates. All three components showed a more rapid decline with increasing age. Gait and strength showed a faster annual rate of change as compared to upper extremity dexterity (Table 2, Change in motor measure/year).
4 DISCUSSION
In a cohort of more than 500 older community-dwelling African American adults, repeated annual objective measures of several motor performances showed that declining motor function accelerates with increasing age. This association was not attenuated when we adjusted for the severity of concomitant health conditions, physical activity or body composition at baseline. Both the level and rate of change in motor function were independently associated with survival and subsequent development of disability. These data suggest that late-life motor impairment is a progressive disorder and is likely to become a larger public health challenge in our aging population.
Motor impairment is a common concomitant of aging and is associated with a wide range of adverse health outcomes.10 Moreover, studies have shown that both the level and rate of change of motor function in older adults are independent predictors of subsequent risk of death and disability.7, 8, 27 However, there are few studies in African Americans which have examined repeated objective measures of different motor performances over time.28–30 The current study found that while there is heterogeneity in the person-specific change in motor function, overall most African Americans show some degree of progressive loss of motor function (Figure 1).
While the progressive loss of motor function which was documented in the current study was common, it is not “benign,” since the rate of motor decline independently predicts risk of death and incident disability in older African Americans. Moreover, the rate of declining motor function in these analyses was higher with increasing age at baseline. The number of adults older than 65 is projected to increase from 40 to 70 million by 2030. Moreover, adults over 65 will be increasingly diverse, as the percentage of older non-hispanic whites will decrease from 80% to 70% and the percentage of older African Americans will increase to comprise 10% of the older population.31 Thus, based on the findings in the current study that motor decline accelerates with age, it can be expected that late-life motor impairment will become an even larger problem in our aging and increasingly diverse population. These findings underscore the need to explicate the biology underlying age-related motor decline so as to provide new strategies and interventions to meet this growing public health challenge.
Older adults show a wide spectrum of loss of motor abilities ranging from mild decreased muscle strength and bulk and reduced speed and dexterity to overt motor impairments with concomitant disability. For example, grip strength and walking speed have been studied extensively across the lifespan, but methodologic differences in the performances assessed and testing protocols make it difficult to compare different studies.32–35 Nonetheless, these studies, based on cross sectional data, show that after midlife, increasing age in adults is associated with lower levels of function. These cross sectional studies may underestimate the rate of age-related motor decline. Based on cross sectional analysis in the current study grip strength would be expected to decline by about −0.35kg/yr similar to a previous study.32 As reported by prior studies, the magnitude of decline documented in the current longitudinal analysis for grip strength, almost 0.70kg/yr, was larger than expected based on the cross-sectional analysis.36–39 While hand grip has been commonly used as a surrogate for muscle strength loss, there may be regional differences in the rate of declining muscle strength.40, 41 Moreover, the varied manifestations of loss of different motor abilities and individual differences in their rates of decline may account in part for the heterogeneity observed in the rate of decline in global motor scores in this study (Figure 1). Thus, some of these factors may account for differences in the rate of change in the individual motor performances used to construct the global motor score. For example, upper extremity dexterity did not decline as rapidly as strength and gait (Table 2). These findings may reflect disparities in the fidelity of the instruments employed to measure motor performances in this study. Nonetheless, they are also likely to reflect differences in the rates of decline in the range of motor abilities which were measured. Recent work by our group has shown that using a whole body sensor when testing a standard motor performance test the Timed Get Up and Go task shows that even this “simple” performance is a complex motor task which can be decomposed into several more basic subtasks that may be differentially affected in older adults.42 A broad range of interventions are likely to be necessary to compensate for these heterogeneous impairments.
The phenotypic heterogeneity of late-life motor impairment and its varied progression is due in part to the complexity of the anatomical substrate underlying motor function as well as due to its varied pathologic basis. Motor control systems reside both within the brain and other CNS regions including the brainstem and spinal cord. Moreover, via peripheral nerves, these motor control systems are integrated together with peripheral musculoskeletal structures and non-neurologic systems such as cardiopulmonary function and systemic metabolism essential for movement. Not only does the anatomical substrate of specific motor abilities differ, but these structures can be differentially affected by diverse diseases and age-related processes. For example, it is well known that focal brain lesions (e.g., stroke, hydrocephalus) and diseases can affect vulnerable populations of neurons in discrete locations in the brainstem (e.g., PD) or spinal cord (e.g., ALS, myelopathy) and can thus impair some aspects of motor function while leaving others intact.43–46 To extend the findings of the current study will require longitudinal objective measures of a wider range of motor abilities in older African Americans and strategies to explicate their underlying pathologic basis.42 These data are crucial for the development of interventions and treatments which are more specific and can be individualized to meet the growing challenge of impaired motor function in older adults, and would be targets for interventions in African Americans who as a population, have greater disability.47
Our study has some limitations. Most importantly, inferences regarding causality must be drawn with great caution from observational studies. Testing of other accepted standardized motor performances like Chair Stand Test or motor assessment batteries like the Timed Get Up and Go would have facilitated comparison of the current results with other studies.33, 50 Many of the health measures employed were based on self-reported data, further studies employing objective measures of vascular risk factors and diseases are needed. Furthermore, objective measures of physical activity are imperative given its importance for health and motor function in particular. Although, participants in this cohort share similar characteristics i.e, BMI, hypertension and smoking, with other older African Americans,48,49 our cohort is a convenience sample of relatively well-educated African Americans, and the current findings will need to be replicated in the general population. Further, direct comparison to non-Hispanic whites will allow us to determine whether these results represent an important health disparity for older African Americans.
Several factors increase confidence in our findings. Perhaps most importantly, the study enjoys high follow-up participation, reducing bias due to attrition. In addition, motor function was evaluated as part of a uniform clinical evaluation which incorporated reliable strength and motor performance measures. Furthermore, strength and motor performance testing was done for the arms and legs. In addition, a relatively large number of older African Americans were studied, so that there was adequate statistical power to identify the associations of interest while controlling for several potentially confounding variables.
Highlights.
We tested whether declining motor function accelerates in older African Americans.
The rate of motor decline increased an additional 4%/year of age at baseline.
Both level and rate of motor decline predicted death and incident disability.
Acknowledgments
This work was supported by National Institute of Health grants [R01AG22018 (LLB); P30AG10161 (DAB); R01NS78009 (ASB)]; and the Illinois Department of Public Health. We thank all the participants in MARS and Rush Clinical Core. We also thank staff employed at the Rush Alzheimer’s Disease Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aron S. Buchman, Email: Aron_S_Buchman@rush.edu.
Robert S. Wilson, Email: rwilson@rush.edu.
Sue E. Leurgans, Email: Sue_E_Leurgans@rush.edu.
David A. Bennett, Email: David_A_Bennett@rush.edu.
Lisa L. Barnes, Email: Lisa_L_Barnes@rush.edu.
References
- 1.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy SE, Gill TM. Factors Associated With Recovery of Independence Among Newly Disabled Older Persons. Archives of Internal Medicine. 2005;165:106–112. doi: 10.1001/archinte.165.1.106. [DOI] [PubMed] [Google Scholar]
- 3.Feinglass J, Song J, Manheim LM, Semanik P, Chang RW, Dunlop DD. Correlates of Improvement in Walking Ability in Older Persons in the United States. American Journal of Public Health. 2009;99:533–539. doi: 10.2105/AJPH.2008.142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shipley BA, Weiss A, Der G, Taylor MD, Deary IJ. Neuroticism, Extraversion, and Mortality in the UK Health and Lifestyle Survey: A 21-Year Prospective Cohort Study. Psychosomatic Medicine. 2007;69:923–931. doi: 10.1097/PSY.0b013e31815abf83. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MD, Whiteman MC, Fowkes GR, Lee AJ, Allerhand M, Deary IJ. Five Factor Model Personality Traits and All-Cause Mortality in the Edinburgh Artery Study Cohort. Psychosomatic Medicine. 2009;71:631–641. doi: 10.1097/PSY.0b013e3181a65298. [DOI] [PubMed] [Google Scholar]
- 6.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proctor DN, Fauth EB, Hoffman L, et al. Longitudinal changes in physical functional performance among the oldest old: insight from a study of Swedish twins. Aging Clin Exp Res. 2006;18:517–530. doi: 10.1007/BF03324853. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch CH, Bůžková P, Robbins JA, Patel KV, Newman AB. Predicting late-life disability and death by the rate of decline in physical performance measures. Age and Ageing. 2012;41:155–161. doi: 10.1093/ageing/afr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9:734–745. doi: 10.2174/156720512801322627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of Motor Measures More Strongly Predict Adverse Health Outcomes in Old Age: The Rush Memory and Aging Project, a Community-Based Cohort Study. BMC Medicine. 2011;9:42. doi: 10.1186/1741-7015-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 12.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 13.Barnes LL, Lewis TT, Begeny CT, Yu L, Bennett DA, Wilson RS. Perceived discrimination and cognition in older African Americans. J Int Neuropsychol Soc. 2012;18:856–865. doi: 10.1017/S1355617712000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RS, Segawa E, Buchman AS, Boyle PA, Hizel LP, Bennett DA. Terminal Decline in Motor Function. Psych & Aging. 2012;4:988–1007. doi: 10.1037/a0028182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchman AS, Yu L, Boyle PA, et al. Microvascular Brain Pathology and Late-Life Motor Impairment. Neurology. 2013;80:712–718. doi: 10.1212/WNL.0b013e3182825116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013;80:2055–2061. doi: 10.1212/WNL.0b013e318294b462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchman AS, Yu L, Boyle PA, et al. Microvascular brain pathology and late-life motor impairment. Neurology. 2013;80:712–718. doi: 10.1212/WNL.0b013e3182825116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology conributes to simultaneous change in physical frailty and cognition in old age. J Gerontol Med Sci. 2014 doi: 10.1093/gerona/glu117. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle PA, Wilson RS, Aggarwal NT, et al. Parkinsonian signs in subjects with mild cognitive impairment. Neurology. 2005;65:1901–1906. doi: 10.1212/01.wnl.0000188878.81385.73. [DOI] [PubMed] [Google Scholar]
- 23.Buchman AS, Wilson RS, Boyle PA, Tang Y, Fleischman DA, Bennett DA. Physical Activity and Leg Strength Predict Decline in Mobility Performance in Older Persons. J Am Geriatr Soc. 2007;55:1618–1623. doi: 10.1111/j.1532-5415.2007.01359.x. [DOI] [PubMed] [Google Scholar]
- 24.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 26.SAS/STAT® Software for Unix, Version (9.18) [computer program] Cary, NC: SAS Institute Inc; 2002–2003. [Google Scholar]
- 27.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55:11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 28.Wolinsky FD, Bentler SE, Hockenberry J, et al. Long-term declines in ADLs, IADLs, and mobility among older Medicare beneficiaries. BMC Geriatr. 2011;11:43. doi: 10.1186/1471-2318-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araujo AB, Chiu GR, Kupelian V, et al. Lean mass, muscle strength, and physical function in a diverse population of men: a population-based cross-sectional study. BMC Public Health. 2010;10:508. doi: 10.1186/1471-2458-10-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes LL, Wilson RS, Hebert LE, Scherr PA, Evans DA, Mendes de Leon CF. Racial differences in the association of education with physical and cognitive function in older blacks and whites. J Gerontol B Psychol Sci Soc Sci. 2011;66:354–363. doi: 10.1093/geronb/gbr016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC. The State of Aging and Health in America 2013. Atlanta, GA: US Department of Health and Human Services; 2013. [Google Scholar]
- 32.Beenakker KG, Ling CH, Meskers CG, et al. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res Rev. 2010;9:431–436. doi: 10.1016/j.arr.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottenbacher KJ. Relationship between test methodology and mean velocity in timed walk tests: a review. Arch Phys Med Rehabil. 2008;89:865–872. doi: 10.1016/j.apmr.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97:182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 37.Desrosiers J, Hebert R, Bravo G, Rochette A. Comparison of cross-sectional and longitudinal designs in the study of aging of upper extremity performance. J Gerontol A Biol Sci Med Sci. 1998;53:B362–368. doi: 10.1093/gerona/53a.5.b362. [DOI] [PubMed] [Google Scholar]
- 38.Dontas AS, Jacobs DR, Jr, Corcondilas A, Keys A, Hannan P. Longitudinal versus cross-sectional vital capacity changes and affecting factors. J Gerontol. 1984;39:430–438. doi: 10.1093/geronj/39.4.430. [DOI] [PubMed] [Google Scholar]
- 39.Forrest KY, Zmuda JM, Cauley JA. Patterns and correlates of muscle strength loss in older women. Gerontology. 2007;53:140–147. doi: 10.1159/000097979. [DOI] [PubMed] [Google Scholar]
- 40.Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 41.Abe T, Loenneke JP, Thiebaud RS, Fukunaga T. Age-related site-specific muscle wasting of upper and lower extremities and trunk in Japanese men and women. Age (Dordrecht, Netherlands) 2014;36:813–821. doi: 10.1007/s11357-013-9600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchman AS, Leurgans SE, Weiss A, et al. Associations between Quantitative Mobility Measures Derived from Components of Conventional Mobility Testing and Parkinsonian Gait in Older Adults. PLoS ONE. 2014;9:e86262. doi: 10.1371/journal.pone.0086262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol. 2011;69:193–197. doi: 10.1002/ana.22165. [DOI] [PubMed] [Google Scholar]
- 44.Longstreth WT, Manolio TA, Arnold A, et al. Clinical Correlates of White Matter Findings on Cranial Magnetic Resonance Imaging of 3301 Elderly People: The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 45.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 46.Browner N, Giladi N. What can we learn from freezing of gait in Parkinson’s disease? Curr Neurol Neurosci Rep. 2010;10:345–351. doi: 10.1007/s11910-010-0127-1. [DOI] [PubMed] [Google Scholar]
- 47.Whitson HE, Hastings SN, Landerman LR, Fillenbaum GG, Cohen HJ, Johnson KS. Black-white disparity in disability: the role of medical conditions. J Am Geriatr Soc. 2011;59:844–850. doi: 10.1111/j.1532-5415.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim CX, Bailey KR, Klee GG, et al. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PLoS One. 2010;5:e9065. doi: 10.1371/journal.pone.0009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng JH, Bierman AS, Elliott MN, Wilson RL, Xia C, Scholle SH. Beyond black and white: race/ethnicity and health status among older adults. The American journal of managed care. 2014;20:239–248. [PMC free article] [PubMed] [Google Scholar]
- 50.Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract. 2008;14:552–562. doi: 10.1111/j.1365-2753.2007.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]