Abstract
The feasibility of a neighboring boronic acid-facilitated facile condensation of an aldehyde is described. This reaction is bio-orthogonal, complete at room temperature within minutes, and suitable for bioconjugation chemistry. The boronic acid group serves the dual purpose of catalyzing the condensation reaction and being a handle for secondary functionalization.
Graphical Abstract

A novel boronic acid facilitated cyclization reaction was developed. This reaction reaches completion at room temperature within minutes and is suitable for coupling and bioconjugation chemistry.
Click chemistry describes reactions that are fast and versatile, require mild conditions, and achieve high yields after minimum purification effort.1 A number of reactions in this category has been reported. Some examples are the well-known copper-catalyzed and strain promoted/copper-free azide-alkyne cycloaddition reactions,2 tetrazine and strained alkyne/alkene Diels-Alder type of reactions,3 and the Staudinger–Bertozzi ligation.4 These types of reactions have found wide-spread utility in a number of applications including bioconjugation, drug delivery, development of libraries of compounds, materials chemistry, and DNA modifications.5 In many such applications, the ability to undergo a secondary reaction will be very useful.6 The idea of orthogonal click-click reactions is also reflected in our earlier effort in tuning reaction rates for staged labeling.3c A recent and very significant report of protecting strained alkynes with Cu(I) also allows for sequential click reactions.7 Incorporation of the azido/alkyne chemistry and cyanobenzothiazole/1,2-aminothiol chemistry pairs into a single molecule would also allow for sequential click reactions and thus staged labeling.8 Herein, we report a simple system, which allows for sequential reactions by utilizing a neighboring boronic acid-mediated click cyclization. The boronic acid functional group can then be used for a secondary modification reaction under mild conditions. The initial cyclization is very fast, complete within minutes in excellent yields (up to > 95 %), does not need an external catalyst, and uses mild/inert conditions. The key aspects of the developed methodology are 1) the reaction is promoted through activation of the aldehyde by an ortho-positioned boronic acid; 2) the reaction is tolerant of various substituents on the formyl aromatic ring; and 3) the benzothiazole product carries a boronic acid moiety that can be used as a handle for further modifications using a large number of well-established conditions.9
In the process of searching for new bioorthogonal click reactions, we are interested in condensations involving aldehydes. It is well known that aldehydes can undergo facile condensation reactions with compounds that have vicinal nucleophiles. Aldehyde condensations with aryl diamines, aminobenzothiols, aminophenols, and cysteine have been previously reported.10 For example, aminobenzothiols and aminophenols have been condensed with an aldehyde in the presence of ZnO nanoparticles10c or in toluene at refluxing temperature; and similar reactions using cysteine analogs took 6–15 hours to complete at room temperature.10a Recently, Ai and colleagues reported a facile oxidative condensation of aldehydes with o-phenylenediamine analogs under acid catalysis.11 This was used for protein conjugation for various applications. Such reactions are also similar to the cyanobenzothiazole-cysteine condensation reactions.8a, 8b, 12 We are interested in taking advantage of this class of reactions by developing mild and facile conjugation chemistry that occurs without the use of acid. In doing so, we were interested in exploring the ability of a boronic acid group to function as a Lewis acid. Our lab has had a long-standing interest in boronic acid chemistry, exploring its applications in sensing,13 catalysis,14 drug design,15 and imaging16 as well as its behavior in mass spectrometry.17 It is well known that a Lewis base/nucleophile in a 1,5-relationship with an arylboronic acid can donate its lone pair electrons to the boron open shell. This has been shown with amino and hydroxyl groups.18 Our lab has also shown that an aldehyde carbonyl oxygen can also engage in such coordination and intermolecular aldehyde-boronic acid interactions facilitate aldehyde reduction.14b In addition, it has been previously reported that o-formyl boronic acids can easily form imines with an amine under physiological conditions.19 Recently, Gillingham and co-workers reported oxime condensation with o-formylphenylboronic acid as a linking tool for various chemical biology applications.20 This could very well be due to the ability for boronic acid to catalyze the reaction through coordination. Another piece of evidence seems to suggest that the boron atom in an o-formyl arylboronic acid tends to stay in the tetrahedral form, suggesting coordination.15b For all these reasons, we envisioned that a boronic acid group ortho to the aldehyde would be able to interact with the aldehyde oxygen atom and thus promote its acid-mediated cyclization reaction. Thus, we examined whether o-formylphenylboronic acid would undergo facile cyclization reactions with compounds containing vicinal nucleophiles at room temperature (Table 1).
Table 1.
Design of boronic acid facilitated “click” reaction.

| ||||
|---|---|---|---|---|
| Compound | X | Y | R | Isolated Yield (%) |
| 1 | NH2 | OH | 2-B(OH)2 | - |
| 2 | NH2 | NH2 | 2-B(OH)2 | - |
| 3 | NH2 | SH | 2-B(OH)2 | 85 |
| 4 | NH2 | SH | 3-B(OH)2 | - |
| 5 | NH2 | SH | 4-B(OH)2 | - |
| 6 | NH2 | SH | H | 10 |
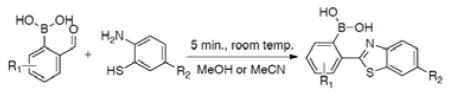
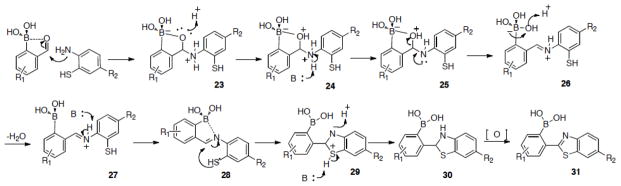
We first tried the reaction with phenylenediamine. Much to our surprise, the reaction generated a complex mixture. We did not examine the detailed composition of the reaction mixture because it was clear that this was not going to be a successful click reaction. However, all indications are that polymerization and cross-linking are possible side reactions. With this in mind, we were interested in using a structural moiety that has only one of the nucleophiles being “divalent.” In such a case, polymerization becomes not possible. With this consideration, we studied the same reaction with o-aminophenol and o-aminobenzothiol. With o-aminophenol, the cyclization product was not isolated. However, when the reaction was conducted with o-aminobenzothiol, it was very fast (Tables 1 and 2). We hypothesize that the open shell of the boron atom can serve as Lewis acid and coordinate with the oxygen of the adjacent aldehyde group in facilitating the first step of the reaction (Scheme 1). Furthermore, we reasoned that the boron atom could also coordinate with the nitrogen of the imine intermediate and thus facilitate the second step of the reaction as well (Scheme 1). In order to examine whether the cyclization was indeed facilitated by the O-B interaction that afforded the enhanced reactivity of the aldehyde group, we examined the same reactions using m- and p-formylphenylboronic acids and benzaldehyde. Not surprisingly, reacting aminobenzothiol and benzaldehyde without the boronic acid moiety gave no more than 10% of the cyclized product 6. Furthermore, the reaction did not reach completion even after 24 h. Indeed, when the boronic acid is not positioned ortho to the aldehyde functional group, no such cyclization happened (Table 1). Thus, the presence of a boronic acid moiety at a position ortho to the aldehyde was crucial to the reaction. The reaction was also examined using cysteine and homocysteine as naturally occurring molecules with two adjacent nucleophiles. However, no new spots or consumption of the starting material was observed by TLC within one hour of stirring at room temperature; consequently, the reaction was not further examined. Thus, a system with two adjacent nucleophiles is needed for the click-like cyclization with o-formyphenylboronic acid to occur.
Table 2.
Scope of the developed cyclization reaction.
| ||||
|---|---|---|---|---|
| Compound | R1 | R2 | Conversion (%) | Isolated Yield (%) |
| 7 | 4-Mea | Ha | 100 | 95 |
| 8 | 4-OCF3a | Ha | 100 | 97 |
| 9 | 3-OBna | Ha | 100 | 60 |
| 10 | 4-Fa | Ha | 100 | 78 |
| 11 | 3-OMea | Ha | 100 | 95 |
| 12 | Ha | CIb | 100 | 84 |
| 13 | 4-Mea | CIb | 100 | 75 |
| 14 | 3-OBna | CIb | 100 | 77 |
| 15 | 4-Fa | CIb | 100 | 52 |
| 16 | 3-OMea | CIb | 100 | 74 |
| 17 | Ha | CF3c | 100 | 56 |
| 18 | 4-Mea | CF3c | 100 | 79 |
| 19 | 4-OCF3a | CF3c | 100 | 56 |
| 20 | 3-OBna | CF3c | 100 | 74 |
| 21 | 4-Fa | CF3c | 100 | 68 |
| 22 | 3-OMea | CF3c | 100 | 94 |
1 equivalence of the reagent used.
2 equivalence of the reagent used.
1.2 equivalence of the reagent used.
Scheme 1.
Proposed mechanism (R1 = H, Me, OMe, OBn, OCF3, F; R2 = H, Cl, CF3).
In order to examine the scope of this reaction, six o-formylbenzylboronic acids, with various substitutions on the aromatic ring, were examined for their reactivity against three different o-aminobenzothiols (Table 2). The substituents did not seem to have much of an effect on the reaction outcome. The difference in the isolated yield of the oxidized final products was largely due to purification issues as TLC indicated full conversion in all reactions. The purification issues arise from the challenge of separating the cyclized intermediate 30 and the oxidized final product 31 (Scheme 1).
It should be noted that the reaction sequence also involves oxidation, which we assume is air-oxidation because no other reagent was added. The oxidation step appears to be much slower compared to the click-like cyclization reaction. Addition of one equivalent of DDQ and stirring for 30 min substantially increased the oxidation rate and isolated yields (data not shown). However, the use of harsh oxidizing reagents is undesirable and simple overnight air oxidation resulted in the desired fully oxidized products (example: compounds 7, 8 11, 22, Table 2). This is also consistent with the condensation work by others that air-oxidation would drive the reaction to produce the fully aromatized system.21
To further explore the scope of the reaction, commercially available 2-amino-4-(trifluoromethyl)thiophenol and 2-amino-4-chlorothiophenol were selected. One of these reagents introduces an electron withdrawing group in the nucleophilic system and the other a slightly electron donating group that can potentially be used as a functionalization site (Table 2, compounds 12–22).
Although aminobenzothiol has two nucleophilic groups, we propose that imine formations happens first (26, Scheme 1), followed by intramolecular cyclization driven by 1) boronic acid activated imine (28) and 2) energetically favorable five-membered ring formation (29, Scheme 1). As previously mentioned, the presence of a boronic acid at a position ortho- to the aldehyde not only is crucial for the cyclization reaction, but also provides a handle for further modifications of the synthesized benzothiazole.
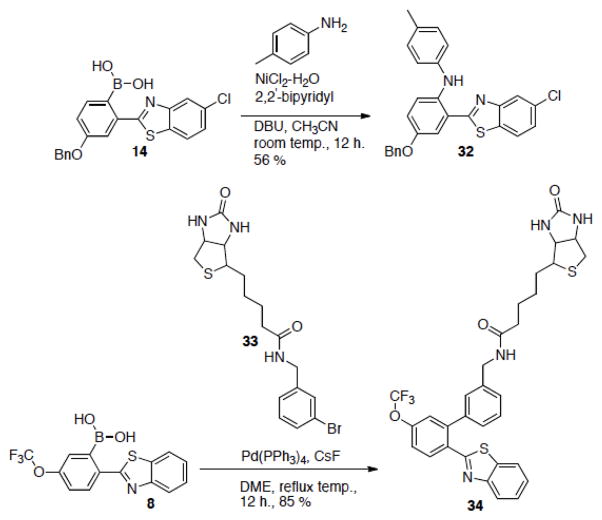
The functionalization of the synthesized boronic acid benzothiazoles can easily be achieved through a number of coupling methods for various applications.8a, 8b, 12 For example, molecules can be conjugated to the benzothiazole via Suzuki coupling or the Chan – Lam reaction (Scheme 2). In one such test, we conjugated biotin to the cyclization product benzothiazole. This can be extremely useful for the evaluation of biologically active compounds in binding studies such as SPR, QCM, QCM-D, and ELISA studies. Therefore, this new neighboring group assisted benzaldehyde cyclization reaction can be a versatile tool for various applications.
Scheme 2.
The use of the boronic acid as a handle for further modifications.
Conclusions
In summary, a facile neighboring group assisted cyclization reaction between formylphenylboronic acid and amino thiol has been developed. The reaction achieves full conversion at room temperature without the use of harsh conditions. The benzothiazole products can easily be modified through various boronic acid-based coupling methodologies for different applications such as bioconjugation, biolabeling, and fluorescent tagging, thus giving two sequential facile reactions.
In addition, benzothiazole compounds have shown a wide array of biological activities including anticancer,22 anti-inflammation,23 antiviral,24 antidiabetic,25 antimicrobial26 and anticonvulsant27 activities. This class of compounds is perhaps most useful in the area of diagnostics where it has been clinically used for amyloid fibrils imaging for Alzheimer’s disease detection.28 More recently, benzothiazole analogs have been utilized in studying the biologically important and naturally occurring gasotransmitter molecules, hydrogen sulfide29 and carbon monoxide.30 Consequently, the new chemistry described here has a wealth of synthetic applications as well.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support from the National Institutes of Health through a grant to BW (GM084933), the Molecular Basis of Disease Program (MBD) at GSU through fellowships to ABD, KW, and DW, the Graduate Assistance in Areas of National Need (GAANN) Fellowship at GSU to JH, and Frontier Scientific Inc.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/c000000x/
References
- 1.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Edit. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Edit. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. P Natl Acad Sci USA. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 3.(a) Chen W, Wang D, Dai C, Hamelberg D, Wang B. Chem Commun. 2012;48:1736. doi: 10.1039/c2cc16716f. [DOI] [PubMed] [Google Scholar]; (b) Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM, Chin JW. J Am Chem Soc. 2012;134:10317. doi: 10.1021/ja302832g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang D, Chen W, Zhang Y, Dai C, Wang B. Org Biomol Chem. 2014;12:3950. doi: 10.1039/c4ob00280f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxon E, Bertozzi CR. Science. 2000;287:2007. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wang K, Wang D, Wang B. Cur Org Chem. 2015;19:1385. [Google Scholar]; (b) Wang K, Wang D, Ji K, Chen W, Zheng Y, Dai C, Wang B. Org Biomol Chem. 2015;13:909. doi: 10.1039/c4ob02031f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nwe K, Brechbiel MW. Cancer Biother Radio. 2009;24:289. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36:1249. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 6.(a) Yuan Z, Kuang GC, Clark RJ, Zhu L. Org Lett. 2012;14:2590. doi: 10.1021/ol300899n. [DOI] [PubMed] [Google Scholar]; (b) Aucagne V, Leigh DA. Org Lett. 2006;8:4505. doi: 10.1021/ol061657d. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S, Hatakeyama Y, Johmoto K, Uekusa H, Hosoya T. J Am Chem Soc. 2014:13590. doi: 10.1021/ja507660x. [DOI] [PubMed] [Google Scholar]
- 8.(a) Ren HJ, Xiao F, Zhan K, Kim YP, Xie HX, Xia ZY, Rao J. Angew Chem Int Edit. 2009;48:9658. doi: 10.1002/anie.200903627. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheng Y, Dai C, Peng H, Chen W, Ni N, Ke B, Wang B. Chem Eur J. 2013;19:4036. doi: 10.1002/chem.201201677. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liang Gl, Ren HJ, Rao JH. Nat Chem. 2010;2:54. doi: 10.1038/nchem.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Kim A, Park JC, Kim M, Heo E, Song H, Park KH. J Nanosci Nanotechnol. 2014;14:1872. doi: 10.1166/jnn.2014.9100. [DOI] [PubMed] [Google Scholar]; (b) Polshettiwar V, Decottignies AL, C, Fihri A. ChemSusChem. 2010;3:502. doi: 10.1002/cssc.200900221. [DOI] [PubMed] [Google Scholar]; (c) Littke AF, DC, Fu GC. J Am Chem Soc. 2000;122:4020. [Google Scholar]; (d) Kirchhoff JH, Netherton MR, Hills ID, Fu GC. J Am Chem Soc. 2002;124:13662. doi: 10.1021/ja0283899. [DOI] [PubMed] [Google Scholar]; (e) Dudnik AS, Fu GC. J Am Chem Soc. 2012;134:10693. doi: 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Maegawa T, Kitamura Y, Sako S, Udzu T, Sakurai A, Tanaka A, Kobayashi Y, Endo K, Bora U, Kurita T, Kozaki A, Monguchi Y, Sajiki H. Chem Eur J. 2007;13:5937. doi: 10.1002/chem.200601795. [DOI] [PubMed] [Google Scholar]; (g) Herradura PS, Pendola KA, Guy RK. Org Lett. 2000;2:2019. doi: 10.1021/ol005832g. [DOI] [PubMed] [Google Scholar]
- 10.(a) Lu Y, Wang Z, Li CM, Chen J, Dalton JT, Li W, Miller DD. Bioorg Med Chem. 2010;18:477. doi: 10.1016/j.bmc.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Riadi Y, Mamouni R, Azzalou R, El Haddad M, Routier S, Guillaumet G, Lazar S. Tetrahedron Lett. 2011;52:3492. [Google Scholar]; (c) Banerjee S, Payra S, Saha A, Sereda G. Tetrahedron Lett. 2014;55:5515. [Google Scholar]
- 11.Ji A, Ren W, Ai HW. Chem Commun. 2014;50:7469. doi: 10.1039/c4cc01551g. [DOI] [PubMed] [Google Scholar]
- 12.Liang GL, Ren HJ, Rao JH. Nature Chemistry. 2010;2:54. doi: 10.1038/nchem.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Karnati V, Gao X, Gao S, Yang W, Sabapathy S, Ni W, Wang B. Bioorg Med Chem Lett. 2002;12:3373. doi: 10.1016/s0960-894x(02)00767-9. [DOI] [PubMed] [Google Scholar]; (b) Kaur G, Lin N, Fang H, Wang B. In: Glucose Sensing. Geddes CD, Lakowicz JR, editors. Springer Press; 2006. pp. 377–397. [Google Scholar]; (c) Wang W, Gao S, Wang B. Org Lett. 1999;1:1209. doi: 10.1021/ol9908732. [DOI] [PubMed] [Google Scholar]; (d) Wang W, Springsteen G, Gao S, Wang B. Chem Commun. 2000:1283. [Google Scholar]; (e) Yang W, Lin L, Wang B. Tetrahedron Lett. 2005;46:7981. [Google Scholar]; (f) Yang W, Yan J, Fang H, Wang B. Chem Commun. 2003:792. doi: 10.1039/b300098b. [DOI] [PubMed] [Google Scholar]
- 14.(a) Latta RP, Springsteen G, Wang B. Synthesis. 2001:1611. [Google Scholar]; (b) Yu H, Wang B. Synth Commun. 2001;31:163. [Google Scholar]
- 15.(a) Ni N, Chou HT, Wang J, Li M, Lu CD, Tai PC, Wang B. Biochem Biophys Res Commun. 2008;369:590. doi: 10.1016/j.bbrc.2008.02.061. [DOI] [PubMed] [Google Scholar]; (b) Ni N, Li M, Chou HT, Choudhary G, Lu CD, Tai PC, Wang B. Chem Biol Drug Design. 2009 in press. [Google Scholar]; (c) Yang W, Gao X, Wang B. Med Res Rev. 2003;23:346. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- 16.(a) Chu Y, Wang D, Wang K, Liu Z, Weston B, Wang B. Bioorg Med Chem Lett. 2013;23:6307. doi: 10.1016/j.bmcl.2013.09.063. [DOI] [PubMed] [Google Scholar]; (b) Dai C, Cazares LH, Wang L, Chu Y, Troyer DA, Semmes OJ, Drake RR, Wang B. Chem Commun. 2011;47:10338. doi: 10.1039/c1cc11814e. [DOI] [PubMed] [Google Scholar]; (c) Yang W, Fan H, Gao S, Gao X, Ni W, Karnati V, Hooks WB, Carson J, Weston B, Wang B. Chem Biol. 2004;11:439. doi: 10.1016/j.chembiol.2004.03.021. [DOI] [PubMed] [Google Scholar]; (d) Yang W, Gao S, Gao X, Karnati VR, Ni W, Wang B, Hooks WB, Carson J, Weston B. Bioorg Med Chem Lett. 2002;12:2175. doi: 10.1016/s0960-894x(02)00339-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Dai C, Burroughs SK, Wang SL, Wang B. Chem Eur J. 2013;19:7587. doi: 10.1002/chem.201204290. [DOI] [PubMed] [Google Scholar]
- 18.(a) Zheng H, Ghanbari S, Nakamura S, Hall DG. Angew Chem Int Edit. 2012;51:6187. doi: 10.1002/anie.201201620. [DOI] [PubMed] [Google Scholar]; (b) Wulff G. Pure Appl Chem. 1982;54:2093. [Google Scholar]; (c) James TD, Sandanayake K, Shinkai S. Angew Chem Int Ed Engl. 1994;33:2207. [Google Scholar]; (d) James TD, Kras S, Shinkai S. Chem Commun. 1994:477. [Google Scholar]; (e) Arimori S, Bosch LI, Ward CJ, James TD. Tetrahedron Lett. 2001;42:4553. [Google Scholar]; (f) Ni W, Kaur G, Springsteen G, Wang B, Franzen S. Bioorg Chem. 2004;32:571. doi: 10.1016/j.bioorg.2004.06.004. [DOI] [PubMed] [Google Scholar]; (g) Franzen S, Ni WJ, Wang BH. J Phys Chem B. 2003;107:12942. [Google Scholar]
- 19.Cal P, Vicente JB, Pires E, Coelho AV, Veiros LF, Cordeiro C, Gois PMP. J Am Chem Soc. 2012;134:10299. doi: 10.1021/ja303436y. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt P, Stress C, Gillingham D. Chem Sci. 2015 doi: 10.1039/c5sc00921a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynn MA, Carlson LJ, Hwangbo H, Tanski JM, Tyler LA. J Mol Struct. 2012;1011:81. [Google Scholar]
- 22.(a) Wang Z, Shi XH, Wang J, Zhou T, Xu YZ, Huang TT, Li YF, Zhao YL, Yang L, Yang SY, Yu LT, Wei YQ. Bioorg Med Chem Lett. 2011;21:1097. doi: 10.1016/j.bmcl.2010.12.124. [DOI] [PubMed] [Google Scholar]; (b) Kumbhare RM, Dadmal T, Kosurkar U, Sridhar V, Rao JV. Bioorg Med Chem Lett. 2012;22:453. doi: 10.1016/j.bmcl.2011.10.106. [DOI] [PubMed] [Google Scholar]; (c) Saeed S, Rashid N, Jones PG, Ali M, Hussain R. Eur J Med Chem. 2010;45:1323. doi: 10.1016/j.ejmech.2009.12.016. [DOI] [PubMed] [Google Scholar]; (d) Havrylyuk D, Mosula L, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R. Eur J Med Chem. 2010;45:5012. doi: 10.1016/j.ejmech.2010.08.008. [DOI] [PubMed] [Google Scholar]; (e) Caputo R, Calabro ML, Micale N, Schimmer AD, Ali M, Zappala M, Grasso S. Med Chem Res. 2012;21:2644. [Google Scholar]; (f) Oanh DTK, Hai HV, Park SH, Kim HJ, Han BW, Kim HS, Hong JT, Han SB, Hue VTM, Nam NH. Bioorg Med Chem Lett. 2011;21:7509. doi: 10.1016/j.bmcl.2011.07.124. [DOI] [PubMed] [Google Scholar]
- 23.Shafi S, Alam MM, Mulakayala N, Mulakayala C, Vanaja G, Kalle AM, Pallu R, Alam MS. Eur J Med Chem. 2012;49:324. doi: 10.1016/j.ejmech.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Nagarajan SR, De Crescenzo GA, Getman DP, Lu HF, Sikorski JA, Walker JL, McDonald JJ, Houseman KA, Kocan GP, Kishore N, Mehta PP, Funkes-Shippy CL, Blystone L. Bioorg Med Chem. 2003;11:4769. doi: 10.1016/j.bmc.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 25.(a) Patil VS, Nandre KP, Ghosh S, Rao VJ, Chopade BA, Sridhar B, Bhosale SV, Bhosale SV. Eur J Med Chem. 2013;59:304. doi: 10.1016/j.ejmech.2012.11.020. [DOI] [PubMed] [Google Scholar]; (b) Ammazzalorso A, Giancristofaro A, D’Angelo A, De Filippis B, Fantacuzzi M, Giampietro L, Maccallini C, Amoroso R. Bioorg Med Chem Lett. 2011;21:4869. doi: 10.1016/j.bmcl.2011.06.028. [DOI] [PubMed] [Google Scholar]; (c) Navarrete-Vazquez G, Ramirez-Martinez M, Estrada-Soto S, Nava-Zuazo C, Paoli P, Camici G, Escalante-Garcia J, Medina-Franco JL, Lopez-Vallejo F, Ortiz-Andrade R. Eur J Med Chem. 2012;53:346. doi: 10.1016/j.ejmech.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 26.(a) Cho Y, Ioerger TR, Sacchettini JC. J Med Chem. 2008;51:5984. doi: 10.1021/jm800328v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Telvekar VN, Bairwa VK, Satardekar K, Bellubi A. Bioorg Med Chem Lett. 2012;22:649. doi: 10.1016/j.bmcl.2011.10.064. [DOI] [PubMed] [Google Scholar]
- 27.(a) Amir M, Asif S, Ali I, Hassan MZ. Med Chem Res. 2012;21:2661. [Google Scholar]; (b) Kumar P, Shrivastava B, Pandeya SN, Tripathi L, Stables JP. Med Chem Res. 2012;21:2428. [Google Scholar]; (c) Jimonet P, Audiau F, Barreau M, Blanchard JC, Boireau A, Bour Y, Coleno MA, Doble A, Doerflinger G, Hu CD, Donat MH, Duchesne JM, Ganil P, Gueremy C, Honore E, Just B, Kerphirique R, Gontier S, Hubert P, Laduron PM, Le Blevec J, Meunier R, Miquet JM, Nemecek C, Pasquet M, Piot O, Pratt J, Rataud J, Reibaud N, Stutzmann JM, Mignani S. J Med Chem. 1999;42:2828. doi: 10.1021/jm980202u. [DOI] [PubMed] [Google Scholar]; (d) Ugale VG, Patel HM, Wadodkar SG, Bari SB, Shirkhedkar AA, Surana SJ. Eur J Med Chem. 2012;53:107. doi: 10.1016/j.ejmech.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 28.(a) Mathis CA, Wang YM, Holt DP, Huang GF, Debnath ML, Klunk WE. J Med Chem. 2003;46:2740. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]; (b) Koole M, Lewis DM, Buckley C, Nelissen N, Vandenbulcke M, Brooks DJ, Vandenberghe R, Van Laere K. J Nucl Med. 2009;50:818. doi: 10.2967/jnumed.108.060756. [DOI] [PubMed] [Google Scholar]; (c) Klunk WE, Engler H, Nordberg A, Wang YM, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Ann Neurol. 2004;55:306. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 29.(a) Jiang Y, Wu Q, Chang X. Talanta. 2014;121:122. doi: 10.1016/j.talanta.2014.01.001. [DOI] [PubMed] [Google Scholar]; (b) Zhang J, Guo W. Chem Commun. 2014;50:4214. doi: 10.1039/c3cc49605h. [DOI] [PubMed] [Google Scholar]
- 30.Carrington SJCI, Bernard JML, Mascharak PK. Med Chem Lett. 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.