Abstract
A 2-year-old domestic shorthair cat was presented with a history of hematuria, stranguria and intermittent urethral obstruction. Urine sediment showed hematuria, pyuria, and yellow-brown, amorphous and spherical crystals. Upon surgical correction of the obstructed urethra by perineal urethrostomy, many dark yellow to grey, irregular, gravel-like to millet grain-sized uroliths, consisting of 100% xanthine by crystallography were found. The urinary xanthine concentration was high. The cat subsequently developed bilateral nephroliths, recurrent urinary tract infection, and chronic kidney failure. Dietary management with a low-purine diet failed in part due to poor compliance, and the cat was euthanized at 6 years of age. Xanthinuria is rare inborn error of metabolism in cats and other species but should be considered as a differential diagnosis in cases of feline urolithiasis. No associated molecular genetic defect has been elucidated, and management of these cases is difficult. In the absence of calculi for analysis, measuring urinary xanthine concentration can help in diagnosing this metabolic defect.
Keywords: urine crystals, feline lower urinary tract disorders (FLUTD), purine metabolism, metabolic disease, nephrolithiasis, uric acid
Introduction
Purines are metabolized to hypoxanthine, and the enzyme xanthine dehydrogenase (XDH) catalyzes conversion from hypoxanthine to xanthine and then from xanthine into uric acid. The hepatic uricase converts uric acid to allantoin, which is renally excreted (Fig. 1). Defects in purine metabolism can result in tissue accumulation and excessive renal excretion of xanthine, with increased hypoxanthine and decreased uric acid concentrations. Xanthine is moderately insoluble at any urine pH and therefore can lead to the formation of xanthine crystals and urolithiasis (Osborne 2010; Jacinto 2013; Raivio 2014).
Fig 1.

Purine metabolic pathway, XDH xanthine dehydrogenase
Xanthinuria is a rare inborn error of metabolism in humans, dogs, and cats. In humans, xanthinuria (Online Mendelian Inheritance in Man 278300) has been well characterized and shown to have an autosomal recessive mode of inheritance with different types (Raivio 2014), while in animals, little is known about the route of expression.
Xanthinuria in dogs (Online Mendelian Inheritance in Animals 001283-9615) has been reported in Cavalier King Charles Spaniels (Van Zullian 1997; Gow 2011) and Dachshunds (Kucera 1997; Flegel 1998). Acquired xanthinuria is also seen in dogs treated with allopurinol, which inhibits the action of the enzyme XDH, and thereby leads to urolithiasis (Ling 1991; Osborne 2010; Torres 2011).
In cats, xanthinuria (Online Mendelian Inheritance in Animals 001283-9685) was previously described in 5 domestic shorthair (DSH) cats (White 1997; Schweighauser 2009; Mestrinho 2013) and 1 Himalayan cat (Tsuchida 2007). Laboratories dealing with urolith analysis reported that 0.1–0.3% of feline calculi analyzed were composed of xanthine (Osborne 2004; Canon 2007; Hesse 2012). Preliminary molecular genetic studies of 1 xanthinuric cat found the allelic composition of the XDH gene to be heterozygous and no obvious coding mutation (Tsuchida 2007).
In this case report, we describe the clinicopathological features of a cat with xanthinuria, crystalluria, urolithiasis, pyuria, bacteriuria and kidney failure.
Case Report
A 2 year-old male neutered DSH cat, weighing 4.25 kg, was presented to a primary care veterinary clinic with a history of intermittent urethral obstruction and red-colored urine of 3 day duration. The cat was housed indoors and was fed a commercial feline dry and canned diet with free access to water. On clinical examination the cat was alert and in good body condition. The caudal abdominal region appeared painful on palpation, and the bladder was of medium size. The cat was sedated, and while passing the catheter through the urethra resistance to forward movement was encountered. This was suspected to be due to small urethral uroliths or plugs but no such material was retrieved in the collected urine. The catheter was left in situ for 2 days and anti-inflammatory (carprofen 2 mg/kg body weight), antibiotic (cefovecin 8 mg/kg) and spasmolytic (phenoxybenzamine 0.5 mg/kg) drugs were administered.
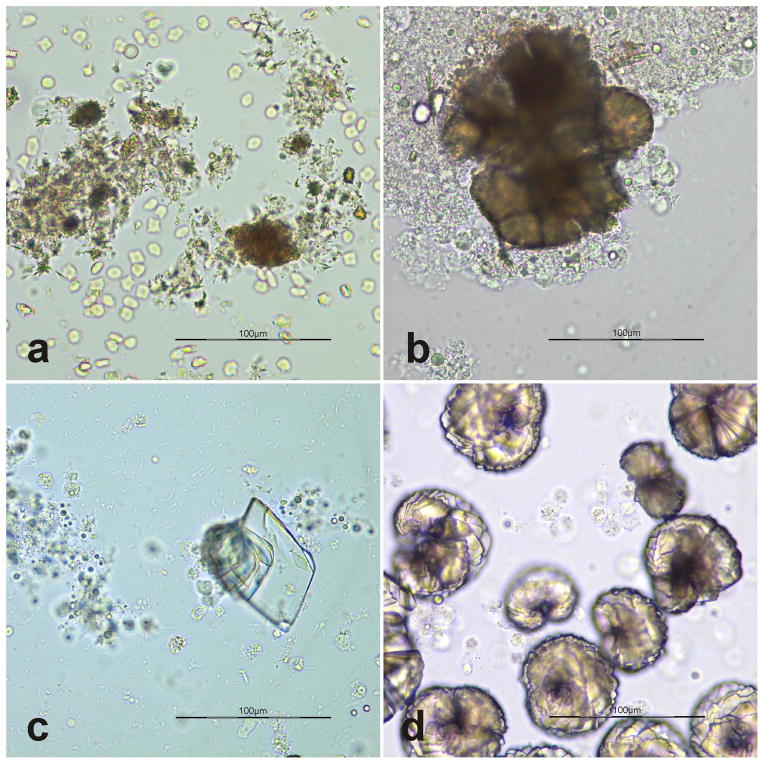
The initial urinalysis revealed light yellow, turbid, inadequately concentrated urine (specific gravity [SG] 1.016) with a notable urine sediment which contained many leukocytes, erythrocytes, and crystals as well as few bacteria. The crystals were yellow-brown, and appeared amorphous to spheroid or needle shaped (Fig. 2a). Ammonium urate crystals were initially suspected, however, because of their similar morphological appearance, ammonium urate, amorphous urate and xanthine crystals cannot be definitively distinguished by light microscopy (Osborne 1999; Sink 2012).
Fig. 2.
Urine sediment on initial (a) and subsequent (b, c week 2; d week 12) presentation of xanthinuric cat unstained wet mounts of urine sediment; bar=100μm. Crystals in a and b are consistent with ammonium urate, amorphous urates or xanthine; and those in c and d are more like uric acid or xanthine.
Two days after removing the indwelling urine catheter the cat was again presented with urethral obstruction. The cat was anesthetized, and multiple yellow-brown, spherical, gravel-like to millet grain-size uroliths were collected from the urethra. A perineal urethrostomy was performed to reduce the risk of further obstruction.
Based upon the quantitative infrared spectroscopic analysis (Harnsteinanalysezentrum Bonn, Germany) the uroliths were pure (100%) xanthine. Abdominal ultrasound 12 weeks after the first presentation revealed multiple small calculi in both renal pelvises. They were assumed to be xanthine nephroliths, but were not removed for further investigation.
Over the next 18 months following initial presentation 5 additional urinalyses were performed from urine collected by catheterization under sedation (week 2, 12, 17, 44 and 84). Aside from finding persistently inadequately concentrated urine (urinary SG 1.012–1.018), the urine sediments contained many leukocytes, none to many erythrocytes, variable degrees of bacteriuria and few to many crystals. The crystals found in the sediment resembled those described for the first urinalysis and low numbers of rhomboid-shaped crystals, morphologically consistent with uric acid crystals were also present (Fig. 2b and c; week 2). Additionally, few rosette-like yellow-brown crystals likely being composed of small rhomboid crystals were also noted (Fig. 2d; week 12).
Furthermore, Pseudomonas aeruginosa was cultured from the urine on week 12 and treatment with marbofloxacin (10mg/kg) was initiated based on the results of the urinary antibiogram. Repeat urinary culture at week 17 still revealed Pseudomonas as well as an anaerobic bacteria, Bacterioides fragilis infection. Ciprofloxacin (15mg/kg) was administered, but no follow up culture of urine was performed. A chronic bacterial urinary tract infection (UTI) secondary to catheterization and surgery was suspected.
During the 18 months follow up period the blood was examined at week 12, 44 and 84. While the results of the complete blood count were unremarkable, the serum biochemistry revealed persistent to progressive, moderate azotemia and hyperphosphatemia, and a mild hypercholesteremia. Together with the not adequately concentrated urine, the cat was diagnosed with secondary chronic kidney failure (CKF).
In order to confirm the xanthinuria, urinary xanthine and hypoxanthine concentrations were measured by high performance liquid chromatography (week 70) and serum and urinary uric acid concentrations were measured on week 12, 17, 44, 70 and 84 (Table 1a and b). Xanthine concentration in spot urine sample were measured in comparison with that of urinary creatinine levels revealing significantly increased levels in the affected cats urine when compared to values from 10 healthy control cats in which hypoxanthine and xanthine was not detected (Table 1a). Urinary uric acid:creatinine ratios were surprisingly increased when compared to values from control cats with the value from the last visit being the highest (Table 1a and b). In contrast, the affected cat’s serum uric acid concentrations remained in the normal range (Table 1b).
Table 1a.
Concentration of purine metabolites measured in the urine of a xanthinuric cat
| Urine ratio | Xanthinuric cat at week 70 | Controls (n=10) |
|---|---|---|
| Xanthine/creatinine (μmol/mmol) | 75.3 | <0.1 |
| Hypoxanthine/creatinine (μmol/mmol) | Not detected | Not detected |
| Uric acid/creatinine ratio (mmol/mmol) | 0.15 | 0.02–0.07 |
Table 1b.
Results of serum uric acid concentrations and urine uric acid/creatinine ratios in a xanthinuric cat during the follow-up period
| Week | Serum uric acid concentration (μmol/l) | Urine uric acid/creatinine ratio |
|---|---|---|
| 12 | 11.9 | 0.16 |
| 17 | Not done | 0.09 |
| 44 | 35.7 | 0.09 |
| 84 | 35.7 | 0.68 |
| Reference Interval | <59.5 | 0.03–0.07 (n=6) |
The cat continued to have intermittent stranguria, pollakiuria, bacteriuria and hematuria which were managed with carprofen (2 mg/kg), after which the clinical signs resolved. The cat was euthanized due to worsening clinical signs at 6 years of age, 4 years after initial diagnosis of xanthinuria. No post-mortem examination was performed.
Discussion
This report describes a young adult castrated DSH cat with xanthinuria similar to 5 previously described cats (White 1997; Tsuchida 2007; Schweighauser 2009; Mestrinho 2013). A diagnosis of hereditary xanthinuria was suspected based upon the urinary crystal morphology and confirmed by analyses showing calculinmade up of 100% xanthine together with high urinary xanthine values. However, this cat additional to the xanthinuria also suffered mild uric aciduria and developed secondary bacterial UTI, and a progressive CKF.
Xanthine crystals have been described in different shapes such as spheroid, amorphous or ovoid structures with yellow-brown color (Osborne 1999; Sink 2012) or rhomboid shape (Althof 2006) and can present a diagnostic challenge, as they cannot be distinguished morphologically from ammonium urate or amorphous urate (Osborne 1999; Sink 2012). Particularly, the rhomboid crystals (Fig. 2c, d) resemble either uric acid (Osborne 1996a) or xanthine (Althof 2006). The crystals seen in the cat of this report also appear similar to those found in dogs treated with allopurinol (Osborne 1999), but the cat had no exposure to that drug. As this cat had both severe xanthinuria and mild uric aciduria, the very polymorphic crystals observed may reflect both metabolites. However, the crystals were not biochemically analyzed, and the reason for the mild uric aciduria was not further investigated and thus remains unexplained.
Similar to the cat in this report all other xanthinuric cats described previously were initially presented for recurrent feline urinary tract disorder (FLUTD) and urinary tract obstruction. At further examination either crystals or calculi or both were found. Of the cats with confirmed xanthinuria, 3 cats had xanthine uroliths (Tsuchida 2007; Schweighauser 2009; Mestrinho 2013) and 1 had xanthine crystalluria (Schweighauser 2009). In addition 1 cat exhibited both uroliths and crystals (White 1997), similar to the cat of this report. Although the uroliths were shown to be 100% xanthine in that cat, urinary xanthine concentrations were not measured, and the crystals were thought to be amorphous phosphates. As the cat reported here had not been exposed to allopurinol or other drugs, a hereditary xanthinuria was the most likely diagnosis.
The present case was complicated by persistent pyuria and bacteriuria. Similarly Escherichia coli was cultured from a xanthine urolith in 1 other cat (White 1997), however, that infection resolved with antibiotic treatment. There are various potential causes of UTI in cases of hereditary urolithiasis, but it is unlikely that nephroliths lead directly to the UTI as hereditary nephroliths are not initially associated with UTI.
Bacterial infections have been reported in cats with crystalluria (13.5%), urolithiasis (5%) or both (7%) among 134 cats with FLUTD (Eggertsdottir 2007). Moreover, the prevalence of UTI was found to be higher in cats which have undergone catheterization or perineal urethrostomy (22–53%; Griffin 1992; Osborne 1996b; Bass 2005; Corgozinho 2007). It appears likely that the Pseudomonas aeruginosa and Bacterioides fragilis infections in the cat reported here developed secondary to repeated catheterization and perineal urethrostomy and may have ascended to the kidneys and thereby persisted.
Metabolically, xanthinuria due to a XDH deficiency is characterized by increased hypoxanthine and xanthine and decreased uric acid concentrations in urine and blood (Osborne 2010; Raivio 2014). This metabolic cross-over pattern has been previously reported in 1 cat (Schweighauser 2009). Similarly increased hypoxanthine and xanthine concentrations in blood and urine were noted in another affected cat, but uric acid concentrations were not measured (Mestrinho 2013). The previously reported xanthinuric Himalayan cat (Tsuchida 2007) had increased serum and urinary xanthine concentrations, but normal serum hypoxanthine levels (urine hypoxanthine was not measured). Moreover, the case reported by Tschuchida had a urinary uric acid:creatinine ratio which was at the upper limited of normal, showing a similar metabolic pattern to the findings in the present case. Interestingly, xanthine uroliths have been reported to contain uric acid in a few other cats (Osborne 2004). It is likely that these cats carried a different metabolic defect than XDH deficiency or the purine metabolism in cats is different from other studied mammals.
Azotemia was present at the first serum evaluation (week 12) and persisted during the follow up period leading to a diagnosis of CKF. Renal failure related to xanthine urolithiasis has been reported in humans (Bradbury 1995; Sikora 2006; Fujiwara 2012) and dogs (Kucera 1997; Van Zuilen 1997; Flegel 1998; Gow 2011), and in 1 xanthinuric cat (Schweighauser 2009). The cat reported by Mestrinho (2013) had bilateral ureteral obstruction with xanthine uroliths and was markedly azotemic at presentation, but after removing the blockage no renal failure was noted. The cause of CRF in the case reported here may have been due to urinary obstruction and persistent UTI. It should be noted that no association was found between different nephrolithiasis and progression of chronic kidney disease or death in cats (Ross 2007).
The recommended management for xanthinuria involves feeding of low protein or low purine diet in order to reduce purine excretion (Tsuchida 2007; Osborne 2010; Gow 2011; Mestrinho 2013), but the effectiveness of dietary management is not established in cats. The current case report describes a cat with hereditary xanthinuria, xanthine uroliths, crystals, pyuria, bacteriuria and renal failure. The crystals, uroliths, catherizations and perineal urethrostomy may have led to recurrent UTI and thereby decreased the cat’s quality of life.
Measuring xanthine concentration in urine is indicated in cases of suspected ammonium urate or amorphous urate or xanthine crystalluria. The presence of uric aciduria may indicate a different metabolic defect than XHD or a different metabolic pattern in cats.
References
- Althof S, Kindler J. Atlas – Untersuchungstechnik – Beurteilung. 7. Thieme; Stuttgart: 2006. Das Harnsediment; pp. 14–40. German. [Google Scholar]
- Bass M, Howard J, Gerber B, Messmer M. Retrospective study of indications for and outcome of perineal urethrostomy in cats. J Small Anim Pract. 2005;46:227–231. doi: 10.1111/j.1748-5827.2005.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Bradbury MG, Henderson M, Brocklebank JT, Simmonds HA. Acute renal failure due to xanthine stones. Pediatr Nephrol. 1995;9:476–477. doi: 10.1007/BF00866732. [DOI] [PubMed] [Google Scholar]
- Cannon AB, Westropp JL, Ruby AL, Kass PH. Evaluation of trends in urolith composition in cats: 5,230 cases (1985–2004) J Am Vet Med Assoc. 2007;15:570–576. doi: 10.2460/javma.231.4.570. [DOI] [PubMed] [Google Scholar]
- Corgozinho KB, de Souza HJM, Pereira AN, Belchior C, da Silva MA, Martins MCL, Damico CB. Catheter induced urethral trauma in cats with urethral obstruction. J Fel Med Surg. 2007;9:481–486. doi: 10.1016/j.jfms.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsdottir AV, Lund HS, Krontveit R, Sørum H. Bacteriuria in cats with feline lower urinary tract disease: a clinical study of 134 cases in Norway. J Fel Med Surg. 2007;9:458–465. doi: 10.1016/j.jfms.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel T, Freistadt R, Haider W. Xanthine urolithiasis in a dachshund. Vet Rec. 1998;143:420–423. doi: 10.1136/vr.143.15.420. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kawakami Y, Shinohara Y, Ichida K. A case of hereditary xanthinuria type 1 accompanied by bilateral renal calculi. Intern Med. 2012;51:1879–1884. doi: 10.2169/internalmedicine.51.6891. [DOI] [PubMed] [Google Scholar]
- Gow AG, Fairbanks LD, Simpson JW, Jacinto AML, Ridyard AE. Xanthine urolithiasis in a Cavalier King Charles Spaniel. Vet Rec. 2011;169:209–210. doi: 10.1136/vr.d3932. [DOI] [PubMed] [Google Scholar]
- Griffin DW, Gregory CR. Prevalence of bacterial urinary tract infection after perineal urethrostomy in cats. J Am Vet Med Assoc. 1992;200:681–684. [PubMed] [Google Scholar]
- Hesse A, Orzekowsky H, Frenk M, Neiger R. Epidemiologische Daten zur Harnsteinerkrankung bei Katzen im Zeitraum 1981–2008. Tierarztl Prax Kleintiere Heimtiere. 2012;40:95–101. in German. [PubMed] [Google Scholar]
- Jacinto AML, Mellanby RJ, Chandler M, Bommer NX, Carruthers H, Fairbanks LD, Gow AG. Urine concentration of xanthine, hypoxanthine and uric acid in UK Cavalier King Charles spaniels. J Small Anim Pract. 2013;54:395–398. doi: 10.1111/jsap.12106. [DOI] [PubMed] [Google Scholar]
- Kucera J, Bulkova T, Rychla JP. Bilateral xanthine nephrolithiasis in a dog. J Small Anim Pract. 1997;38:302–305. doi: 10.1111/j.1748-5827.1997.tb03471.x. [DOI] [PubMed] [Google Scholar]
- Ling GV, Ruby AL, Harrold DR, Johnson DL. Xanthine-containing urinary calculi in dogs given allopurinol. J Am Vet Med Assoc. 1991;198:1935–1940. [PubMed] [Google Scholar]
- Mestrinho LA, Goncalves T, Parreira PB, Niza MM, Hamaide AJ. Xanthine urolithiasis causing bilateral ureteral obstruction in a 10-month-old cat. J Fel Med and Surg. 2013;15:911–916. doi: 10.1177/1098612X13477413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CA, Bartages JW, Lulich JP, Albasan H, Weiss C. Canine purine urolithiasis: causes, detection, management and prevention. In: Hands MS, Thatcher CD, Remillard RL, et al., editors. Small animal clinical nutrition. 5. Topeka, KS: Mark Morris Institute; 2010. pp. 833–853. [Google Scholar]
- Osborne CA, Bartges JW, Lulich JP, Ulrich L, Carpenter K, Koehler L. Feline xanthine urolithiasis: a newly recognized cause of urinary tract disease. Proceedings of the ACVIM Forum; Charlotte, NC. 2004. pp. 781–782. [Google Scholar]
- Osborne CA, Caywood DD, Johnston GR, Polzin DJ, Lulich JP, Kruger JM, Ulrich LK. Feline perineal urethrostomy: a potential cause of feline lower urinary tract disease. Vet Clin North Am Small Anim Pract. 1996b;26:535–549. doi: 10.1016/s0195-5616(96)50083-5. [DOI] [PubMed] [Google Scholar]
- Osborne CA, Lulich JP, Ulrich LK, Bird KA. Feline crystalluria. Detection and interpretation. Vet Clin North Am Small Anim Pract. 1996a;26:369–391. doi: 10.1016/s0195-5616(96)50217-2. [DOI] [PubMed] [Google Scholar]
- Osborne CA, Stevens JB. Urine Sediment: Under the microscope. In: Osborne CA, editor. A clinical guide to compassionate patient care. Bayer Corporation; Leverkusen: 1999. pp. 125–179. [Google Scholar]
- Raivio KO, Saksela M, Lapatto R. Xanthine oxidoreductase—Role in human pathophysiology and in hereditary xanthinuria. In: Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM, Mitchell G, editors. The online metabolic and molecular bases of inherited disease. New York, NY: The McGraw-Hill Companies, Inc; 2014. [Accessed 6 October 2014]. http://www.ommbid.mhmedical.com/ommbid.13/1. [Google Scholar]
- Ross SJ, Osborne CA, Lekcharoensuk C, Koehler LA, Polzin DJ. A case-control study of the effects of nephrolithiasis in cats with chronic kidney disease. J Am Vet Med Assoc. 2007;230:1854–1869. doi: 10.2460/javma.230.12.1854. [DOI] [PubMed] [Google Scholar]
- Schweighauser A, Howard J, Malik Y, Francey T. Xanthinuria in a domestic shorthair cat. Vet Rec. 2009;164:91–92. doi: 10.1136/vr.164.3.91. [DOI] [PubMed] [Google Scholar]
- Sikora P, Pijanowska M, Majewski M, Bienia B, Borzecka H, Zajczkowska M. Acute renal failure due to bilateral xanthine urolithiasis in a boy with Lesch-Nyhan syndrome. Pediatr Nephrol. 2006;21:1045–1047. doi: 10.1007/s00467-006-0149-8. [DOI] [PubMed] [Google Scholar]
- Sink CA, Weinstein NM. Routine urinalysis: microscopic elements. 1. Wiley; West Sussex: 2012. Practical veterinary urinalysis; pp. 55–112. [Google Scholar]
- Torres M, Bardagí X, Zanna G, Ravera I, Ferrer L. Long term follow-up of dogs diagnosed with leishmaniosis (clinical stage II) and treated with meglumine antimoniate and allopurinol. Vet J. 2011;188:346–351. doi: 10.1016/j.tvjl.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Tsuchida S, Kagi A, Hidekazu K, Tagawa M. Xanthine urolithiasis in a cat: a case report and evaluation of a candidate gene for xanthine dehydrogenase. J Fel Med Surg. 2007;9:503–508. doi: 10.1016/j.jfms.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zuilen CD, Nickel RF, van Dijk TH, Reijngoud DJ. Xanthinuria in a family of Cavalier King Charles Spaniels. Vet Q. 1997;19:172–174. doi: 10.1080/01652176.1997.9694766. [DOI] [PubMed] [Google Scholar]
- White RN, Tick NT, White HL. Naturally occurring xanthine urolithiasis in a domestic shorthair cat. J Small Anim Pract. 1997;38:299–230. doi: 10.1111/j.1748-5827.1997.tb03470.x. [DOI] [PubMed] [Google Scholar]