Modern African great apes, even the so-called “savanna chimpanzees,” have relatively restricted diets consisting largely of leaves and fruits from C3 plants (1). In contrast, fossil members of the genus Homo, like their modern descendants, are believed to have had a rather eclectic diet, consuming foods from C3, C4, and crassulacean acid metabolism plants, while also complementing their diet with calorie-rich animal foods (2). Deeper in the fossil record, stable carbon isotope data suggest that at 4.4 Ma, the putative early hominin taxon Ardipithecus had a diet similar to modern chimpanzees, mostly consuming resources derived from C3 plants (3). Thus, the timing of significant C4 inclusion in the hominin diet following evolutionary divergence from the great apes at ca. 6–7 Ma is critical, because the event represents a milestone in human evolution that may relate to major shifts in early hominin habitat, ecology, and even physiology. In PNAS, Levin et al. (4) contribute to a better understanding of these issues in early hominins.
Over the past decade, research into primate and particularly hominin paleodiets has gained momentum, due to a proliferation of fieldwork resulting in the discovery and naming of many new species. These efforts have been enhanced by the development of stable isotope techniques that can be done with the extraction of only a negligible amount of dental enamel. Our knowledge of hominin diets has improved considerably with the resultant outpouring of new stable isotopic data, which have allowed us to test long held ideas and formulate more complex hypotheses. For example, although it has been customary to link craniodental features of extinct species to their dietary adaptations since the pioneering work of Robinson (5), recent isotopic studies and dental microstructure observations have made it abundantly clear that such approaches may not always be warranted. An increasing body of evidence has shown that the relationships between diet, dental microstructure, δ13C values, food material properties, and craniodental features are more complex than hitherto understood. The paper by Levin et al. (4) reports on stable isotope results for fossils from the Pliocene site of Woranso-Mille in Ethiopia, and is directly relevant to these issues. New data from the current study support the emerging concept that C4 food consumption in primates may not always require “assumed” dentognathic adaptations, and that observed morphological features in primates may not necessarily reflect the consumption of certain types of food.
Despite the wealth of fossil evidence, stable isotope data were missing for most eastern African fossil primates, particularly for the most relevant early hominins, until recently. Our knowledge was thus limited to stable isotope data from South African cave sites, which served as proxies by necessity. Pioneering researchers working in South Africa (6, 7) showed that both Australopithecus africanus and Paranthropus robustus fed mostly on C3 foods but also consumed significant amounts of C4 plants. These findings were intriguing, particularly in light of Robinson’s landmark dietary hypothesis (5), which distinguished the two taxa at the generic level based mainly on morphological distinctions ostensibly reflecting contrasting dietary adaptations. Owing to its megadontia and robust jaw structure, P. robustus was believed to have depended on a more abrasive and hard diet compared with the more gracile A. africanus. Later isotopic work in eastern Africa (8) showed a very high proportion of C4 plants in the diet of Paranthropus boisei and began to suggest to some that the hypermasticatory apparatus characterizing this taxon was due to its adaptation to processing large quantities of low-quality vegetation rather than hard objects, as had been asserted for decades. Further, microwear studies cast doubt on assertions that P. robustus and P. boisei had similar diets, supporting the notion that phrenetic features may not necessarily reflect the presumed type of diet consumed (9).
The recent explosion in stable isotopic studies of eastern African fossils, including the study by Levin et al. (4), serves as a checkpoint to question previous assumptions and revisit old hominin paleobiological conundrums. The first isotopic study on eastern African fossils from Tanzania showed that P. boisei and Homo had very different diets (10), and it was followed by other work concluding that the former species’ diet included more C4 food (up to 80%) than the diet of any other known hominin taxon (8). More recently, isotopic studies were expanded to larger eastern African datasets and took our knowledge of hominin diet to a different level in terms of depth and temporal extent (11–13). This compilation identified key dietary transitions in the hominin lineage and established patterns and major trends across taxa, allowing for a synthesis of the state of our knowledge today (13) (Fig. 1B).
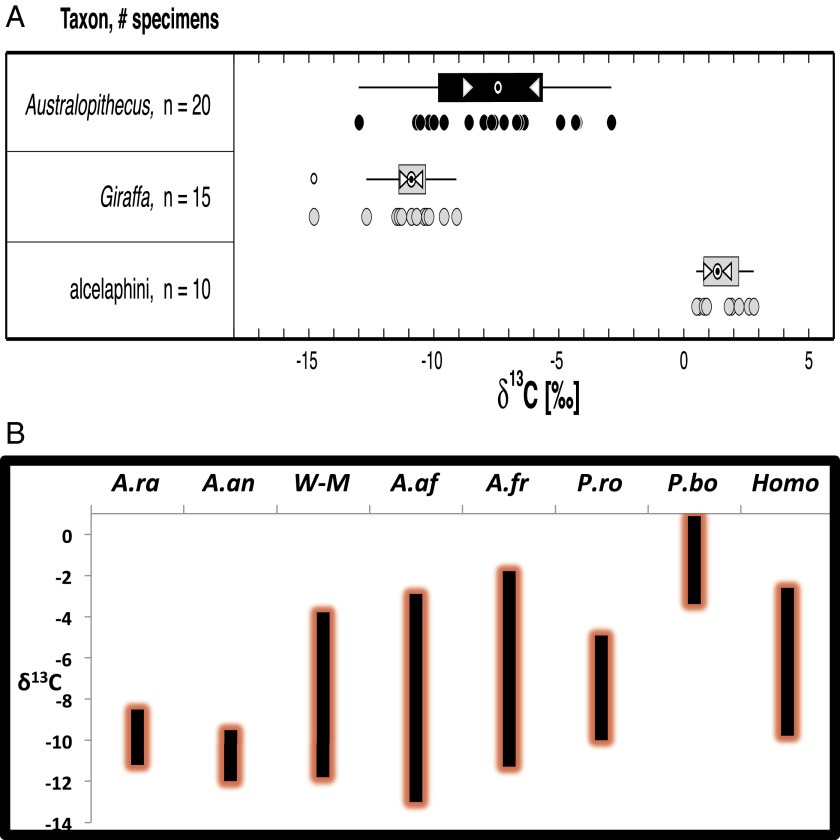
Fig. 1.
(A) Box and whisker plot showing the carbon isotopic composition (δ13C value) of tooth enamel from A. afarensis and two end member taxa (C3 browser: Giraffa, C4 grazer: alcelaphini) [from Wynn et al. (11)] showing that diagenesis is not an issue. (B) Ranges in δ13C values for relevant hominin species showing Woranso-Mille (W-M) hominins and A. afarensis expanded their diet substantially by including a higher amount of C4 food in contrast to what is seen in A. anamensis. Note also the striking difference between P. robustus and P. boisei in C4 consumption despite their morphological similarity. A.af (A. afarensis), A.an (A. anamensis), A.ra (A. ramidus), Homo, P.bo (P. boisei), P.ro (P. robustus), and W-M refers to hominin species from Woranso-Mille [data used are from Sponheimer et al. (13)]. W-M range refers to the range reported by Levin et al. (4).
In this vein, the work by Levin et al. (4) used carbon isotope data from diverse groups of mammals, particularly two key primate taxa, hominins and Theropithecus, to identify temporal trends in C4 food consumption. Levin et al. (4) found the isotopic composition of many mammalian taxa from Woranso-Mille to be in accordance with both field observations and previously reported data, in which giraffes, on one hand, and Alcelaphines and other presumed grass grazers, on the other hand, consistently occupied the two end points of the mammalian carbon isotopic spectrum (C3- and C4-dominated). Such observations confirm suggestions that diagenesis is not playing any obvious role in the carbon isotopic composition of well-selected tooth enamel (11, 12) (Fig. 1A). These new results also show that early hominins began incorporating considerable amounts of C4 foods in their diet as early as 3.76 Ma. This finding mirrors the feeding strategy of the papionin lineage, Theropithecus oswaldi, which also began consuming a C4-rich diet at 3.76 Ma, coinciding with its first known appearance datum in the fossil record. The new data from Levin et al. (4) highlight the notions that some aspects of primate dietary adaptation, their evolutionary time frames, and how they relate to observed morphologies are not as simple as previously thought. In the first place, the data from Woranso-Mille extend the timing of early hominin C4 food consumption in eastern Africa to 3.76 from ca. 3.4 Ma, which was previously documented at Dikika and Hadar (11) (Fig. 1B). This result suggests that early hominins became generalists exploiting both C4 and C3 foods in different types of environments earlier than had been previously recorded. Second, the study shows that the consumption of a C4 diet by the earliest members of the Theropithecus lineage at 3.76 Ma preceded the suite of dental specializations that were long considered to have been essential for feeding on such a diet. This primate did not acquire these morphofunctional configurations until much later, which may suggest that they are related to the intensification, rather than the beginning, of C4 food consumption.
What is more, the conclusions of Levin et al. (4) are consistent with the recently published eastern African stable isotopic dataset (11–13). These papers had already presented tantalizing evidence that separated Australopithecus afarensis from Australopithecus anamensis based on their carbon isotopic composition, despite their morphological similarities (Fig. 1B). The study by Wynn et al. (11) had documented that A. afarensis was among the first species in eastern Africa to venture into the C4 isotopic niche as early as 3.4 Ma, a date that is now pushed back by Levin et al. (4) to 3.76 Ma. This finding placed A. afarensis in contrast to its ancestor A. anamensis, which consumed mostly C3 plants, as did Ardipithecus and as do modern chimpanzees (1, 2). However, the link made by Levin et al. (4) between increased C4 diet and committed bipedality is not supported, because A. afarensis and A. anamensis have similar locomotor repertoires (14), whereas the latter’s isotopic signature is more similar to the isotopic signature of Ardipithecus ramidus (12, 13), which had a significantly, if not preponderantly, arboreal lifestyle (15, 16).
Finally, the Woranso-Mille site has yielded multiple hominin species, including A. afarensis, Australopithecus deyiremeda, some intermediate forms between A. anamensis and A. afarensis, and possibly another species to which the enigmatic foot from Burtele belongs (17–20). At this point, it is not clear to which of these species the reported carbon values pertain or whether the values reflect the range for multiple species. The observed range for the combined Woranso-Mille sample is −11.8 to −3.8‰, with a median value of −7.9‰. This range is slightly narrower but still comparable to the observed range of A. afarensis (−13 to −2.9‰, median of −7.4‰) (11). A better understanding of the taxonomy of the hominin remains from Woranso-Mille could help our knowledge of the wide isotopic space occupied by the various taxa, and the role of their potentially overlapping ecological niches in the co-occurrence of several species. Testing for such a distinction between coeval but clearly identified species may be particularly important in light of the foot anatomy of the taxon from Burtele, which, based on existing evidence, is more Ardipithecus-like than any other species, at least in its locomotor adaptation.
Footnotes
The author declares no conflict of interest.
See companion article on page 12304.
References
- 1.Schoeninger MJ, Moore J, Sept JM. Subsistence strategies of two “savanna” chimpanzee populations: The stable isotope evidence. Am J Primatol. 1999;49(4):297–314. doi: 10.1002/(SICI)1098-2345(199912)49:4<297::AID-AJP2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 2.Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain N. The raw and the stolen: Cooking and the ecology of human origins. Curr Anthropol. 1999;40(5):567–594. [PubMed] [Google Scholar]
- 3.White TD, et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science. 2009;326(5949):87–93. [PubMed] [Google Scholar]
- 4.Levin NE, et al. Dietary change among hominins and cercopithecids in Ethiopia during the early Pliocene. Proc Natl Acad Sci USA. 2015;112:12304–12309. doi: 10.1073/pnas.1424982112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson JT. Prehominid dentition and hominid evolution. Evolution. 1954;8:324–334. [Google Scholar]
- 6.Lee-Thorp JA, Van der Merwe NJ, Brain CK. Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J Hum Evol. 1994;27:361–372. [Google Scholar]
- 7.Sponheimer M, Lee-Thorp JA. Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science. 1999;283(5400):368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 8.Cerling TE, et al. Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grine FE, Sponheimer M, Ungar PS, Lee-Thorp J, Teaford MF. Dental microwear and stable isotopes inform the paleoecology of extinct hominins. Am J Phys Anthropol. 2012;148(2):285–317. doi: 10.1002/ajpa.22086. [DOI] [PubMed] [Google Scholar]
- 10.van der Merwe NJ, Masao FT, Bamford MK. Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. S Afr J Sci. 2008;104:153–155. [Google Scholar]
- 11.Wynn JG, et al. Diet of Australopithecus afarensis from the Pliocene Hadar Formation, Ethiopia. Proc Natl Acad Sci USA. 2013;110(26):10495–10500. doi: 10.1073/pnas.1222559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerling TE, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc Natl Acad Sci USA. 2013;110(26):10501–10506. doi: 10.1073/pnas.1222568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sponheimer M, et al. Isotopic evidence of early hominin diets. Proc Natl Acad Sci USA. 2013;110(26):10513–10518. [Google Scholar]
- 14.Ward CV, Leakey MG, Walker A. The new hominid species Australopithecus anamensis. Evol Anthropol. 1999;7:197–205. [Google Scholar]
- 15.White TD, et al. Ardipithecus ramidus and the paleobiology of early hominids. Science. 2009;326(5949):75–86. [PubMed] [Google Scholar]
- 16.Wood B, Harrison T. The evolutionary context of the first hominins. Nature. 2011;470(7334):347–352. doi: 10.1038/nature09709. [DOI] [PubMed] [Google Scholar]
- 17.Haile-Selassie Y, et al. An early Australopithecus afarensis postcranium from Woranso-Mille, Ethiopia. Proc Natl Acad Sci USA. 2010;107(27):12121–12126. doi: 10.1073/pnas.1004527107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haile-Selassie Y, Saylor BZ, Deino A, Alene M, Latimer BM. New hominid fossils from Woranso-Mille (Central Afar, Ethiopia) and taxonomy of early Australopithecus. Am J Phys Anthropol. 2010;141(3):406–417. doi: 10.1002/ajpa.21159. [DOI] [PubMed] [Google Scholar]
- 19.Haile-Selassie Y, et al. A new hominin foot from Ethiopia shows multiple Pliocene bipedal adaptations. Nature. 2012;483(7391):565–569. doi: 10.1038/nature10922. [DOI] [PubMed] [Google Scholar]
- 20.Haile-Selassie Y, et al. New species from Ethiopia further expands Middle Pliocene hominin diversity. Nature. 2015;521(7553):483–488. doi: 10.1038/nature14448. [DOI] [PubMed] [Google Scholar]