Significance
This report documents that beta cells from islets of Langerhans normally transfer some of their secretory granules to resident phagocytes. The transfer involves a close contact interaction between live beta cells and phagocytes, increases upon glucose stimulation, and requires mobilization of intracellular Ca++. In autoimmune diabetes, the CD4 T cells to various peptides of the insulin B chain recognize the transferred antigens in the phagocytes represented in islets by macrophages and a subset of dendritic cells. We have identified a process whereby antigens become available for recognition by autoreactive T cells in type 1 diabetes.
Keywords: autoimmune diabetes, autoimmunity, insulin reactivity, insulin-reactive T cells
Abstract
Beta cells from nondiabetic mice transfer secretory vesicles to phagocytic cells. The passage was shown in culture studies where the transfer was probed with CD4 T cells reactive to insulin peptides. Two sets of vesicles were transferred, one containing insulin and another containing catabolites of insulin. The passage required live beta cells in a close cell contact interaction with the phagocytes. It was increased by high glucose concentration and required mobilization of intracellular Ca2+. Live images of beta cell–phagocyte interactions documented the intimacy of the membrane contact and the passage of the granules. The passage was found in beta cells isolated from islets of young nonobese diabetic (NOD) mice and nondiabetic mice as well as from nondiabetic humans. Ultrastructural analysis showed intraislet phagocytes containing vesicles having the distinct morphology of dense-core granules. These findings document a process whereby the contents of secretory granules become available to the immune system.
An important issue in understanding the initiation of the autoimmune diabetic process is the manner in which the products of the secretory granule, in particular insulin, are handled by the immune system and made available to the autoreactive T cells. Autoreactivity to insulin is evident in type 1 diabetics and is a major component of the spontaneous diabetes of the NOD mouse (reviewed in refs. 1, 2). In the nonobese diabetic (NOD) mouse, CD4 T cells recognizing insulin peptides are found among the T cells that infiltrate the islets of Langerhans early in the autoimmune process. Such T cells induce disease when isolated and injected into nondiabetic mice (3–5). Altogether, a persuasive case has been made for insulin autoreactivity as driving forward diabetic autoimmunity (6–10). Our previous investigations showed that the islets of Langerhans of NOD mice contained resident phagocytes represented by two lineages: typical macrophages composed the majority, whereas a minority was made up of a subset of dendritic cells (DCs) expressing the CD103 integrin—that is, the CD103+/CD8α+ DC lineage under the control of the Batf3 transcription factor (11). Islet phagocytes were found to be in intimate contact with both beta cells and the vessel walls, throwing small projections into the vessel lumen (12). Importantly, when isolated they were strong antigen-presenting cells (APCs), particularly of insulin epitopes (5, 13). Furthermore, an initial ultrastructural analysis showed dense-core secretory granules inside vacuoles of the islet phagocytes residing in the islets (13), and by immunofluorescence islet phagocytes were shown to contain products from the beta cells (5, 13, 14). Direct evidence of insulin peptides within the beta cells and in islet phagocytes was obtained using a monoclonal antibody that was exclusively reactive with an insulin B chain peptide segment and not with native insulin (5). The passage of insulin to APCs took place even in nondiabetic mice; for a telling example, it was evident in NOD.Rag1−/− mice that have no lymphocytes and do not develop diabetes. In brief, inflammation was not a requisite for the acquisition of secretory granule antigens by the APCs. [A structural study in active diabetic rats also showed granules inside monocyte-like cells (15).] In toto, these results indicated that the products of the secretory granule had been passed to the APCs in vivo. These findings point to a scenario in which beta cells donate the antigenic epitopes that trigger autoreactive T cells to the local phagocytes as an initial stage before the sensitization of the draining pancreatic node (16–21; as reviewed in ref. 22).
This study further characterizes the interactions between beta cells and APCs probing with CD4 T cells to insulin. It examines the conditions that generate the insulin peptides recognized by diverse CD4 T cells and documents the passage of vesicles containing the peptides to APCs in a contact reaction dependent on live beta cells. Importantly, it documents that such peptides are also generated from human beta cells, further highlighting the importance of insulin immunoreactivity in T1D.
Results and Discussion
A Functional Assay to Examine the Passage of Insulin Epitopes to Phagocytes.
We previously characterized two distinct sets of insulin-reactive CD4 T cells (5, 8, 23). One set recognizes a peptide resulting from insulin processing by APCs and encompasses residues 13–21 of the B chain. In NOD diabetes, these T cells are poorly represented, most likely because of thymic deletional effects. We have referred to them as “type A,” represented here by the IIT-3 clone. The second set recognizes primarily the 12–20 segment of the insulin B chain, a one-amino acid shift of the binding register. This peptide is not expressed after the processing of insulin by APCs but is found when APCs react with denatured insulin or insulin peptides. These clones are relatively abundant in NOD diabetes. We have referred to these T cells as “type B,” herein represented by the 8F10 clone. Therefore, comparing the response of these two T cells is useful, in that the T cells directed to the 12–20 segment probe the presentation of denatured insulin or the insulin catabolites but not the native insulin molecule.
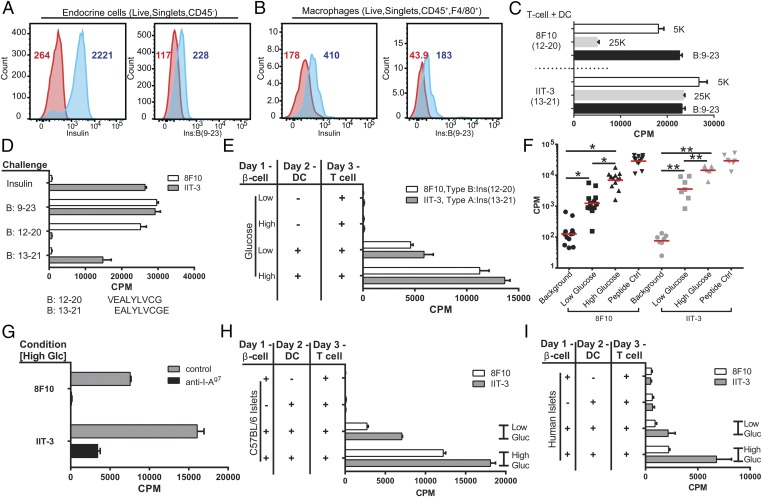
In an initial report, we had stained whole isolated islets with antibodies to insulin and also to the insulin B:9–23 peptide (5). The antibody to B:9–23 identified positive granules in beta cells that were larger than the insulin+ granules and stained positive with anti-Lamp2 antibodies, suggesting that B:9–23 granules may compose a distinct pool of granules (figure 3 in ref. 5). The intraislet APCs contained B:9–23-positive granules at an average of about 10 per cell. To quantitate our previous findings using flow cytometry, islet cells were isolated and examined using antibodies to insulin and the B:9–23 peptide (5). We found insulin and B:9–23 reactivity both in beta cells (Fig. 1A) and in CD45+ CD11c+ F4/80+ macrophages (Fig. 1B), importantly from islets of nondiabetic NOD.Rag1−/− mice. Similar results were found in islets from 4–6-wk-old NOD mice or C57BL/6 mice. In a different manipulation, beta cells were isolated from NOD.Rag1−/− mice and subjected to subcellular fractionation of the secretory granules using differential centrifugation of vesicles (24, 25). The granules were then added to CD11c+ DCs isolated from FMS-like tyrosine kinase 3 ligand (FLT-3L)-treated mice, and presentation to the two T cells was examined. (The DCs comprised two sets, a SIRPα+ and a CD24+; the two phagocytes in the islets are a macrophage-SIRPα+ and the CD103+DC. In this report, we do not differentiate between the two sets.) The 25,000 × g fraction that contains the mature secretory granules stimulated the IIT-3 T cells that recognized insulin epitopes and had a much smaller reactivity to 8F10 that only recognizes peptides or denatured insulin. The reverse was found for the 5,000 × g fraction that stimulated strongly the 8F10 (Fig. 1C). In sum, beta cells contain vesicles that are immunoreactive to B chain-specific antibody; the intraislet APCs likewise show positive reactivity with both B chain peptide as well as insulin-specific antibodies.
Fig. 1.
Beta cells transfer immunogenic insulin to phagocytes. Flow cytometry plots of isolated endocrine cells (A) or intraislet macrophages (B) stained with antibodies reactive to insulin and to the B:9–23 peptide. Cells were gated as indicated in the panels. Red histograms correspond to the isotype control antibody staining. Blue histograms are either antiinsulin or anti-B:9–23 peptide staining. Numbers in each plot indicate the mean fluorescence intensity for their respective histogram. (C) Secretory granules were isolated by differential centrifugation from beta cells isolated from NOD.Rag1−/− mice and offered to spleen DCs, and the response of the 8F10 T cell or IIT-3 T cell was then assayed. Shown are the responses to the 5,000 and 25,000 × g fractions (in 5K and 25K, respectively) and as a control to the B:9–23 peptide. (D) The characterization of the two CD4 T cells to insulin (8). The FLT-3L DCs were incubated with insulin or with the B:9–23 peptide, each at 10 μM. 8F10 reacts with peptides B:9–23 or B:12–20 (sequence shown below the graph) but not with insulin or B:13–21. IIT-3 reacts with insulin and peptides B:9–23 and B:13–21. (E) A representative assay (of n > 25). Indicated are the cells used in the assay. Beta cells were from 6-wk-old NOD.Rag1−/− mice; the APCs were DCs obtained from the spleen of mice previously injected with FLT-3L. Background response of the T cells never exceeds 150 cpm. (F) Summary of the first series of experiments. The explanation is in the text. (G) Antibody to I-Ag7 inhibits the transfer. The culture included the presence or absence of 10 μg/mL of the antibody Ag2.42.67 specific for I-Ag7. (H) Same as in A but testing islets from B6 mice. Shown is a representative experiment of two experiments. (I) As in A but testing human islets. The results are pooled from two experiments.
A culture assay was developed to examine the transfer of insulin immunogenic material from beta cells to phagocytes. Endocrine cells harvested from isolated islets were placed in culture in different media from short time periods of 1–3 h to overnight, after which DCs were added for several hours. (We refer to the endocrine cells as beta cells, as we are probing only insulin transfer.) Lastly, the presence in the DCs of the peptide bound to the I-Ag7 class II MHC molecule was probed using either of the two insulin-reactive CD4 T cells.
Testing Beta Cells from Multiple Sources.
Fig. 1D shows the specificity of the T cells used in these experiments: The CD4 T-cell 8F10 only recognizes the 12–20 insulin peptide and not the peptide resulting from insulin processing, whereas IIT-3 recognizes the segment 13–21 derived from either insulin processing or free peptide. Knowing the specificity of our T cells as antigenic probes, we sampled their response following beta cell–DC interaction. Beta cells were obtained from NOD.Rag1−/− mice that do not develop diabetes. As expected, in the absence of additional DCs, the T cells never responded to beta cells, because they lack expression of MHC-II molecules (Fig. 1E).
Consistently, the addition of DCs after the first 24 h of culture of beta cells in media containing 5 mM glucose resulted in a variable low level of presentation (low glucose in the figures). (Identical results were also found with 2.5 mM glucose in the media.) Both T cells reacted on average about 10-fold higher over the background stimulation (Fig. 1 E and F). Culturing beta cells with 25 mM glucose resulted in a significant increase in insulin presentation by DCs. The response of 8F10 (i.e., to B:12–20 peptide) increased by 352% (n = 13 experiments); with IIT-3 (i.e., to B:13–21 peptide), there was a similar increase (351%, n = 8 experiments) (Fig. 1 E and F). The age of the mice did not influence the transfer. In various experiments, beta cells from 4–16-wk-old NOD.Rag1−/− mice presented equally. Similar results were obtained examining beta cells from young prediabetic NOD mice 6, 8, and 14 wk of age. Not shown are results indicating similar findings with bone marrow-derived macrophages as the capture APCs.
The presentation of insulin peptides, as expected, depended on the interaction with class II MHC molecules and was inhibited by addition of a blocking monoclonal antibody to the I-Ag7 protein (Fig. 1G). The specificity of the insulin reactivity was shown by testing islets from mice that express only insulin-2 with a mutation at Tyr-16 of the beta chain (6). This is a nonimmunogenic insulin known to be nonreactive with either type A or type B T cells (6–8, 23); their beta cells did not elicit a positive response. (The results were as follows. The mean cpm response of T cells alone, T cells plus beta cells and DCs, and the control of beta cells plus DCs plus B:9–23 peptides were for 8F10, 58, 47, and 38,610, respectively, and for IIT-3, 35, 46, and 41,491, respectively.)
C57BL/6 mice do not develop spontaneous diabetes. Their beta cells incubated with DCs from NOD mice transferred the immunogenic peptides under low glucose media, whereas the transfer was potentiated by incubating them in high glucose media (Fig. 1H). In brief, there was no difference in the generation of peptides between beta cells of a different MHC haplotype or diabetes-susceptible status. The same transfer took place testing human islet preparations obtained from two recently deceased nondiabetic patients. The segment of the insulin B chain that stimulates the murine 12–20- and 13–21-reactive CD4 T cells is identical between human and mouse (Fig. 1D). As expected, in both situations, the DCs and the CD4 T cells were from NOD mice—that is, syngeneic—based on MHC restriction rules. Clearly two events are taking place: One is the transfer of the antigenic material from the beta cells, and the second is the DC–T cell interaction, the latter requiring the diabetes-susceptible MHC molecule.
Nature of the Transfer.
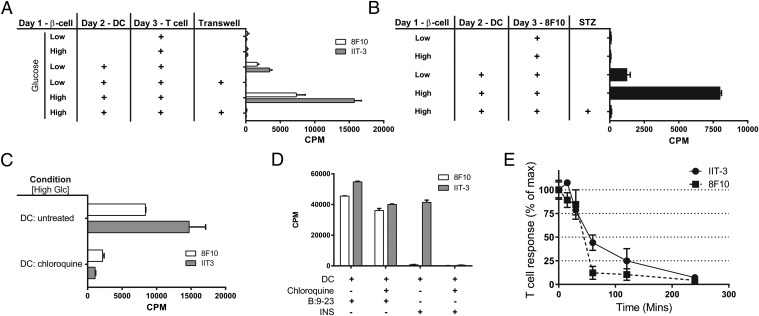
Phagocytes could take up extracellular insulin or insulin peptides and then present them to the T cells. Separation of the beta cells from the DC by a cell-impermeable membrane abolished presentation, indicating that close cell contact was required (Fig. 2A). About 30% of beta cells invariably died after the first 24 h of culture, but the dead beta cells were not responsible for the transfer of peptides to the DCs. Indeed, there was a complete lack of presentation when all beta cells were killed by streptozotocin before the addition of the DCs (Fig. 2B).
Fig. 2.
Transfer requirements and life of the peptide–MHC complex. (A) Same as in Fig. 1E but adding one variable: the separation of beta cells and DCs by a 0.4-μm polycarbonate filter, which results in the lack of transfer. (B) Examination of dead beta cells after streptozotocin (STZ) treatment, 50 mM overnight, showing a complete lack of transfer. (C) DCs were treated with chloroquine (100 μM) for 2 h and washed extensively to remove the drug, and then the DCs were incubated with the beta cells for 4 h and tested with the T cells. (D) Control experiments using DCs incubated with insulin or peptide in the presence of chloroquine. (E) DCs were incubated with beta cells for 1 h, after which the DCs were separated. T-cell hybridomas were added to the separated DCs at the indicated times; the 100% numbers for 8F10 and IIT-3 are 5,030 cpm and 20,447 cpm, respectively.
Presentation required the processing of the granules by the DCs. DCs were pretreated with chloroquine, the drug was washed after 1 h, and then the DCs were added to the culture for a short period (because the effects of the drug are reversible). There was significant inhibition by the chloroquine pretreatment, indicating that the insulin vesicle had to be taken to acidic compartments of the phagocyte in order for their contents to be presented to the T cells (Fig. 2C). The control manipulations testing APCs pulsed with insulin proteins or peptides established, as expected, that presentation of the free peptide was not affected by chloroquine, whereas the presentation of insulin was completely inhibited (Fig. 2D). Presentation of the peptide–MHC complex from the transferred vesicles, however, was short. Following a 1-h period of incubation of beta cells with DCs, the DCs were separated and T cells were added at various times. There was a progressive drop in presentation so that by 1–2 h there was a 50–75% decrease in the response (Fig. 2E). In sum, the transfer granules need to enter an acidic compartment of the APCs to release their contents of insulin or insulin peptide, but their time available for immune recognition is relatively short.
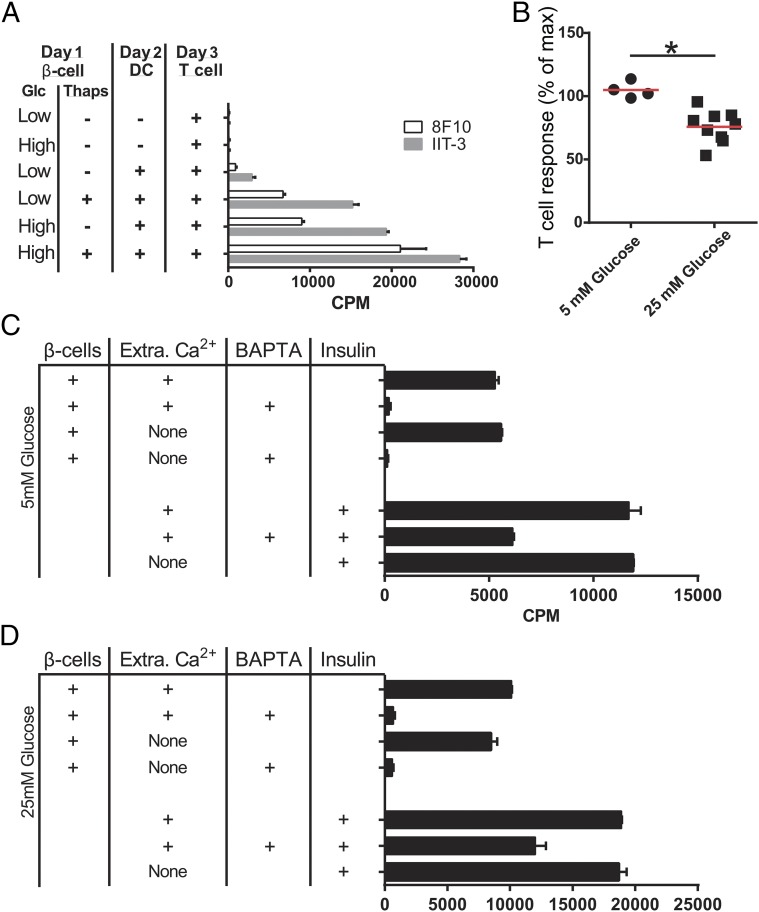
The previous findings indicate a close membrane-to-membrane interaction between beta cells and phagocytes that results in the transfer of the granules containing insulin or insulin products. We therefore investigated the potential role of Ca2+ in the mobilization of granules. We first examined if thapsigargin, a well-established inhibitor of the sarco/endoplasmic reticulum (ER) Ca2+ ATPase, may modulate the transfer of the granules. Antigen presentation was significantly increased by thapsigargin treatment of beta cells in low glucose media and was further enhanced in high glucose media (Fig. 3A). [In 10 different experiments, addition of thapsigargin increased by 211% over that of high glucose media (Fig. 3A).]
Fig. 3.
Effects of thapsigargin and evaluation of Ca2+ requirements. (A) Beta cells were treated with thapsigargin (10 μM) for 12 h in media with 5 or 25 mM glucose, after which the drug was removed and DCs and T cells were added to the culture. The experiment is representative of 10 experiments. (B) The beta cells were cultured in the absence of extracellular Ca2+, either in media prepared without Ca2+ or media containing 10 μM EGTA–. The results were identical and have been pooled. The T cells were IIT-3; mean cpm at 5 mM glucose was 5,686; at 25 mM, glucose was 11,738. (C and D) Both beta cells and DCs were treated with BAPTA-AM for 1 h, and then the cells were separated. The passage to the DCs was then probed with IIT3. In C and D, cells were in media with 5 mM or 25 mM glucose, respectively. Results are representative of two experiments. In the lower portion of the graph, the DCs were treated with BAPTA for the same time, after which the drug was washed off and insulin was added. The processing of insulin was consistently inhibited by about 30%.
Both thapsigargin and high glucose increase cytoplasmic Ca2+ levels by depleting ER Ca2+ (26), raising the possibility that Ca2+ leakage from the ER, not the Ca2+ influx from the extracellular space, increases the transfer of granules. To test this idea, the assay was changed to study the requirements for extracellular or intracellular Ca2+. Beta cells were incubated with DCs for a limited time of 1 h, after which the DCs were separated from the beta cells: The content of immunoreactive insulin was then probed on the isolated DCs with the T cells. At 5 mM glucose concentration, the transfer was not affected by the absence of extracellular Ca2+ (n = 4). At 25 mM glucose, the transfer was inhibited by about 25% (n = 9) (Fig. 3B). In contrast, chelation of intracellular Ca2+ using 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA) resulted in complete inhibition, either at low or high glucose concentrations (Fig. 3 C and D). The effect of BAPTA chelation was on the beta cells. The isolated DCs were removed and then pulsed with insulin presented to the T cells. This meant that had insulin been transferred in the beta cells and DCs plus BAPTA culture, it would have been presented when the DCs were separated. This conclusion was confirmed in the imaging experiments described below. Collectively, these results suggest that the transfer of granules required an increase in intracellular Ca2+ levels caused by ER Ca2+ depletion.
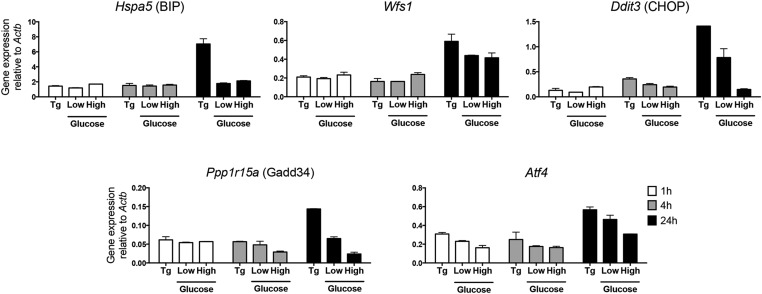
ER Ca2+ depletion is known to induce ER stress and activate the unfolded protein response. We therefore considered the possibility that the unfolded protein response may be involved in the transfer of granules. In our culture assay, during the several hours of culture of DCs with beta cells, we did not find up-regulation in the expression of any of the ER stress sensors: We evaluated a panel of canonical ER stress markers that are highly transcriptionally up-regulated during ER stress responses (27, 28) (Fig. S1 shows one representative result). In data not presented, the addition to the culture of tunicamycin, a drug known to increase the ER stress response, did not enhance presentation of insulin epitopes to T cells. (In unrelated experiments, the addition to beta cells of each of four cytokines—IFN-alpha A, IFN-gamma, tumor necrosis factor, and interleukin-1 beta—had no effect on the transfer to either of the two CD4 T cells.)
Fig. S1.
ERS markers evaluation. Beta cells from NOD.Rag1−/− mice were incubated in high or low glucose media for 1 h, 4 h, and 24 h. cDNA was synthesized from extracted mRNA. Hspa5 (BIP), Wfs1, Ddit3 (CHOP), Ppp1r15a (GADD34), and Atf4 were amplified with specific primers by quantitative RT-PCR. The fold change in gene expression was calculated using 2–ΔCT. Bars are mean ± SD of biological duplicates. Thapsigargin (Tg), 0.1 μM, was a positive control for ERS induction. Results are representative of three experiments.
Live Imaging of Beta Cells and APCs and Electron Microscopy Examination of Islets.
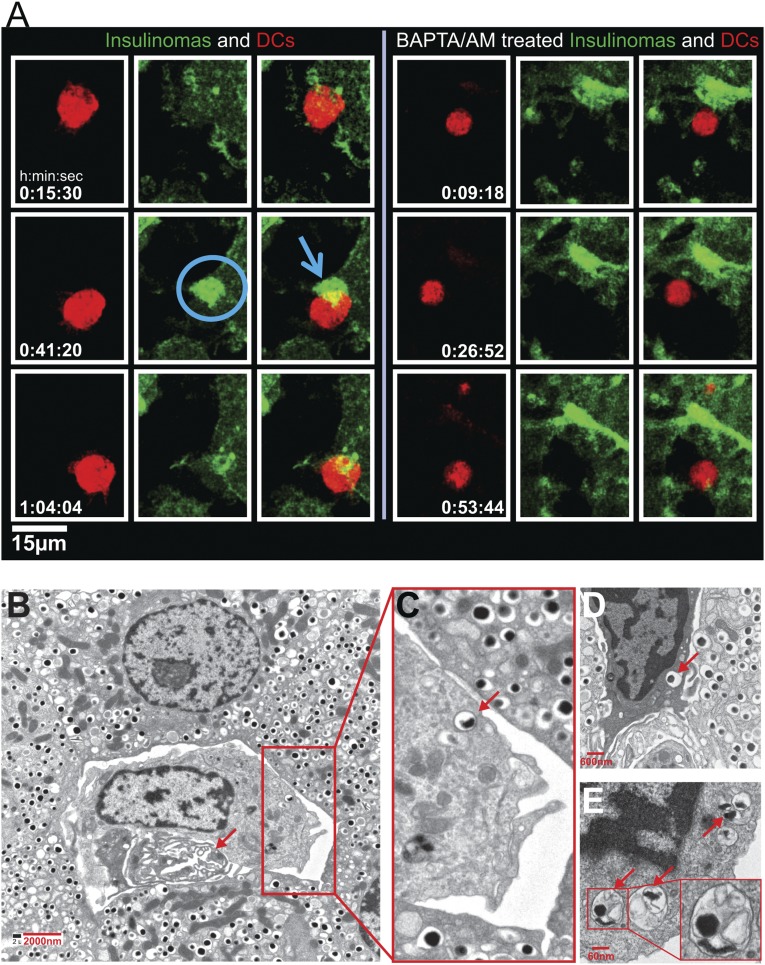
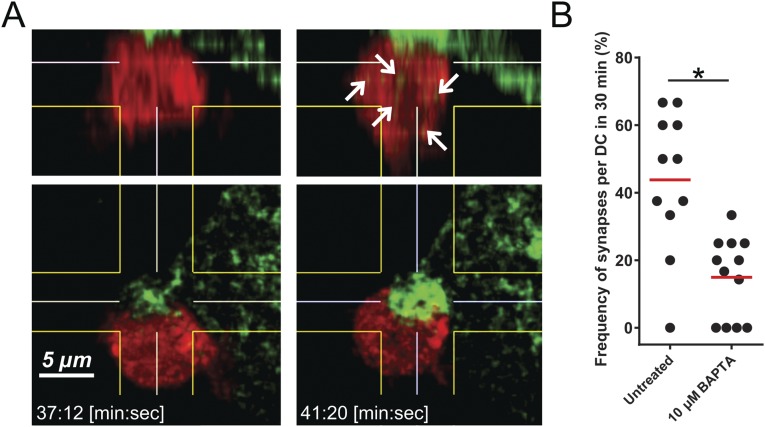
The passage of granules was also documented by live images of the insulinoma line NIT expressing granules bearing ZnT8 bound to green fluorescent protein (GFP) (29). We had reported in a previous study on interactions between the insulinoma cell line NIT with DCs and T cells directed to ZnT8 the islet-specific membrane transport for Zn ions (29). DCs were labeled with CellTrace Violet (false colored red) and added to NIT-ZnT8-GFP cells. Images showed the DCs contacting the NIT: There was a striking and progressive enrichment of GFP+ granules to the contact area (Fig. 4A and Movie S1) in a synapse-like structure. After a contact period, some of the GFP+ granules appeared in the DCs, which eventually detached from the beta cells. The time of contact ranged between 20 and 30 min. Such images were found invariably in all DC–NIT interactions. A 3D reconstruction shown in Fig. S2 documents the contact area and passage of the granules to the DCs. DCs were examined after contact with NIT-ZnT8-GFP treated with BAPTA. There were few granules in the contact area and limited transfer of them into the DCs (Fig. 4A, Movie S2, and Fig. S2). The contact time in the NIT treated with BAPTA was considerably shorter—just a few minutes. These results paralleled those made in the transfer assay described above (Fig. 1 E and F). A similar image of granule transfer was made in the single experiment done with bone marrow-derived macrophages (Movie S3).
Fig. 4.
Imaging and electron microscopy. (A) DCs labeled with CellTrace Violet (red) were added to NIT GFP-ZnT8-Insulinomas. Left panel (Movie S1) shows the contact area of NIT insulinomas in green with the DCs. Note the accumulation of GFP+ granules at the contact area. No movement of granules was observed in BAPTA/AM-treated NIT cells (Right panel and Movie S2). (B–E) Electron micrographs. B represents an islet from an 8-wk-old female NOD; C–E are islets taken from NOD.Rag1−/− mice at 14 wk of age. The arrow in B indicates a vessel. In the enlarged area, one of the dense-core granules is indicated by an arrow. In panel D a phagocyte is shown in between beta cells, and an arrow points to a dense-core granule. Panel E shows a portion of a phagocyte with endocytosed material in the form of vesicles containing an electron-dense core, with others containing amorphous content.
Fig. S2.
(A and B) Granule accumulation in insulinomas at the contact side of DCs (“synapse”). A is a 3D reconstruction and computational sectioning of DCs at the beginning of synapse formation (Left) and at the point of a mature synapse (Right) (related to Fig. 4 and Movie S1). Green particles increase dramatically inside the DCs during the process of synapse generation (white arrows). B is a quantitation of synapse per DC at 30 min.
Islets from young NOD mice including those from NOD.Rag1−/− mice were examined by electron microscopy. The intraislet phagocytes were found in close contact with beta cells and always next to blood vessels (Fig. 4B). All of the phagocytes had cytoplasmic vesicles, many of which contained material identical to beta cell secretory vesicles—that is, with a central electron-dense material, a light surrounding the zone, and a thin membrane; others had vesicles with irregular electron-dense material (Fig. 4C). The structural features indicate that the entire secretory vesicles from beta cells have been taken into the phagocytes. Fig. 4 B and C is from an NOD 8 wk of age; Fig. 4 D and E is from 14-wk-old NOD.Rag1−/− mice.
Summary.
We show here that beta cells pass the contents of their secretory granules to phagocytes. The presentation assays indicated that the passage involved close interaction between live cells that took place under conditions of low glucose. The passage increased by addition of a high glucose concentration or the drug thapsigargin, both of which increase cytosolic Ca2+. The passage took place even in beta cells from mice not undergoing an immunological intervention such as those from NOD.Rag1−/− mice or C57BL/6 mice; it also took place with human islets obtained from nondiabetic subjects. The passage of granules and/or insulin antigens to phagocytes was also documented in vivo by ultrastructural analysis and by live imaging of beta cells and islet APCs (Fig. 4) (13,14). In toto, these findings point to a scenario in which secretory granules are being transferred in a constitutive process to the islet resident phagocytes.
The biological relevance of the beta cell–phagocyte interaction outside the context of an autoimmune reaction needs to be evaluated because it may be key in maintaining islet homeostasis. Secretory granules contain a number of bioactive molecules that could well modulate the biology of the phagocytes; phagocytes including tissue macrophages are known to release cytokines and chemokines. In the absence of macrophages, islets are of much smaller size, suggesting a trophic effect of macrophages on islets (12, 30, 31). We envision a symbiotic interaction between both cells: Experiments in progress indicate that islet macrophages are in an activated state (11, 31).
The nature of the passage of vesicles during the beta cell–APC interaction is of particular interest. In contrast to the release of insulin granule content taking place under glucose-regulated secretion, the passage involved the whole secretory granule, as was evident from three different results. The ultrastructural analysis showed images compatible with intact granules inside the phagocytes, without any evidence of death of beta cells or inflammation within the islets. The imaging studies pointed to a flow of ZnT8-GFP+ granules to areas of contact with the APCs and a rapid flow of the granules into them. The immunological assays probing with the T cells to insulin required a chloroquine-sensitive stage in the APCs, indicating a catabolic process required to release the granule contents. Had free denatured insulin or B chain peptides passed to the APCs, there would not have been a requirement for a chloroquine-sensitive step (Fig. 2C). This finding is made more evident by the reactivity of the 8F10 T cell that recognizes catabolites of the insulin B chain. APCs that take up soluble denatured insulin or B chain peptide do not require intracellular catabolism, as is well established for diverse protein antigens. These findings are pointing thus to a close membrane interaction between the intraislet phagocytes that stimulated the movement of granules and its passage from one cell to another.
A number of the vesicles passed to the APCs must have contained insulin catabolites, some in the form of denatured insulin chains or peptides from the insulin B chain. We base this conclusion on the fact that we probed with a T cell that recognizes only insulin B chain catabolites offered exogenously to an APC. This T cell does not react with insulin epitopes after the processing of insulin by an APC (5, 6) (Fig. 1D). Moreover, the monoclonal antibody to insulin B chain peptide identified positive material inside the beta cell as well as in the islet APC. The importance of this finding is that the antibody exclusively reacts with the B chain peptide and not with native insulin, indicating that some intracellular vesicles contain degradative products of insulin. Extensive studies have unequivocally shown that beta cells contain vesicles bearing insulin catabolites, in a pathway referred to as crinophagy (32–36). It indicates an intracellular degradation of excess insulin granules, as a mode of controlling the homeostasis of insulin granule content (32, 37). The impression one obtains from these findings is that the process of generation of the peptide-positive—crinophagic—granules is taking place also constitutively, albeit increased by high glucose. We expect that as active diabetogenesis ensues this process may be modulated, such as a result of the stress conditions that accompanies diabetogenesis (38–40). The issue from the perspective of autoimmunity is that the insulin catabolic products constitute the immunogens that select for autoreactive T cells that bypass insulin processing and escape thymus regulation (5, 23).
We suggest that the findings made here represent the first stage by which immunogenic autoantigens of beta cells are made available to the immune system. We previously reported that insulin-reactive T cells, such as the 8F10 used here, entered islets to contact the intraislet APCs, not requiring a prior activation state in pancreatic lymph nodes (23). Subsequently, presentation of antigens in the pancreatic node represented an active amplification step for diabetogenesis. It is likely that the node is supplied by the movement of islet phagocytes. Recently we reported the seminal role of the CD103+ DCs in such a process (11).
Materials and Methods
Islet Isolation, T-Cell Assays.
Islets were isolated following standard procedures described before (11). In brief, collagenase was injected into the pancreas via the common bile duct, after which islets were selected under microscopy with a pipettor. The islet cells were dispersed nonenzymatically and plated in a 12-well tissue culture plate at a density of 2.5 × 105 cells/mL in either Dulbecco’s minimal essential media with 10% (vol/vol) fetal calf sera containing either 25 mM or 5 mM glucose (referred to as high or low glucose, respectively). Depending on the experiment, cells were cultured for a few hours or overnight at 37 °C. Islets were obtained in most experiments from NOD.Rag1−/− mice of either sex 4–6 wk of age. Islets also were obtained from NOD mice 4–16 wk of age and C57BL/6 mice 8 wk of age. Two human islets were examined, both obtained from Prodo Laboratories. The patients were a 43-y-old male and a 34-y-old female, neither of whom had a history of diabetes. In most assays, 2 × 104 beta cells were incubated with 5 × 104 CD11c+ DCs in a total volume of 150 μL of a 96-well tissue culture plate for several hours (from 1 to 24 h depending on the nature of the experiment). T cells were then added for 24 h, and their response was assayed.
We used two reporter hybridoma T-cell lines: IIT-3, which recognizes INS protein and the INS B:13–21 peptide, and 8F10, which recognizes INS B:12–20. The production of IL-2 was measured by the stimulation of the IL-2–dependent line CTLL-2 (5). DCs were harvested from spleens of mice killed 10 d after injections with FLT-3L (10 µg for 3 consecutive days). The spleen cells were positively selected for CD11c+ cells using MACS beads (Miltenyi Biotec).
The following changes in the transfer assay of beta cells–APCs were made. To examine (i) the effects of anti–I-Ag7 antibodies, beta cells and DCs were cultured for a day, after which the anti–I-Ag7 antibody AG2.42.7 at 10 μg/mL and the T cells were added for another 24 h. To examine (ii) whether the transfer process involves cell-to-cell contact, a transwell system was used in which beta cells and DCs were separated via a 96-well transwell plate containing a 0.4-µm polycarbonate membrane (Corning). In control wells, beta cells were incubated with DCs in the lower chamber of the transwell. To examine (iii) the effects of beta cell viability, the beta cells were cultured with streptozocin (50 mM) first for 12 h before addition of the DCs. To examine (iv) the role of acidification of vesicles in the DCs, the DCs were treated with 100 µM chloroquine (Sigma) for 2 h at 37 °C and then collected, washed, and added to the beta cells and the T cells for overnight culture. To examine (v) the effects of extracellular Ca2+, beta cells and DCs were incubated in media prepared without any added Ca2+ ions or in media having 10 μM EGTA for 1 h, after which the DCs were isolated and cultured with the T cells. To examine (vi) the effects of chelation of intracellular Ca2+, a culture of beta cells and DCs were treated with 10 μM BAPTA-AM for 1 h, and then the DCs were separated from the beta cells and tested with the T cells. As a control, isolated DCs after the BAPTA treatment were then offered insulin at 10 μM together with the T cells. In steps v and vi, we mostly used the IIT3 line, which is a more sensitive T-cell line. The beta cells (at 105 cells) and the DCs (at 6 × 105 cells) were incubated in a total volume of 500 µL media. The combined cells were passed through MACS columns, separating the DCs from beta cells.
Microscopy.
For electron microscopy, islets were isolated as described above, fixed in Karnovsky fixative, and processed by standard procedures. For live imaging two-photon microscopy, we used the NIT-ZnT8-GFP insulinomas reported before (29). The cells were cultured in F12 Ham’s nutrient mixture (Sigma) supplemented with 10% dialyzed FBS (Sigma), 1.5 g/L sodium bicarbonate, and 2 mM l-glutamine. Before the examination, we replaced the media with CO2-independent media (Gibco)/10% FBS. CD11c+ DCs were collected from mice injected with FLT-3L as described previously. The DCs were stained with 2 µM Cell Trace Violet (Molecular Probes) for 30 min at 37 °C in serum-free CO2-independent media. Plated NIT-GFP ZnT8 cells were placed under the two-photon scope. After focusing on the plated cells, DCs were added to the plate. Once DCs appeared in the area, imaging began. We acquired 18–25 z-sections (1.25 µm apart) every minute using a customized SP8 two-photon microscope (Leica) equipped with a 25×/0.95 N.A. water-dipping objective and a Mai Tai HP DeepSee Laser (Spectra-Physics) tuned to 885 nm. Fluorescence emission was separated by high-efficiency custom dichroic mirrors (Semrock) and directed to supersensitive external detectors. CellTrace Violet (Molecular Probes) was detected below 484 nm and GFP between 509 and 562 nm. Image sequences were then processed with Imaris (Bitplane) and Adobe Premiere software. The 3D reconstructions and sectioning were performed with Imaris software (Bitplane). For experiments studying the effects of BAPTA-AM on granule transfer, the above was modified slightly. NIT cell growth media was removed and replaced with fresh growth media containing 10 µM BAPTA-AM. The cells were incubated at 37 °C for 2 h. BAPTA containing media was removed from the dish and replaced with fresh growth media for 15 min at 37 °C. The DCs were then added as detailed above.
In experiments examining for ERS sensors, total RNA was isolated with Ambion RNAqueous-Micro Kit (Life Technologies). cDNA was obtained from total RNA using First Strand Synthesis Protocol with Reverse Transcriptase (New England BioLabs). TaqMan PCR was performed using TaqMan Fast Universal PCR Master Mix. Primers for quantitative RT-PCR were designed using the PrimeTime predesigned quantitative PCR assays (IDT DNA). PrimeTime primers used 5′-nuclease detection. StepOne 2.1 software was used to perform quality control and the relative expression quantification. We examined an Hsp70 family member (BIP, Hspa5), the ER-calcium regulator Wolfram syndrome 1 protein (Wfs1), the transcription factor C/EBP-homologous protein (CHOP, Ddit3), the activating transcription factor 4 (Atf4), and the growth arrest and DNA damage-inducible protein-34 (GADD34, Ppp1r15a).
Supplementary Material
Acknowledgments
Katherine Fredericks, Karen Green, Lindsay Moore, and Brian T. Saunders provided invaluable technical support. We thank Dr. Robert Schmidt for help in the reading of the electron micrographs. This research was supported by National Institutes of Health Grants DK058177 and DK020579, Juvenile Diabetes Research Foundation Grant 2-SRA-2014-293-Q-R, and 17-2013-512 award cofunded by the Helmsley Charitable Trust. C.V. is supported by a joint Washington University and Universidad Nacional de Colombia program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515954112/-/DCSupplemental.
References
- 1.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20(1):111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [DOI] [PubMed] [Google Scholar]
- 3.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol. 1994;24(8):1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 4.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25(4):1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 5.Mohan JF, et al. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11(4):350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama M, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435(7039):220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriyama H, et al. Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc Natl Acad Sci USA. 2003;100(18):10376–10381. doi: 10.1073/pnas.1834450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an insulin peptide-MHC complex allows diabetogenic T cells to escape thymic deletion. J Exp Med. 2011;208(12):2375–2383. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French MB, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes. 1997;46(1):34–39. doi: 10.2337/diab.46.1.34. [DOI] [PubMed] [Google Scholar]
- 10.Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol. 2004;5(10):1028–1035. doi: 10.1038/ni1120. [DOI] [PubMed] [Google Scholar]
- 11.Ferris ST, et al. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41(4):657–669. doi: 10.1016/j.immuni.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderon B, Carrero JA, Miller MJ, Unanue ER. Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans. Proc Natl Acad Sci USA. 2011;108(4):1561–1566. doi: 10.1073/pnas.1018973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci USA. 2008;105(16):6121–6126. doi: 10.1073/pnas.0801973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melli K, et al. Amplification of autoimmune response through induction of dendritic cell maturation in inflamed tissues. J Immunol. 2009;182(5):2590–2600. doi: 10.4049/jimmunol.0803543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.In’t Veld PA, Pipeleers DG. In situ analysis of pancreatic islets in rats developing diabetes. Appearance of nonendocrine cells with surface MHC class II antigens and cytoplasmic insulin immunoreactivity. J Clin Invest. 1988;82(3):1123–1128. doi: 10.1172/JCI113669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höglund P, et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189(2):331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of β cell reactive T cells in NOD mice. J Exp Med. 2002;196(3):369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, et al. In situ beta cell death promotes priming of diabetogenic CD8 T lymphocytes. J Immunol. 2002;168(3):1466–1472. doi: 10.4049/jimmunol.168.3.1466. [DOI] [PubMed] [Google Scholar]
- 19.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological β cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198(10):1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levisetti MG, Suri A, Frederick K, Unanue ER. Absence of lymph nodes in NOD mice treated with lymphotoxin-β receptor immunoglobulin protects from diabetes. Diabetes. 2004;53(12):3115–3119. doi: 10.2337/diabetes.53.12.3115. [DOI] [PubMed] [Google Scholar]
- 21.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7(1):83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calderon B, Carrero JA, Unanue ER. The central role of antigen presentation in islets of Langerhans in autoimmune diabetes. Curr Opin Immunol. 2014;26:32–40. doi: 10.1016/j.coi.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan JF, Calderon B, Anderson MS, Unanue ER. Pathogenic CD4⁺ T cells recognizing an unstable peptide of insulin are directly recruited into islets bypassing local lymph nodes. J Exp Med. 2013;210(11):2403–2414. doi: 10.1084/jem.20130582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell SL, Fink CJ, Lacy PE. Isolation and properties of secretory granules from rat islets of Langerhans. I. Isolation of a secretory granule fraction. J Cell Biol. 1969;41(1):154–161. doi: 10.1083/jcb.41.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goginashvili A, et al. Insulin granules. Insulin secretory granules control autophagy in pancreatic β cells. Science. 2015;347(6224):878–882. doi: 10.1126/science.aaa2628. [DOI] [PubMed] [Google Scholar]
- 26.Hara T, et al. Calcium efflux from the endoplasmic reticulum leads to β-cell death. Endocrinology. 2014;155(3):758–768. doi: 10.1210/en.2013-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 28.Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011;22(7):266–274. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayak DK, Calderon B, Vomund AN, Unanue ER. ZnT8-reactive T cells are weakly pathogenic in NOD mice but can participate in diabetes under inflammatory conditions. Diabetes. 2014;63(10):3438–3448. doi: 10.2337/db13-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banaei-Bouchareb L, et al. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76(2):359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 31.Calderon B, et al. The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med. 2015;212(10):1497–1512. doi: 10.1084/jem.20150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halban PA, Wollheim CB. Intracellular degradation of insulin stores by rat pancreatic islets in vitro. An alternative pathway for homeostasis of pancreatic insulin content. J Biol Chem. 1980;255(13):6003–6006. [PubMed] [Google Scholar]
- 33.Halban PA, Renold AE. Influence of glucose on insulin handling by rat islets in culture. A reflection of integrated changes in insulin biosynthesis, release, and intracellular degradation. Diabetes. 1983;32(3):254–261. doi: 10.2337/diab.32.3.254. [DOI] [PubMed] [Google Scholar]
- 34.Orci L, et al. Insulin, not C-peptide (proinsulin), is present in crinophagic bodies of the pancreatic B-cell. J Cell Biol. 1984;98(1):222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnell AH, Borg LAH. Lysosomes and pancreatic islet function. Glucose-dependent alterations of lysosomal morphology. Cell Tissue Res. 1985;239(3):537–545. doi: 10.1007/BF00219232. [DOI] [PubMed] [Google Scholar]
- 36.Schnell AH, Swenne I, Borg LAH. Lysosomes and pancreatic islet function. A quantitative estimation of crinophagy in the mouse pancreatic B-cell. Cell Tissue Res. 1988;252(1):9–15. doi: 10.1007/BF00213820. [DOI] [PubMed] [Google Scholar]
- 37.Arvan P, Halban PA. Sorting ourselves out: Seeking consensus on trafficking in the beta-cell. Traffic. 2004;5(1):53–61. doi: 10.1111/j.1600-0854.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 38.Tersey SA, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61(4):818–827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engin F, et al. Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci Transl Med. 2013;5(211):211ra156. doi: 10.1126/scitranslmed.3006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans-Molina C, Hatanaka M, Mirmira RG. Lost in translation: Endoplasmic reticulum stress and the decline of β-cell health in diabetes mellitus. Diabetes Obes Metab. 2013;15(Suppl 3):159–169. doi: 10.1111/dom.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.