Significance
Microvillus inclusion disease (MVID) is a rare intestinal enteropathy resulting in severe diarrhoea in neonates. Here, we have generated an intestine-specific knockout mouse model for Myosin Vb, the gene causing MVID in the majority of human patients. Our mouse model completely recapitulates the intestinal human MVID phenotype, including severe diarrhoea, loss of microvilli, occurrence of microvillus inclusions, and subapical secretory granules in villus enterocytes. In addition, we identify a newly identified role of Myo5b in trafficking of basolateral proteins, in the apical localization of the brush border membrane fusion protein syntaxin 3 (STX3), and in early differentiation of enterocytes. Our data indicate a role of MYO5B in regulating polarity of epithelial cells and have important implications for future treatment options for MVID patients.
Keywords: mouse model, myosin Vb, epithelila polarity, intestinal enteropathy, microvillus inclusion disease
Abstract
Microvillus inclusion disease (MVID) is a rare intestinal enteropathy with an onset within a few days to months after birth, resulting in persistent watery diarrhea. Mutations in the myosin Vb gene (MYO5B) have been identified in the majority of MVID patients. However, the exact pathophysiology of MVID still remains unclear. To address the specific role of MYO5B in the intestine, we generated an intestine-specific conditional Myo5b-deficient (Myo5bfl/fl;Vil-CreERT2) mouse model. We analyzed intestinal tissues and cultured organoids of Myo5bfl/fl;Vil-CreERT2 mice by electron microscopy, immunofluorescence, and immunohistochemistry. Our data showed that Myo5bfl/fl;Vil-CreERT2 mice developed severe diarrhea within 4 d after tamoxifen induction. Periodic Acid Schiff and alkaline phosphatase staining revealed subapical accumulation of intracellular vesicles in villus enterocytes. Analysis by electron microscopy confirmed an almost complete absence of apical microvilli, the appearance of microvillus inclusions, and enlarged intercellular spaces in induced Myo5bfl/fl;Vil-CreERT2 intestines. In addition, we determined that MYO5B is involved not only in apical but also basolateral trafficking of proteins. The analysis of the intestine during the early onset of the disease revealed that subapical accumulation of secretory granules precedes occurrence of microvillus inclusions, indicating involvement of MYO5B in early differentiation of epithelial cells. By comparing our data with a novel MVID patient, we conclude that our mouse model completely recapitulates the intestinal phenotype of human MVID. This includes severe diarrhea, loss of microvilli, occurrence of microvillus inclusions, and subapical secretory granules. Thus, loss of MYO5B disturbs both apical and basolateral trafficking of proteins and causes MVID in mice.
Microvillus inclusion disease (MVID) is a rare intestinal enteropathy with autosomal recessive inheritance, which was first described in 1978 (1). MVID patients cannot take up any nutrients and are often completely dependent on parenteral nutrition. The disease is characterized by villus atrophy, (partial) loss of microvilli on the apical plasma membrane of intestinal epithelial cells, and accumulation of intracellular vesicles/vacuoles, containing apical proteins and microvilli (2, 3). In addition, some studies also show mislocalization of apical and basolateral proteins, occasional crypt hyperplasia, and villus fusion (4–6).
In the great majority of patients, MVID is caused by mutations in MYO5B, encoding the motorprotein, myosin Vb (5). In two patients, mutations in syntaxin 3 (STX3) caused a variant form of MVID (7). More than 41 unique mutations along the different regions in MYO5B have been identified in MVID patients, including deletions and nonsense, missense, and splice-site mutations (8–10). MYO5B is coding for the actin-based myosin 5b motor protein, which regulates apical membrane trafficking (5, 11). MYO5B functions as a homodimer and has three functional domains: an N-terminal motor domain, a calmodulin-binding domain, and a C-terminal tail, which binds cargo through association with the small GTPases RAB8A and/or RAB11A (12, 13). Altered expression of myosin Vb affects the apical membrane trafficking mechanism in epithelial cells, causing mislocalization of apical brush border proteins, such as villin (vil), CD10, or alkaline phosphatase (ALP) in the cytoplasm of duodenal enterocytes (2, 3, 5), and an increased apical localization of transferrin receptor (5, 14).
Although mouse models mimicking certain features of MVID have previously been described, such as Rab8 (15), Cdc42 (16, 17), and Rab11a knockout (KO) mice (18, 19), no mutations in the coding regions of those genes have been reported in human MVID patients. Current in vitro models to study apical trafficking and polarization-associated diseases such as MVID are the parental Caco2 cell line, Caco-BBE, and LS174 W4 cells, in which polarization can be induced in vitro (4, 8, 12, 20). Although valuable knowledge about the function of MYO5B in polarization was gained in these models, the direct relevance of the colon cancer cell lines for the disease is questionable, and diverging results have been obtained with knockdown of MYO5B in the parental Caco2 cells compared with the more polarized Caco-BBE cells (8, 12, 20). As such, we here present an inducible MVID mouse model that recapitulates the genetic defects in man, which allows analysis of the role of MYO5B in a physiological setting and the sequence of events in MVID pathophysiology.
Results
Generation of Mice.
To study the consequence of Myo5b ablation in an in vivo model, we generated a Cre-inducible floxed Myo5b mouse in which LoxP sites were placed around exon 4 (Fig. 1A). Targeted ES cell clones were checked for homologous recombination by Southern blotting (Fig. S1A). Cre-induced deletion of exon 4 in our mouse model results in a frame shift and a premature stop codon leading to a truncated protein of 111 amino acids (a full-length protein is 1,818 amino acids) and loss of the MYO5B protein. To generate an intestine-specific inducible KO mouse model for Myo5b, we have crossed homozygous Myo5bfl/fl mice with a Vil-CreERT2 mouse strain (21). Vil-CreERT2 mice express the Cre enzyme under the control of the Vil promoter, which drives stable and homogeneous expression of the Cre recombinase in all epithelial cells of the small and large intestine. Because the Cre recombinase is fused to the estrogen receptor ERT2, it remains retained in the plasma membrane of the epithelial cells. Only upon induction with tamoxifen, the Cre-ERT2 protein is translocated to the nucleus, where it can promote recombination of the LoxP sites and thereby inactivate the conditional Myo5b allele. The resulting Myo5bfl/fl;Vil-CreERT2 mice were genotyped by PCR (Fig. S1B).
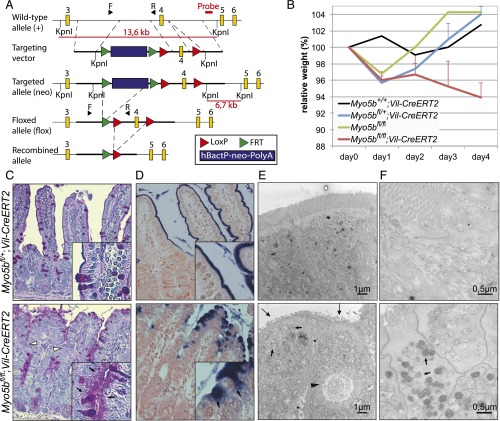
Fig. 1.
Myo5b deficiency causes an MVID phenotype in mice. (A) Diagram depicting the strategy for the conditional KO of exon 4 of the Myo5b gene. The probe used for Southern blot analysis (indicated in red) hybridizes with a 13.6-kb KpnI fragment from the wild-type allele and with a 6.7-kb KpnI fragment from the targeted allele. F and R indicate the location of the primers used for genotyping. Exons are indicated with yellow squares. LoxP sites and FRT sites are represented by red and green triangles, respectively. (B) Relative weight curves of homozygous Myo5bfl/fl;Vil-CreERT2 mice (n = 2–8 per time point), heterozygous Myo5bfl/+;Vil-CreERT2 control mice (n = 2–6 per time point), one Myo5b+/+;Vil-CreERT2, and one Myo5b+/+ control mouse. All mice were induced with 4.5 mg tamoxifen at 8–10 wk of age. Graph shows means + SEM. (C–E) At day 4 after induction, Myo5bfl/fl;Vil-CreERT2 mice showed (C) subapical accumulation of PAS-positive vesicles (small arrows) and villus fusion (white arrowheads) and (D) mislocalization of the apical marker ALP to the cytoplasm (arrows). (E and F) TEM revealed (E) erosion and loss of apical microvilli (large arrows), subapical accumulation of vesicles (small arrows), intracellular microvillus inclusions (black arrowhead), A/Ls, and widened intercellular spaces (*) in villus epithelial cells of Myo5bfl/fl;Vil-CreERT2 mice. (F) EM-cytochemistry shows epithelial cells at the crypt–villus transition with PAS-positive, classical secretory granules (small arrows).
Fig. S1.
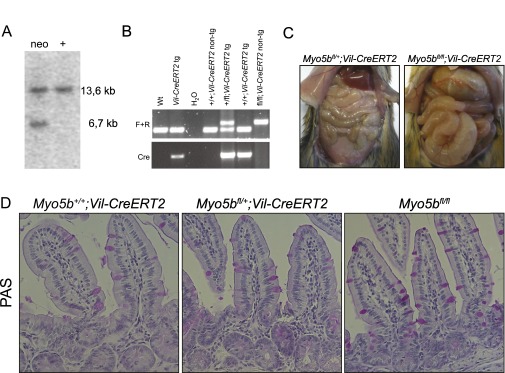
Analysis of conditional Myo5bfl/fl;Vil-CreERT2 mice. (A) Southern blotting analysis of the targeted ES clones. Genomic DNA isolated from ES cell clones was digested with KpnI for hybridization with the probe as indicated in Fig. 1A, showing a correctly recombined clone (neo) and a nonrecombined wild-type clone (+). (B) Genotyping PCRs of genomic DNA isolated from mouse tails. Primers indicated as F and R in Fig. 1A were used to detect the floxed Myo5b allele, and Cre primers were used to detect the Vil-CreERT2 transgene. (C) Intestines of Myo5bfl/fl;Vil-CreERT2 at day 4 after injection with tamoxifen were swollen and filled with watery diarrhea compared with control mice. (D) PAS staining showed a normal intestinal phenotype of tamoxifen-induced Myo5b+/+;Vil-CreERT2 mice, Myo5bfl/+;Vil-CreERT2 mice, and Myo5bfl/fl mice on day 4 after tamoxifen injections.
To determine the intestine-specific phenotype of loss of Myo5b, we have injected Myo5bfl/fl;Vil-CreERT2 mice and Myo5b+/+;Vil-CreERT2, Myo5bfl/+;Vil-CreERT2, and Myo5bfl/fl control mice with tamoxifen and measured their weight every day. Starting from day 3 after tamoxifen induction, only Myo5bfl/fl;Vil-CreERT2 mice displayed severe weight loss compared with controls (Fig. 1B). Mice were killed no later than day 4 after induction, due to severe weight loss and dehydration as a result from watery diarrhea (Fig. S1C). We observed that Myo5b+/+;Vil-CreERT2, Myo5bfl/+;Vil-CreERT2, and Myo5bfl/fl;Vil-CreERT2 control mice did not show any phenotype, as confirmed by weight curves, Periodic Acid Schiff (PAS) staining and EM (Fig. 1B and Fig. S1D). Absence of myosin Vb upon tamoxifen induction was confirmed by mRNA expression and immunohistochemistry (Fig. S2 A and B).
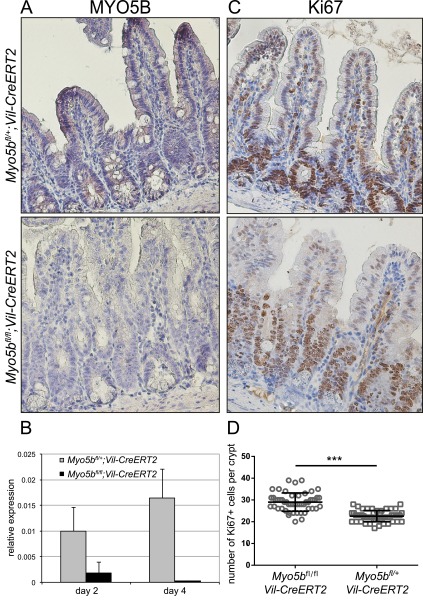
Fig. S2.
(Immuno)histochemical analysis of Myo5bfl/fl;Vil-CreERT2 intestines. On day 4 after induction with tamoxifen, Myo5bfl/fl;Vil-CreERT2 intestines displayed (A) loss of MYO5B protein in intestinal epithelial cells, (B) loss of Myo5b mRNA, and (C) elongated crypts with an increased number of Ki67-positive proliferating cells. (D) Numbers of Ki67+ cells were counted in 50 crypts from one Myo5bfl/+;Vil-CreERT2 and one Myo5bfl/fl;Vil-CreERT2 mouse. Graphs show means ± SEM; ***P < 0.0001.
Loss of Myo5b Causes MVID Phenotype in Mice.
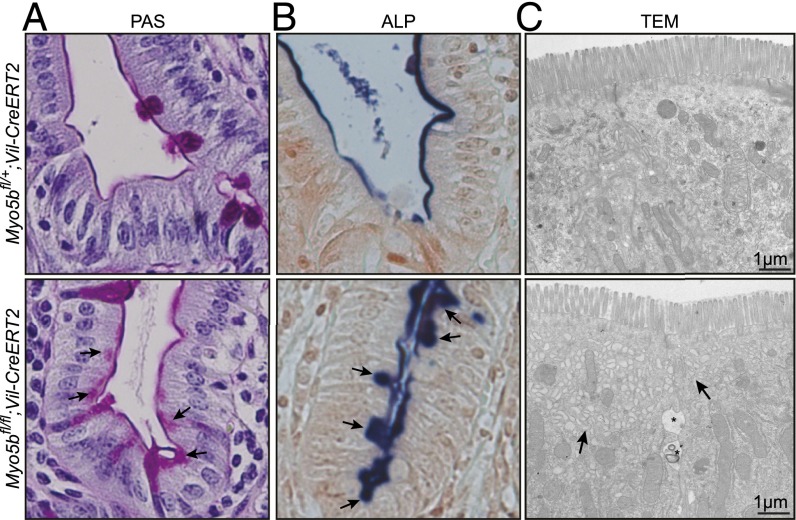
The histological analysis of the intestine of the tamoxifen-induced Myo5bfl/fl;Vil-CreERT2 mice on day 4 showed villus atrophy and crypt hyperplasia, which was confirmed by a significantly (P < 0.0001) increased amount of Ki67-positive crypt cells (Fig. 1 C and D and Fig. S2 C and D). We have counted 23 ± 0.4 and 29 ± 0.6 Ki67+ cells in 50 crypts from a Myo5bfl/+;Vil-CreERT2 and a Myo5bfl/fl;Vil-CreERT2 mouse, respectively (Fig. S2D). Furthermore, PAS and ALP staining revealed subapical accumulation of intracellular vesicles in villus enterocytes. Finally, fusion of villi was observed, a feature only recently reported from MVID patients (4) (Fig. 1 C and D).
Pathognomonic features of MVID are the so-called microvillus inclusions that are found in ∼10% of mature enterocytes at the tips of the villi of these patients (3, 22). Analysis of the intestines of induced Myo5bfl/fl;Vil-CreERT2 mice by electron microscopy (EM) showed a severe reduction to almost complete absence of apical microvilli on day 4 after induction. Furthermore, we found conspicuous clusters of subapical vesicles as well as (auto)lysosomes (A/Ls) and microvillus inclusions in mature villus enterocytes (Fig. 1E). Finally, we observed enlarged intercellular spaces between enterocytes and occasionally basolateral microvilli. Strongly PAS-positive vesicles, representing the classical electron-dense secretory granules (3), occurred in crypt enterocytes and at the crypt–villus transition (Fig. 1F). The features described above have been reported to be the main pathological features of MVID patients (1, 3, 5, 8, 11, 14), indicating that our mouse model phenocopies human MVID. Additionally, we have quantified the phenotypic characteristics of our mouse model in Table 1.
Table 1.
Quantification of TEM characteristics
| MVID characteristics | Myo5bfl/+;Vil-Cre (n = 50) | Myo5bfl/fl;Vil-Cre (n = 100) | P value |
| Microvillus inclusions | 0% | 19% | 0.0004 |
| Apical microvilli | 100% | 20% | <0.0001 |
| Basolateral microvilli | 0% | 20% | 0.0002 |
| A/Ls | 30% | 78% | <0.0001 |
| Subapical accumulation of vesicles | 4% | 73% | <0.0001 |
| Increased intercellular space | 6% | 50% | <0.0001 |
Five Myo5bfl/fl;Vil-Cre and two Myo5bfl/+;Vil-Cre mice were injected with tamoxifen and killed 4 d later. TEM was performed, and 50–100 enterocytes per mouse strain were analyzed for MVID characteristics.
STX3 and RAB11A Define Microvillus Inclusions as Apical Domains.
Previously, it has been shown that MYO5B regulates localization of apical proteins toward the plasma membrane (2, 4, 5, 8, 9, 14, 20, 23, 24). In concordance with previous findings, ALP staining showed subapical accumulation in villus enterocytes of Myo5bfl/fl;Vil-CreERT2 mice (Fig. 1D). Furthermore, phosphorylated Ezrin–Radixin–Moesin (pERM), which connects the apical plasma membrane to F-actin filaments to stabilize microvilli (25–27), was markedly decreased at the apical membrane and was found in the cytoplasm of Myo5bfl/fl;Vil-CreERT2 mice (Fig. 2 A and B).
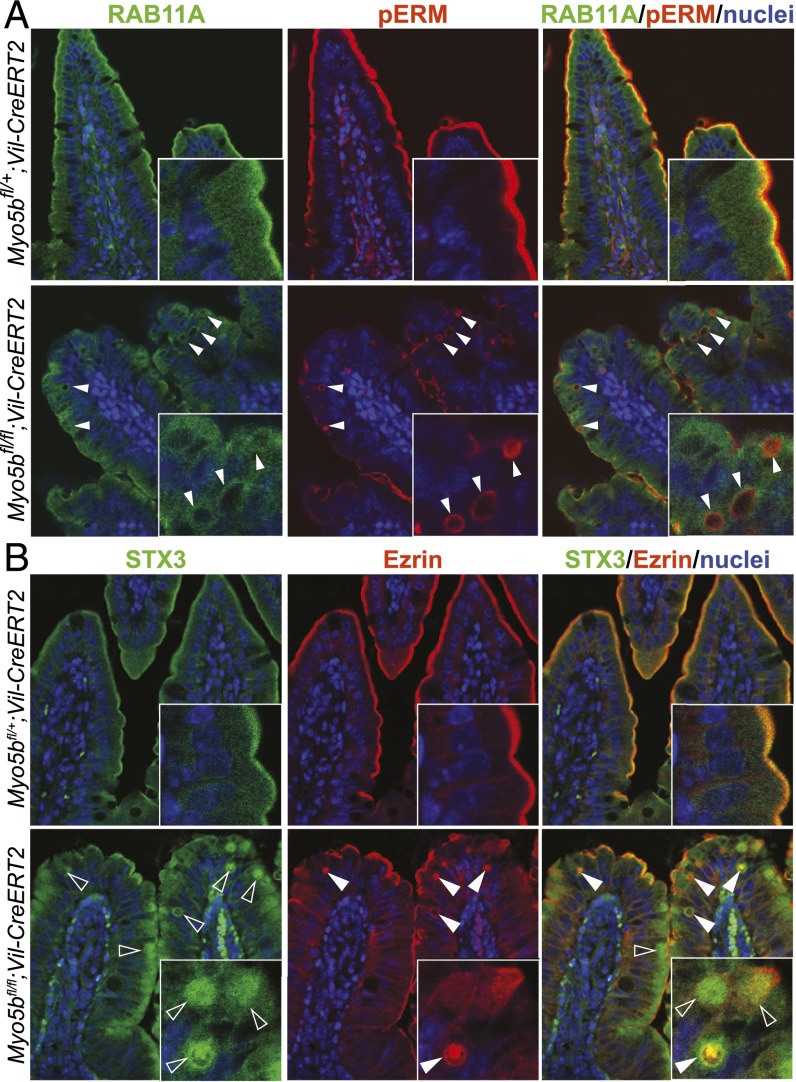
Fig. 2.
Apical proteins mislocalize to inclusions that are surrounded by RAB11A. Coimmunofluorescence staining for (A) pERM with RAB11A and (B) ezrin with STX3 on day 4 after Cre induction revealed a loss of apical pERM (arrows) and occurrence of pERM/ezrin-positive microvillus inclusions (closed arrowheads) in Myo5bfl/fl;Vil-CreERT2 mice, which costained with STX3 (open arrowheads) and are surrounded by RAB11A.
To perform its function, MYO5B associates with RAB8A and RAB11A, working as a tether and connecting Rab-associated vesicles to actin filaments (9, 12). Depending on the association with either RAB8A or RAB11A, MYO5B is involved in distinct trafficking pathways. MYO5B association with RAB8A has been linked to direct trafficking from the Golgi to the plasma membrane, whereas association of MYO5B with RAB11A is crucial for apical endosomal recycling (13).
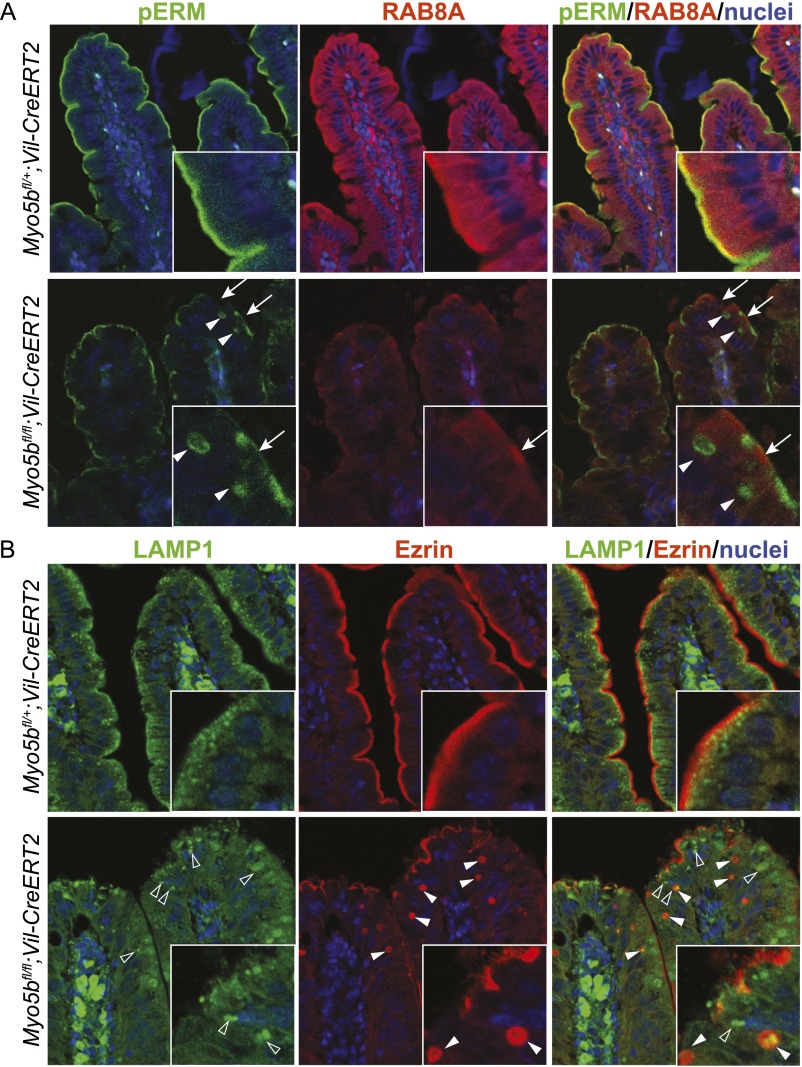
Here, we show that control mice show diffuse staining of RAB8A and subapical staining of RAB11A. In contrast, Myo5bfl/fl;Vil-CreERT2 mice show reduced RAB8A staining with a few subapical foci (Fig. S3A). RAB11A was lost at the apical membrane and showed a more diffuse cytoplasmic staining. In addition, several intracellular ezrin-positive inclusions were surrounded by RAB11A-positive vesicles (Fig. 2A). Lysosome-associated membrane protein 1 (LAMP1), which normally localizes subapically, was diffusely distributed in the cytoplasm of Myo5bfl/fl;Vil-CreERT2 enterocytes (Fig. S3B). These data indicate a disturbed trafficking and recycling machinery in Myo5b-deficient cells.
Fig. S3.
pERM-positive inclusions are not associated with Rab8a and occasionally colocalize with LAMP1. Coimmunofluorescence staining for pERM (A) with RAB8A and (B) with LAMP1 on day 4 after Cre induction revealed a loss of apical pERM (arrows) and occurrence of pERM/ezrin-positive inclusions (closed arrowheads) in Myo5bfl/fl;Vil-CreERT2 mice, which were not associated with RAB8A and occasionally colocalize with LAMP1 (open arrowheads).
Previously, we have shown that, next to MYO5B, the target-soluble N-ethylmaleimide–sensitive factor attachment protein receptor (t-SNARE) protein STX3 is also involved in MVID pathogenesis, as we described two patients with a variant form of MVID having mutations in STX3 (7). Furthermore, in a Rab11-KO mouse model, it was reported that microvillus inclusions were associated with STX3 (18). Here, we show that the ezrin-positive inclusions in our Myo5b-KO mouse model colocalize with STX3 (Fig. 2B). This indicates that microvillus inclusions present as ectopic apical domains positive for the apical t-SNARE STX3 and being surrounded by RAB11A-positive vesicles.
Loss of Myo5b Causes Disturbed Basolateral Polarity.
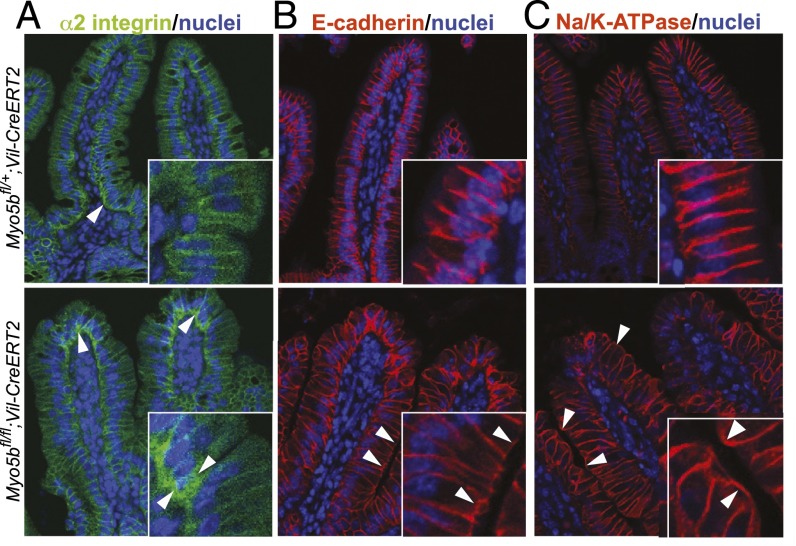
Data on basolateral markers in different MVID patients or MYO5B-deficient cell lines are not consistent, and basolateral polarity seems not always to be disturbed (2, 5, 12, 14). We analyzed the localization of selected basolateral markers and found that 4 days after tamoxifen induction, Myo5bfl/fl;Vil-CreERT2 mice showed a slightly abnormal distribution of α2-integrin. This integrin subunit is mainly expressed on basolateral membranes of lower villus enterocytes. Notably, in Myo5bfl/fl;Vil-CreERT2 mice, enterocytes at the tip of the villus showed stronger staining at the basal plasma membrane and near the nucleus (Fig. 3A). E-cadherin and Na/K-ATPase, which are normally only located at the basolateral membrane, were found on apical surfaces of some, but not all, Myo5b-deficient cells (Fig. 3 B and C).
Fig. 3.
Basolateral proteins are mislocalized. On day 4 after induction with tamoxifen, Myo5bfl/fl;Vil-CreERT2 intestines displayed mislocalization of the basolateral markers (A) α2 integrin to the basolateral cytoplasm of enterocytes at the tip of the villus (arrowheads) and (B) E-cadherin and (C) Na/K-ATPase to the apical membrane (arrowheads).
Myo5bfl/fl;Vil-CreERT2 Mice Phenocopy Human MVID.
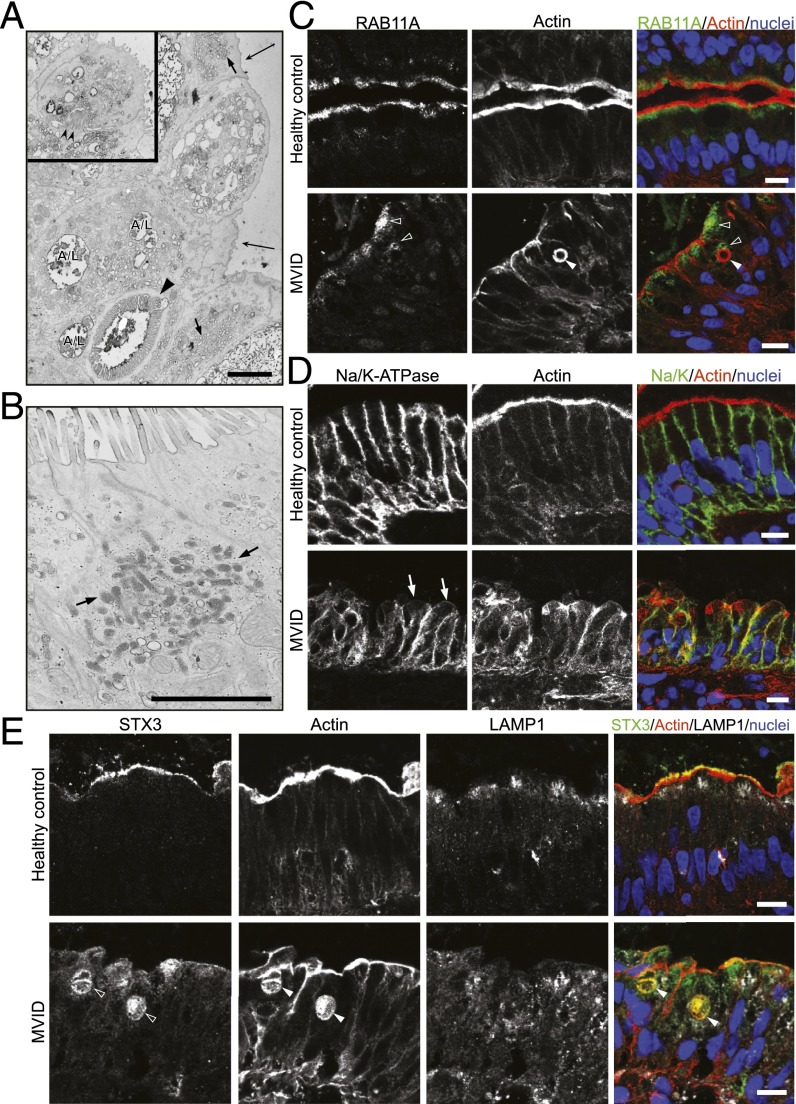
As summarized in Table 1, all histological characteristics found in MVID patients were phenocopied in our Myo5bfl/fl;Vil-CreERT2 mouse model. To better compare mouse and human disease phenotypes, we additionally show the analysis of a newly identified MVID patient, harboring a homozygous c.1323–2A > G splice-site mutation in MYO5B, identified previously in MVID (5). EM shows loss of microvilli, subapical accumulation of vesicles/secretory granules, basolateral microvilli, and microvillus inclusions (Fig. 4A). EM-cytochemistry for PAS-reactive material revealed strongly positive vesicles at the crypt–villus transition of the MVID patient, which represents classical (“dark”) secretory granules (Fig. 4B). Furthermore, costainings on cryosections show that intracellular actin-positive inclusions are surrounded by RAB11A (Fig. 4C) and the basolateral Na/K-ATPase was partly mislocalized to the apical membrane (Fig. 4D). Actin-positive inclusions colocalized with STX3 (Fig. 4E), and LAMP1 was distributed more diffusely throughout the cytoplasm of MVID enterocytes (Fig. 4E).
Fig. 4.
Phenotypic analysis of a newly identified human MVID patient with a MYO5B 1323–2A > G mutation. (A) TEM shows loss of microvilli (big arrows), subapical accumulation of translucent vesicles (small arrows), A/Ls, and microvillus inclusions (arrowhead). The Inset shows basolateral microvilli (double arrowhead). (B) EM-cytochemistry for PAS-positive material shows classical electron-dense, secretory granules (arrows) in epithelial cells at the crypt–villus transition. (C–E) Costainings on crysections show that actin-positive inclusions (arrowheads) are (C) surrounded by RAB11A-positive vesicles (open arrowheads) and (D) colocalizing with STX3. (E) LAMP1 is localized more diffusely in the cytoplasm and does not colocalize with actin inclusions in the MVID patient. [Scale bar, (A and B) 2 µm and (C–E) 10 µm.]
In conclusion, the analysis of the MVID patient fully confirms the validity of the mouse model presented here presented (see Table S1 for a summary).
Table S1.
Typical MVID features found in our MVID mouse model were compared with a newly identified MYO5B mutant MVID patient
| MVID characteristics | MVID mouse model | Novel MVID patient |
| Apical microvilli | Erosion to complete loss | Erosion to complete loss |
| Microvillus inclusions | ∼20% of villus enterocytes | Frequent in villus enterocytes |
| Basolateral microvilli | Yes | Yes |
| Subapical vesicles/secretory granules | Translucent in villus, electron dense in crypt | Translucent, electron dense in crypt |
| PAS/ALP | Subapical | Subapical |
| RAB11A | Subapical | Subapical |
| RAB8A | Almost absent, more diffuse in cytoplasm | n.a. |
| LAMP1 | Almost absent, more diffuse in cytoplasm | More diffuse in cytoplasm |
| Ezrin/pERM/Actin | Cytoplasmic inclusions | Cytoplasmic inclusions |
| STX3 | More diffuse in cytoplasm; cytoplasmic inclusions | More diffuse in cytoplasm; cytoplasmic inclusions |
| E-cadherin | Basolateral and apical | Basolateral and apical |
| Na/K-ATPase | Basolateral and apical | Basolateral and apical |
Loss of Myo5b First Affects Immature Enterocytes and Consecutively Leads to Disturbed Recycling in Mature Enterocytes.
In MVID patients, the most severe phenotype is observed in mature villus enterocytes at the tip of the villi, whereas crypt cells appear less affected (5, 14). In our mouse model, we analyzed the effects of loss of Myo5b in immature and mature epithelial cells over time. On day 1 after induction, we still observed MYO5B protein by immunohistochemistry in the intestines, and as such we did not observe any phenotype.
We confirmed the loss of Myo5b from day 2 after induction onwards. On day 2, cells in the upper part of the villus displayed a normal morphology, whereas the more immature cells at the crypt–villus transition and lower villus cells showed intracellular accumulation of PAS-positive material (Fig. 5A), cytoplasmic ALP staining (Fig. 5B), and accumulation of subapical vesicles (Fig. 5C). However, at this time point, we did not observe pERM-positive microvillus inclusions. In contrast, at day 4 after induction, all enterocytes along the villi were affected.
Fig. 5.
Lower villus cells are the first cells affected by loss of MYO5B. Myo5bfl/+;Vil-CreERT2 and Myo5bfl/fl;Vil-CreERT2 mice were killed 2 d after tamoxifen injection. (A) PAS and (B) ALP staining located subapically in epithelial cells at the crypt–villus transition and lower villus (arrows). (C) TEM confirmed subapical accumulation of vesicles (arrows) and widened intercellular space (*) in lower villus cells of Myo5bfl/fl;Vil-CreERT2 mice.
Collectively, these data show that loss of Myo5b first affects differentiation at the crypt–villus transition, leading to subapical accumulation of secretory granules. Consecutively, Myo5b deficiency disturbs apico-basolateral polarity, reflected by the occurrence of pERM/Ezrin/STX3-positive microvillus inclusions and defective RAB11A-dependent recycling.
Myo5bfl/fl;Vil-CreERT2 Organoids Represent an in Vitro Model for MVID and Reveal Impaired Functionality of Intestinal Epithelial Cells.
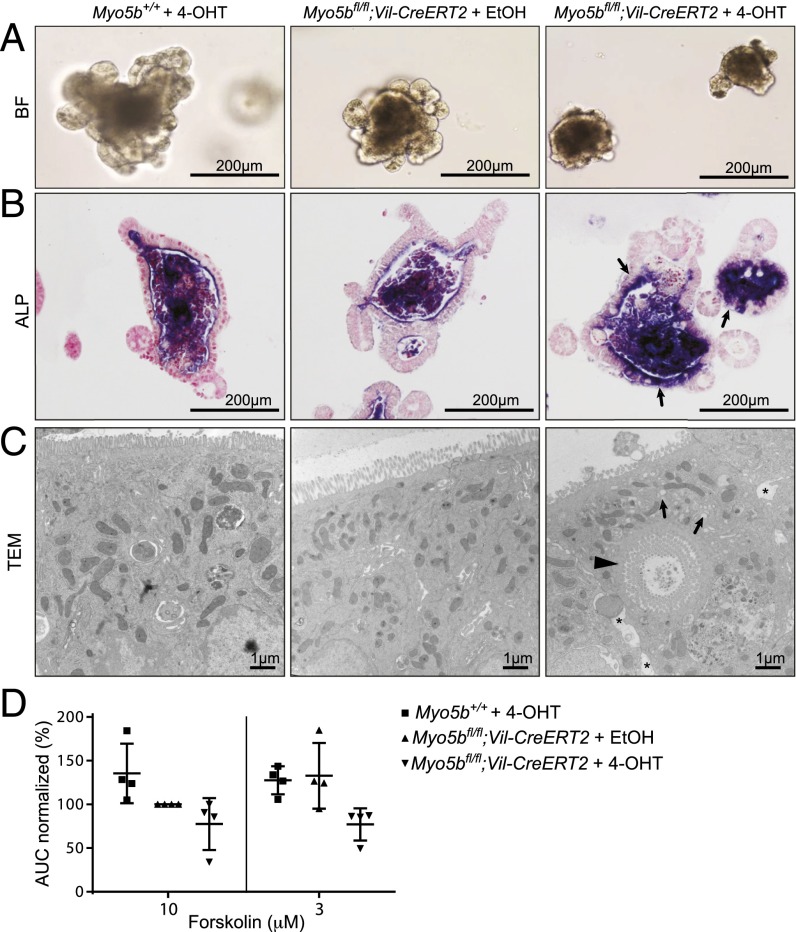
To perform detailed cellular and functional analysis of Myo5b-deficient intestinal epithelial cells, we established intestinal organoids from Myo5bfl/fl;Vil-CreERT2 mice (28). Noninduced organoids from Myo5bfl/fl;Vil-CreERT2 mice showed the same morphology as wild-type control organoids (Fig. 6A). Upon in vitro activation of Cre recombinase with 4-hydroxytamoxifen (4-OHT) for 15 h, the organoids appeared more compact with less budding structures and more cell death (Fig. 6A). By histology and TEM, we confirmed that the induced organoids reflect MVID, including intracellular accumulation of apical proteins such as ALP (Fig. 6B), microvillus inclusions, and absence of microvilli (Fig. 6C).
Fig. 6.
Myo5b-deficient organoids represent an in vitro model for MVID. Myo5bfl/fl and Myo5bfl/fl;Vil-CreERT2 organoids were treated in vitro with 1 µM 4-OHT or vehicle control (EtOH) for 15 h and analyzed 3 d later for (A) morphology by brightfield (BF) microscopy, (B) ALP staining showing subapical accumulations (arrows), and (C) TEM showing subapical accumulation of vesicles (small arrows), intracellular microvillus inclusions (arrowhead), and intercellular space (*) in 4-OHT–treated Myo5bfl/fl;Vil-CreERT2 organoids. (D) FIS assays were performed 24 h after in vitro treatment with 4-OHT or EtOH. CFTR-mediated swelling of the organoids was measured after 40 min of incubation with 10 or 3 µM forskolin. Swelling was calculated relative to the area under the curve (AUC) of Myo5bfl/fl;Vil-CreERT2 organoids that were treated with EtOH and 10 µM forskolin. Graph shows means ± SD of quadruplicate measurements of four independent experiments.
We applied this in vitro model for MVID to assess the functional capacities of Myo5b-deficient intestinal epithelial cells. It was previously shown that apical localization of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in polarized epithelial cells is MYO5B-dependent (2, 24). As such, we have performed Forskolin-Induced Swelling (FIS) assays, which measure the functional activity of the CFTR anion channel (29). Upon stimulation with forskolin, cAMP/protein kinase A-mediated phosphorylation of CFTR, for instance, enables opening of various ion channels, thereby facilitating transport of anions and fluids into the lumen of the organoids, which leads to swelling. Here we show that within 24 h upon Cre induction, Myo5b-deficient organoids are less able to swell upon stimulation with forskolin compared with control organoids (Fig. 6D). These data indicate that loss of Myo5b affects functional capacities of intestinal epithelial cells and myosin Vb is involved in the forskolin-dependent activation of CFTR and perhaps other basolateral ion channels or transporters required for the cAMP-induced secretory response.
Discussion
In this study, we describe an intestine-specific conditional Myo5b KO mouse model, in which loss of Myo5b can be induced by tamoxifen injection. We showed that our model completely recapitulates the disease phenotype of human MVID patients carrying MYO5B mutations (Table S1).
Patients usually present with severe diarrhea within the first days after birth (3). Similarily, Myo5b-deficient mice developed watery diarrhea within 4 days after induction (Fig. 1B and Fig. S1C). We demonstrated that loss of Myo5b led to an almost complete absence of enterocyte brush border, accumulation of intracellular vesicles, and occurrence microvillus inclusions (Fig. 1 C–E). Myo5b is known to interact with RAB8A or RAB11A (13). Both GTPases are involved in epithelial polarity. Furthermore, Rab8a and Rab11a KO mice displayed MVID-like phenotypes (15, 18, 19). However, the phenotypes observed in these mouse models were less severe than in our Myo5b-deficient mice, with a shortening but no absence of apical microvilli, and mice dying only within 2 wk (Rab11 KO) or 5 wk (Rab8 KO) after birth (15, 18, 19).
This suggests that Myo5b acts in different trafficking pathways, dependent on its association with either RAB8A or RAB11A, and that the severe phenotype observed in patients and in our mouse model results from the combined disturbance of both pathways.
In our inducible mouse model, we observed dispersal of both RAB8A and RAB11A. Similar RAB11A staining patterns were observed in the MVID patient. Interestingly, in Myo5bfl/fl;Vil-CreERT2 mice, only RAB11A but not RAB8A accumulated around pERM/Ezrin-positive microvillus inclusions (Figs. 2 and 4 and Fig. S3). This is in concordance with previous findings that only the loss of MYO5B binding to RAB11A caused the formation of microvillus inclusions in Caco-BBE cells (12). Because normal apical recycling through the apical recycling endosome is RAB-dependent, we suggest that this process is disturbed in MVID. Furthermore, we conclude that microvillus inclusions are an ectopic apical domain within the cytoplasm of enterocytes, as they contain the apical t-SNARE STX3 and are surrounded by RAB11A-positive vesicles, which are usually found subapically.
Recently, it was suggested that stabilization of initial cell polarity might be a future target for therapy of MVID patients (12, 17). Here, we confirmed that a normal pERM-containing brush border was present at an early time point (day 2) after induction, and microvillus inclusions were mainly formed in mature enterocytes at a late time point (day 4). However, despite formation of brush border, apical polarity was already affected in the immature enterocytes at the crypt–villus junction on day 2 after induction, represented by subapical accumulation of ALP- and PAS-positive vesicles (Fig. 5). Moreover, using organoids from Myo5bfl/fl;Vil-CreERT2 mice, we show that already on day 1 after induction, the functionality of the epithelial cells was impaired, as they showed reduced swelling upon stimulation with forskolin (Fig. 6). Importantly, at that time point after splitting, the organoids do not contain mature enterocytes yet (28), confirming again that immature enterocytes are also affected in polarity and function upon loss of MYO5B. Stabilization of early brush border formation might therefore not be effective, and a stem cell-based therapy might be necessary as a future treatment for MVID patients.
Materials and Methods
A detailed description of materials and methods can be found in the SI Materials and Methods.
Generation of Mice.
We generated a conditional Myo5b allele by inserting two LoxP sites around exon 4. Male chimaeras were subsequently crossed to C57BL/6 females, and germ-line transmission was confirmed by PCR. The neomycin expression cassette was excised in vivo by crossing the mice with Flp1 transgenic mice (29). For intestinal epithelium-specific KO, Myo5bfl/fl mice were crossed to a Vil-CreERT2 line. All animal procedures were performed in accordance with local animal welfare laws and guidelines and were reviewed by the Animal Ethics Committee of the Royal Dutch Academy of Sciences.
Human Material.
We studied biopsies from a 4-wk-old female Turkish patient. The study was approved by the Ethics committee of the Medical University of Innsbruck (study no. UN3987), and written informed consent from the parents was obtained.
(Immuno)histochemistry and Immunofluorescence.
Stainings were performed as described previously (17). An overview of all antibodies used, their dilutions, incubation times, and antigen retrieval can be found in Table S2.
Table S2.
Antibodies and protocols used for immunohistochemistry and immunofluorescence
| Antibody | Company | Catalogue number | Antigen retrieval | Dilution | Incubation |
| Anti-α2 integrin | Abcam | ab133557 | Citrate pH6 | 1/1,000 | Overnight; 4 °C |
| Anti–E-cadherin-647 | BD | 560062 | Citrate pH6 | 1/50 | Overnight; 4 °C |
| Anti-Ezrin | Abcam | ab41672 | Citrate pH6 | 1/100 | 2 h; RT |
| Anti-Ki67 | Monosan | MONX10283 | Citrate pH6 | 1/1,000 | Overnight; RT |
| Anti-Lamp1 | Abcam | ab24170 | Citrate pH6 | 1/100 | 2 h; RT |
| (Mouse samples) | |||||
| Anti-Lamp1 | DSHB | H4A3 | n.a. | 1/200 | 2 h; RT |
| (Human samples) | |||||
| Anti-Myo5b | Novus Biologicals | NBP1-87746 | Citrate pH6 | 1/100 | Overnight; 4 °C |
| Anti–Na/K-ATPase (mouse samples) | Millipore | 05–369 | Tris-EDTA pH9 | 1/100 | 2 h; RT |
| Anti–Na/K-ATPase (human samples) | GeneTex | GTX61640 | n.a. | 1/100 | 2 h; RT |
| Anti-pERM | Cell Signaling | 3149S | Citrate pH6 or Tris-EDTA pH9 | 1/200 | 2 h; RT |
| Anti-Rab8a | BD | 610845 | Tris-EDTA pH9 | 1/100 | Overnight; 4 °C |
| Anti-Rab11a | Invitrogen | 71–5300 | Tris-EDTA pH9 | 1/50 | 2 h; RT |
| Anti-STX3 | Abcam | ab133750 | Citrate pH6 | 1/100 | Overnight; 4 °C |
| Phalloidin-Alexa568 | Invitrogen | A12-380 | 1/500 | 1 h; RT | |
| Goat anti-rabbit Alexa488 | Life Technologies | A11070 | 1/400 | 1 h; RT | |
| Donkey anti-mouse Alexa568 | Life Technologies | A10037 | 1/400 | 1 h; RT | |
| Goat anti-mouse Alexa647 | Invitrogen | A-21236 | 1/1,000 | 1 h; RT |
Transmission Electron Microscopy.
Transmission electron microscopy (TEM) was performed as described previously (30).
Organoid Culture.
Small intestinal organoids were established from WT and Myo5bfl/fl;Vil-CreERT2 mice as described previously (26).
Note Added in Proof.
During the review and revision process, the following paper was published, reporting another Myo5b mouse model for MVID (31).
SI Materials and Methods
Generation of Mice.
We generated a conditional Myo5b allele by inserting two LoxP sites around exon 4. Exon 4, a 2-kb 5′ homology arm and a 3.4-kb 3′ homology arm were generated by PCR from a 129S7/AB2.2-derived BAC clone (clone bMQ110n22) and cloned into the adapted PL451 plasmid and sequence-verified. The targeting construct (100 µg) was linearized with SacII and transfected into 129/Ola-derived IB10 embryonic stem (ES) cells by electroporation (0,8 kV, 3 µF). Recombined clones were selected by addition of 200 µg/mL G418 (gentamycin sulfate) to the culture medium. DNA from ∼300 ES cell clones was isolated, digested with KpnI, and analyzed for homologous recombination by Southern blot. About 1% of the isolated clones showed homologous recombination. Two independent correctly recombined clones were injected into C57BL/6 blastocysts using standard procedures and then transplanted into foster mothers (F1, C57BL/6 × CBA). Male chimaeras were subsequently crossed to C57BL/6 females, and germ-line transmission was confirmed by PCR. The neomycin expression cassette was excised in vivo by crossing the mice with Flp1 transgenic mice (30). For intestinal epithelium-specific KO, Myo5bfl/fl mice were crossed to a Vil-CreERT2 line (21), which allows gene deletion in all epithelial cells of the intestine upon tamoxifen administration. The histological analysis of the intestine of mice obtained of the two independent ES cell lines gave completely identical results.
Generation, Genotyping, and Induction of the Vil-CreERT2 Myo5bfl/fl Compound Mice.
The transgenic line Vil-CreERT2 was crossed with Myo5bfl/fl mice to obtain the Vil-CreERT2 Myo5bfl/fl as well as various genotypic controls. Primers used for genotyping PCR were as follows: Myo5b_fw, ACCCCCCAAACTCCTA; Myo5b_rev, GCACGGAAGCCTATGT; Vil-CreERT2_fw, CAAGCCTGGCTCGACGGCC; and Vil-CreERT2_rev, CGCGAACATCTTCAGGTTCT.
The Cre enzyme was induced by a single i.p. injection of 4.5 mg tamoxifen (Sigma Aldrich) dissolved in sunflower oil (30 mg/mL). The 8–10-wk-old mice were killed on day 1, 2, 3, and 4 after Cre induction, and the intestines were isolated.
All animal procedures were performed in accordance with local animal welfare laws and guidelines and were reviewed by the Animal Ethics Committee of the Royal Dutch Academy of Sciences.
Human Material and Mutation Analysis.
The female Turkish patient studied here was 4 wk old at the time of establishing a diagnosis of MVID by ultrastructural and molecular investigations. Written informed consent from the parents was obtained, and snap biopsies from jejunal, ileal, and colonic sites were obtained. Genetic counseling was provided to the parents, and written informed consent for testing of MVID genes was obtained from them. The study was approved by the Ethics committee of the Medical University of Innsbruck (study no. UN3987).
Genomic DNA from leukocytes was extracted using the MagAttract DNA Mini M48 Kit. Sanger sequencing of the complete coding region (40 exons) and all flanking exon–intron boundaries of the MYO5B gene (encoding myosin Vb) was performed in the patient; exon 11 was sequenced in both parents. Results are based on National Center for Biotechnology Information mRNA reference sequence NM_001080467, in which the A of the ATG translation initiation codon was nucleotide 1. Primers and conditions are available from the authors upon request.
(Immuno)histochemistry and Immunofluorescence.
Mouse intestines and organoids were fixed in 4% (vol/vol) formalin overnight and 4% (vol/vol) neutral buffered formalin for 45 min at room temperature (RT), respectively. The tissue was then embedded into paraffin, and 4-µm sections were cut. Human biopsy material was embedded in Tissue-Tek O.C.T. Compound (Hartenstein) and frozen at –80 °C. We fixed 10-µm cryostat sections for 20 min in 4% buffered paraformaldehyde (PFA) solution at RT or in methanol at –20 °C.
Stainings were performed as described previously (6, 7, 32). An overview of all antibodies used, their dilutions, incubation times, and (optional) antigen retrieval can be found in Table S1.
For coimmunofluorescent stainings where both primary antibodies were raised in rabbit, the anti-pERM or anti-Ezrin antibody was directly labeled using the Zenon Tricolor Rabbit IgG Labeling Kit #1 (Molecular Probes) before incubation on the tissue. Immunofluorescent slides were counterstained with 2 µg/mL DAPI (Sigma) and mounted with Fluorsafe (Millipore). Confocal images were recorded with a LSM 710 confocal laser scanning microscope (Carl Zeiss) and analyzed with Volocity software version 6.3 (Perkin-Elmer).
TEM.
TEM was performed as described previously (7). In short, 0.5-cm pieces of mouse intestine or 1-mm3 duodenum biopsies of the MVID patient were fixed in 2% formaldehyde and 2.5% glutaraldehyde in 0.1 M Pipes Hepes EDTA MgCl2 buffer (60 mM Pipes, 25 mM Hepes, 10 mM EDTA, 2 mM MgCl2, pH 6.9) on ice for 2 h and then overnight at RT. Organoids were fixed with 4% PFA solution (Electron Microscopy Sciences) in 0.1 M PHEM buffer for 4 h at RT. All samples were then stored in 1% PFA in 0.1 M PHEM buffer at 4 °C. Samples were then postfixed with 1% OsO4, dehydrated, and embedded in Epon epoxy resin (Polysciences). Ultrathin sections of 60 nm were contrasted with uranyl acetate and lead citrate using the AC20 (Leica) and examined with a Jeol 1010 electron microscope (Jeol Europe).
TEM cytochemistry for PAS-positive substances was performed as described previously (8).
Quantification of TEM Data.
To quantify the TEM data, we counted the amount of cells with apical microvilli, basolateral microvilli, intracellular microvillus inclusions, subapical accumulation of electron-light endosomal vesicles, A/Ls, and enlarged intercellular spaces. Samples from five Myo5bfl/fl;Vil-Cre mice and second Myo5bfl/+;Vil-Cre mice, which were treated with tamoxifen and killed 4 d later, were prepared for TEM as described above. Electron micrographs of 50 enterocytes from Myo5bfl/+;Vil-Cre control mice (25 per mouse) and 100 enterocytes from Myo5bfl/fl;Vil-Cre mice (20 per mouse) were taken randomly and across different villi. Only cells with a clear perpendicular cut (where the apical and basolateral side were clearly visible) were included. Apical microvilli were considered to be present even when the length or abundance was reduced.
Organoid Culture.
Small intestinal organoids were established from WT and Myo5bfl/fl;Vil-CreERT2 mice as described previously (28). Organoids were maintained in medium containing 50 ng/mL murine recombinant EGF (Peprotech), R-spondin1 (conditioned medium, 5% final volume), and noggin (conditioned medium, 10% final volume) and were passaged weekly. For in vitro activation of Cre recombinase, organoids were treated for 15 h with 1 µM 4-OHT (Sigma-Aldrich) dissolved in ethanol. Control organoids were treated with corresponding amounts of ethanol. All organoids were harvested for analysis by immunohistochemistry and TEM 3 d after in vitro induction.
Quantitative Real-Time PCR.
mRNA expression of Myo5b was quantified by qRT-PCR as described previously (32) with the following primers: Myo5b_fw, ACCCCCACATCTTTGC; Myo5b_rev, GGGCTGGATGCTAGGA.
FIS Assay.
Small intestinal mouse organoids (wild type and Myo5bfl/fl;Vil-creERT2) were cultured and in vitro activated as described above. Twenty-four hours after removal of tamoxifen and ethanol, the organoids were stained using 10 µM calcein green (Life Technologies). Upon forskolin stimulation (10 or 3 µM, Sellekchem) or DMSO, CFTR-dependent swelling of organoids was followed for 40 min, as previously described (29). Each condition was measured in quadruple per experiment, and four independent experiments were performed.
Statistics.
We counted Ki67+ cells in 50 crypts from Myo5bfl/+;Vil-CreERT2 and Myo5bfl/fl;Vil-CreERT2 mice and performed a two-tailed, unpaired Student’s t test to determine the P value. Statistical significance of the TEM quantification was calculated using a two-tailed Fisher’s exact test.
Acknowledgments
We thank Jeroen Korving for blastocyst injections and George Posthuma, Cecilia de Heus, Karin Gutleben, Barbara Witting, Caroline Hermann, and animal caretakers for (technical) assistance. This work is supported by Netherlands Organisation for Scientific Reserach (NWO) VIDI 016.146.353 (to S.M.) and NWO Translational Adult Stem Cell Research 40-41400-98-1108 (to H.C. and E.E.S.N.) and by the Molecular Cell Biology and Oncology PhD program of the Austrian Research Funds (to L.A.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516672112/-/DCSupplemental.
References
- 1.Davidson GP, Cutz E, Hamilton JR, Gall DG. Familial enteropathy: A syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology. 1978;75(5):783–790. [PubMed] [Google Scholar]
- 2.Ameen NA, Salas PJ. Microvillus inclusion disease: A genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic. 2000;1(1):76–83. doi: 10.1034/j.1600-0854.2000.010111.x. [DOI] [PubMed] [Google Scholar]
- 3.Cutz E, et al. Microvillus inclusion disease: An inherited defect of brush-border assembly and differentiation. N Engl J Med. 1989;320(10):646–651. doi: 10.1056/NEJM198903093201006. [DOI] [PubMed] [Google Scholar]
- 4.Dhekne HS, et al. Myosin Vb and Rab11a regulate phosphorylation of ezrin in enterocytes. J Cell Sci. 2014;127(Pt 5):1007–1017. doi: 10.1242/jcs.137273. [DOI] [PubMed] [Google Scholar]
- 5.Müller T, et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet. 2008;40(10):1163–1165. doi: 10.1038/ng.225. [DOI] [PubMed] [Google Scholar]
- 6.Thoeni CE, et al. Microvillus inclusion disease: Loss of Myosin vb disrupts intracellular traffic and cell polarity. Traffic. 2014;15(1):22–42. doi: 10.1111/tra.12131. [DOI] [PubMed] [Google Scholar]
- 7.Wiegerinck CL, et al. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology. 2014;147(1):65–68.e10. doi: 10.1053/j.gastro.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Ruemmele FM, et al. Loss-of-function of MYO5B is the main cause of microvillus inclusion disease: 15 novel mutations and a CaCo-2 RNAi cell model. Hum Mutat. 2010;31(5):544–551. doi: 10.1002/humu.21224. [DOI] [PubMed] [Google Scholar]
- 9.Szperl AM, et al. Functional characterization of mutations in the myosin Vb gene associated with microvillus inclusion disease. J Pediatr Gastroenterol Nutr. 2011;52(3):307–313. doi: 10.1097/MPG.0b013e3181eea177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Velde KJ, et al. An overview and online registry of microvillus inclusion disease patients and their MYO5B mutations. Hum Mutat. 2013;34(12):1597–1605. doi: 10.1002/humu.22440. [DOI] [PubMed] [Google Scholar]
- 11.Erickson RP, Larson-Thomé K, Valenzuela RK, Whitaker SE, Shub MD. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am J Med Genet A. 2008;146A(24):3117–3119. doi: 10.1002/ajmg.a.32605. [DOI] [PubMed] [Google Scholar]
- 12.Knowles BC, et al. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J Clin Invest. 2014;124(7):2947–2962. doi: 10.1172/JCI71651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roland JT, et al. Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci USA. 2011;108(7):2789–2794. doi: 10.1073/pnas.1010754108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato T, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448(7151):366–369. doi: 10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- 15.Melendez J, et al. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology. 2013;145(4):808–819. doi: 10.1053/j.gastro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamori R, et al. Cdc42 and Rab8a are critical for intestinal stem cell division, survival, and differentiation in mice. J Clin Invest. 2012;122(3):1052–1065. doi: 10.1172/JCI60282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knowles BC, et al. Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes. J Cell Sci. 2015;128(8):1617–1626. doi: 10.1242/jcs.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobajima T, et al. Rab11a is required for apical protein localisation in the intestine. Biol Open. 2014;4(1):86–94. doi: 10.1242/bio.20148532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kravtsov D, et al. Myosin 5b loss of function leads to defects in polarized signalling: Implication for Microvillus Inclusion Disease pathogenesis and treatment. Am J Physiol Gastrointest Liver Physiol. 2014;307(10):G992–G1001. doi: 10.1152/ajpgi.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.el Marjou F, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39(3):186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 21.Reinshagen K, Naim HY, Zimmer KP. Autophagocytosis of the apical membrane in microvillus inclusion disease. Gut. 2002;51(4):514–521. doi: 10.1136/gut.51.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277(52):50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 23.Swiatecka-Urban A, et al. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem. 2007;282(32):23725–23736. doi: 10.1074/jbc.M608531200. [DOI] [PubMed] [Google Scholar]
- 24.Garbett D, LaLonde DP, Bretscher A. The scaffolding protein EBP50 regulates microvillar assembly in a phosphorylation-dependent manner. J Cell Biol. 2010;191(2):397–413. doi: 10.1083/jcb.201004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci USA. 2004;101(51):17705–17710. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6(6):855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Dukes JD, et al. Functional ESCRT machinery is required for constitutive recycling of claudin-1 and maintenance of polarity in vertebrate epithelial cells. Mol Biol Cell. 2011;22(17):3192–3205. doi: 10.1091/mbc.E11-04-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 29.Dekkers JF, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19(7):939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 30.Raymond CS, Soriano P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS One. 2007;2(1):e162. doi: 10.1371/journal.pone.0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cartón-Garcia F, et al. Myo5b knockout mice as a model of microvillus inclusion disease. Sci Rep. 2015;5:12312. doi: 10.1038/srep12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middendorp S, et al. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells. 2014;32(5):1083–1091. doi: 10.1002/stem.1655. [DOI] [PubMed] [Google Scholar]