Significance
Crosstalk between histone modifications regulates transcription by establishing spatial and temporal relationships between histone marks. Despite discoveries of reader domains that physically associate with chromatin-modifying enzymes, the mechanisms by which recognition of one modification triggers other kinds of modifications have remained elusive. Gcn5 is the catalytic subunit of the Spt-Ada-Gcn5 acetyltransferase (SAGA) histone acetyltransferase (HAT) module, which also recognizes histone 3 lysine 4 trimethylation (H3K4me3) through the tandem Tudor domain-containing protein Sgf29. Although previous studies could not connect H3K4me3 recognition to differences in acetylation by Gcn5, we report enhanced processivity by the HAT module on methylated substrates using a previously unpublished histone color-coding assay. Our work defines a mechanism for histone crosstalk that may account for genome-wide patterns of Gcn5-mediated acetylation.
Keywords: acetyltransferase, histone crosstalk, histone modifications, transcription, coactivator
Abstract
The Spt-Ada-Gcn5 acetyltransferase (SAGA) coactivator complex hyperacetylates histone tails in vivo in a manner that depends upon histone 3 lysine 4 trimethylation (H3K4me3), a histone mark enriched at promoters of actively transcribed genes. SAGA contains a separable subcomplex known as the histone acetyltransferase (HAT) module that contains the HAT, Gcn5, bound to Sgf29, Ada2, and Ada3. Sgf29 contains a tandem Tudor domain that recognizes H3K4me3-containing peptides and is required for histone hyperacetylation in vivo. However, the mechanism by which H3K4me3 recognition leads to lysine hyperacetylation is unknown, as in vitro studies show no effect of the H3K4me3 modification on histone peptide acetylation by Gcn5. To determine how H3K4me3 binding by Sgf29 leads to histone hyperacetylation by Gcn5, we used differential fluorescent labeling of histones to monitor acetylation of individual subpopulations of methylated and unmodified nucleosomes in a mixture. We find that the SAGA HAT module preferentially acetylates H3K4me3 nucleosomes in a mixture containing excess unmodified nucleosomes and that this effect requires the Tudor domain of Sgf29. The H3K4me3 mark promotes processive, multisite acetylation of histone H3 by Gcn5 that can account for the different acetylation patterns established by SAGA at promoters versus coding regions. Our results establish a model for Sgf29 function at gene promoters and define a mechanism governing crosstalk between histone modifications.
The different patterns of histone posttranslational modifications distributed across the genome are proposed to constitute a “histone code” that orchestrates distinct transcriptional programs by recruiting specific effector proteins (1–4). The phenomenon of histone code “crosstalk,” whereby one type of histone modification directs the establishment of another, or through which several histone modifications are recognized in tandem, has emerged as an important and widespread mechanism regulating chromatin-templated processes (5, 6). The multifunctional complexes that activate transcription are thought to mediate crosstalk through “reader” domains that recognize particular chromatin marks as well as through catalytic subunits that deposit or remove histone modifications (5, 7, 8). As a result, distinct combinations of histone posttranslational modifications that correlate with transcriptional output cluster across the genome (9–11). High levels of histone 3 lysine 4 trimethylation (H3K4me3) and histone H3 hyperacetylation are present at the promoters of actively transcribed genes (9, 11, 12), and multiple lines of evidence support the existence of regulatory mechanisms coupling the two modifications. Studies using tandem mass spectrometry to sequence whole histone tails have shown that H3K4me3 is highly correlated with hyperacetylation of the same H3 tail (13, 14). Deleting the yeast Set1 methyltransferase, which trimethylates H3K4, leads to dramatically lower levels of histone H3 tail acetylation overall (13, 15). These results are consistent with a role for H3K4 methylation in triggering hyperacetylation of histone H3; indeed, a number of histone acetyltransferase (HAT) complexes also contain reader modules that recognize the H3K4me3 mark (16, 17).
The SAGA (Spt-Ada-Gcn5 acetyltransferase) complex is a highly conserved transcriptional coactivator (18) that is involved in the transcription of nearly all yeast genes (19, 20) and mediates crosstalk between H3K4me3 and histone hyperacetylation. The HAT activity of SAGA resides in a four-protein subcomplex known as the “HAT module” (21, 22), which contains the catalytic subunit Gcn5 (23–25), Ada2, Ada3 (26, 27), and Sgf29 (21, 28). The association of Gcn5 with the other HAT module subunits modulates Gcn5 activity and specificity. Gcn5 on its own acetylates free histones but not nucleosomes (22, 23, 25) and preferentially modifies histone H3 at K14 (25, 29). A complex containing Gcn5 bound to Ada2 and Ada3 is more active overall and modifies nucleosomal substrates (22, 25). Binding to Ada2/Ada3 also broadens the lysine specificity of Gcn5 to acetylate multiple lysine residues on the histone H3 tail (22, 25). Sgf29 contains a tandem Tudor domain that binds to H3K4me3 (17, 30) and is required for maintaining wild-type levels of histone H3 acetylation in vivo (30–32). However, in vitro studies comparing acetylation kinetics by the SAGA complex on peptide substrates in the presence or absence of Sgf29 do not show a rate enhancement when the H3K4me3 modification is present (30). Because these experiments were limited in scope to peptide substrates and measured acetylation using relatively insensitive end-point assays (30), the full impact of H3K4me3 on HAT module activity may be much greater than previously determined. Thus, although multiple lines of evidence suggest that H3K4me3 regulates histone acetylation by SAGA in yeast and mammalian cells (17, 30, 31, 33), in vitro studies have failed to explain how H3K4me3 recognition by Sgf29 gives rise to different patterns of histone acetylation. As a result, the underlying mechanism governing crosstalk between H3K4 trimethylation and acetylation by SAGA remains poorly understood.
To elucidate the mechanism underlying crosstalk between HAT module binding to H3K4me3 and histone acetylation, we developed a histone color-coding assay for measuring acetylation rates on distinct histone populations within a single mixture of methylated and unmodified nucleosomes. We find that the HAT module preferentially acetylates H3K4me3 nucleosomes in a mixture and that this preference depends on the Sgf29 tandem Tudor domain. Compared with unmodified nucleosomes, acetylation by the HAT module is processive on nucleosomes containing the H3K4me3 modification, thereby triggering histone H3 hyperacetylation. These data reveal the mechanism by which the Sgf29 tandem Tudor domain within the SAGA HAT module facilitates crosstalk between Gcn5 acetyltransferase activity and H3K4me3 recognition and provide an explanation for how SAGA can establish different patterns of acetylation at gene promoters versus coding regions.
Results
Effect of H3K4me3 on Histone Peptide Acetylation.
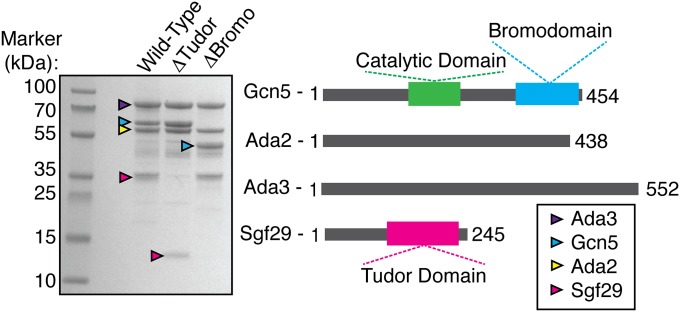
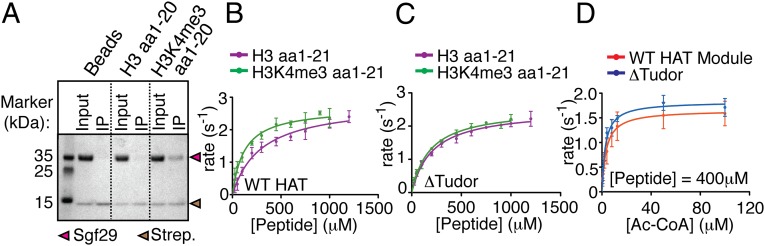
To investigate how H3K4 trimethyl recognition may contribute to HAT module activity on methylated versus unmethylated substrates, we used recombinant HAT module from Schizosaccharomyces pombe, which can be isolated as a stable four-protein complex containing Sgf29, Gcn5, Ada2, and Ada3. The full-length S. pombe HAT module and variants lacking either the Sgf29 tandem Tudor domain or the Gcn5 bromodomain were expressed as complexes in Escherichia coli and purified to homogeneity (Fig. 1). We first confirmed that S. pombe Sgf29 interacts specifically with H3K4me3 peptides in a pull-down assay. As shown in Fig. 2A, streptavidin beads coated with H3K4me3 peptides efficiently retain Sgf29, whereas unmodified H3 peptides do not.
Fig. 1.
Domain architecture of HAT module complexes. (Left) SDS/PAGE gel of purified S. pombe HAT module complexes containing different domain truncations in which all four subunits are present in roughly equimolar quantities. (Right) Location of the catalytic domain, tandem Tudor domain, and bromodomain within the HAT module.
Fig. 2.
The presence of H3K4me3 increases overall acetylation by the HAT module on peptides. (A) Pull-down using biotinylated histone peptides containing H3K4 trimethylation and Sgf29. (B–D) Steady-state kinetic titrations using an enzyme-coupled assay comparing the activity of the wild-type HAT module on unmodified versus H3K4me3 peptides (B), the ΔTudor HAT module on unmodified versus H3K4me3 peptides (C), or the wild-type versus ΔTudor HAT module (D) using a constant amount of H3K4me3 peptide and varying the acetyl-CoA concentration.
To determine the effect of H3K4me3 on the kinetics of peptide acetylation by Gcn5, we measured initial acetylation rates by the HAT module at increasing concentrations of either unmodified or H3K4me3 peptides in the presence of saturating amounts of acetyl-CoA. Compared with unmodified peptides, the presence of the H3K4me3 modification increases kcat/Km by 3.1-fold (Fig. 2B and Table 1). This change in kcat/Km is caused solely by a decrease in Km from 250 ± 40 μM to 77 ± 10 μM, with no corresponding change in kcat (Fig. 2B and Table 1). Deletion of the Sgf29 tandem Tudor domain (ΔTudor) eliminates the difference in acetylation kinetics on H3K4me3 versus unmodified peptides (Fig. 2C and Table 1), as is consistent with a role for H3K4me3 binding in the observed difference in kcat/Km. We also ruled out the possibility that the H3K4me3 modification affects kcat or Km for acetyl-CoA (Fig. 2D and Table 2); our finding is consistent with the proposed Gcn5 reaction mechanism, in which the cofactor binds before the peptide (34, 35).
Table 1.
Summary of steady-state kinetic parameters fit from titrations in which the concentration of the histone H3 amino acids 1–21 peptide was varied and the acetyl-CoA concentration was held constant at 100 μM
| HAT module | Histone H3 peptide amino acids 1–21 | kcat, s−1 | Km, μM | kcat/Km, μM−1⋅s−1 |
| Wild type | Unmod | 2.5 ± 0.1 | 250 ± 40 | 0.010 ± 0.002 |
| ΔTudor | Unmod | 2.5 ± 0.1 | 210 ± 30 | 0.012 ± 0.002 |
| Sgf29 T202A/Y205V | Unmod | 3.4 ± 0.2 | 320 ± 50 | 0.011 ± 0.002 |
| ΔBromodomain | Unmod | 1.8 ± 0.07 | 380 ± 40 | 0.0047 ± 0.001 |
| Wild type | H3K4me3 | 2.4 ± 0.08 | 77 ± 10 | 0.031 ± 0.004 |
| ΔTudor | H3K4me3 | 2.5 ± 0.09 | 190 ± 20 | 0.013 ± 0.001 |
Table 2.
Summary of steady-state kinetic parameters fit from titrations in which the concentration of the H3K4me3 amino acids 1–21 peptide was held at 400 μM and the acetyl-CoA concentration was varied
| HAT module | Substrate | kcat, s−1 | Km, μM | kcat/Km, μM−1⋅s−1 |
| Wild type | Ac-CoA | 1.7 ± 0.1 | 3.4 ± 0.7 | 0.50 ± 0.1 |
| ΔTudor | Ac-CoA | 1.8 ± 0.1 | 2.8 ± 0.3 | 0.64 ± 0.08 |
To confirm that acetylation by Gcn5 is not directly affected by truncating the Sgf29 tandem Tudor domain, we compared kcat and Km values of wild-type and ΔTudor HAT module complexes for unmodified histone peptides and nucleosomes. Because the kinetic parameters for the two complexes are nearly the same for peptides (Table 1) and nucleosomes (Table 3), differences observed with the ΔTudor HAT module must be related to H3K4me3 recognition. We note that the rate enhancement observed resulting from H3K4me3 differs from the results of an earlier study that had found no role for Sgf29 in governing SAGA activity on H3K4me3 versus unmodified peptides in vitro (30). However, the previously reported conclusions were drawn from end-point assays (30), whereas the data presented here are fit from a full steady-state titration and therefore are more sensitive to differences in kinetic parameters.
Table 3.
Summary of steady-state kinetic parameters fit from titrations in which the concentration of the nucleosome was varied and the acetyl-CoA concentration was held constant at 25 μM
| HAT module | Nucleosome | kcat, s−1 | Km, μM | kcat/Km, μM−1⋅s−1 |
| Wild type | Unmod | 1.3 ± 0.1 | 1.9 ± 0.3 | 0.68 ± 0.1 |
| ΔTudor | Unmod | 1.9 ± 0.2 | 2.8 ± 0.5 | 0.68 ± 0.1 |
| Wild-type | H3K4me3 | 1.0 ± 0.05 | 0.41 ± 0.05 | 2.4 ± 0.3 |
H3K4me3 Stimulates HAT Module Activity on Nucleosomes.
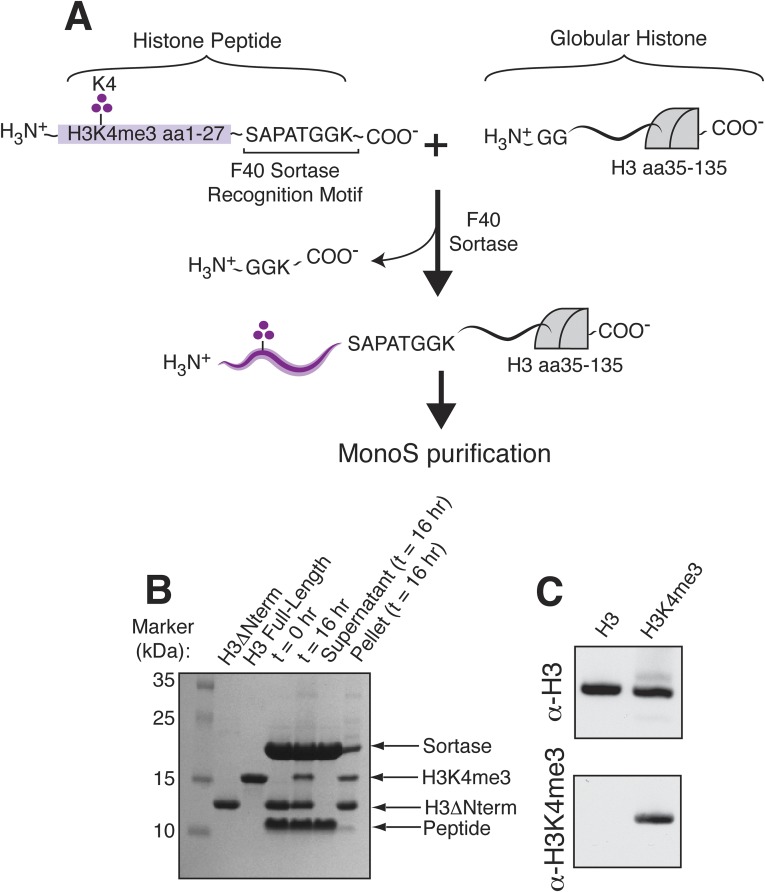
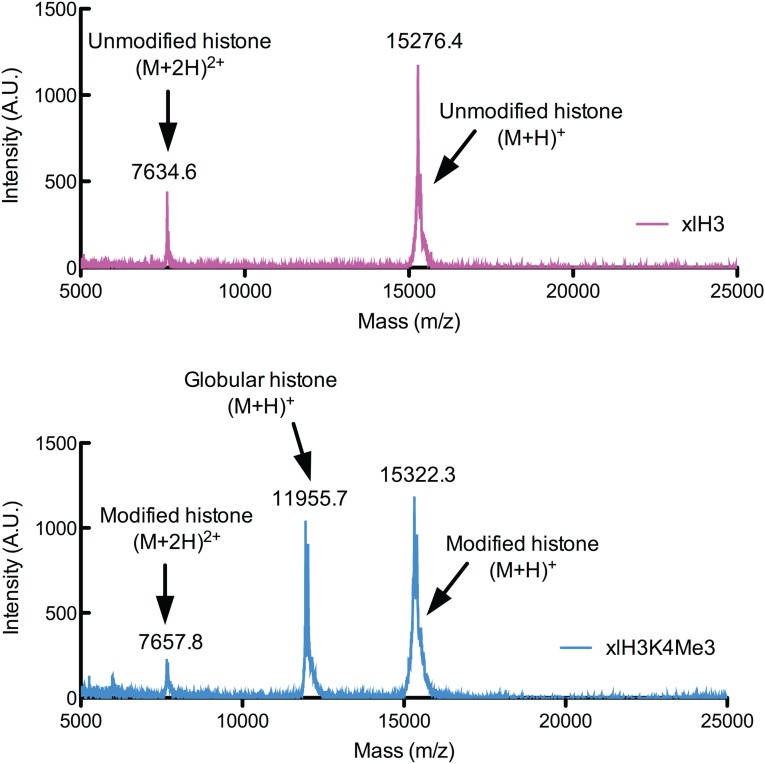
The experiments described above and previously reported studies (30) used peptide substrates, leaving open the possibility that H3K4 trimethylation might have a greater impact on acetylation by the SAGA HAT module in a nucleosomal context. We therefore generated nucleosomes containing recombinant full-length histone H3 that was homogeneously modified with H3K4me3. To incorporate this modification, we performed enzyme-mediated ligation using an engineered variant of the bacterial transpeptidase, Sortase A (F40 SrtA) (36, 37), which splices an N-terminal synthetic peptide onto recombinant histone H3 (Fig. S1A) to yield fully native histone H3 uniformly trimethylated at H3K4 (Fig. S1B). We confirmed the presence of the trimethyl modification using Western blotting with an antibody specific for the H3K4me3 modification (Fig. S1C) as well as MALDI-TOF mass spectrometry (Fig. S2). The modified histone H3 was reconstituted with unmodified histones H2A, H2B, and H4 into nucleosome core particles, thus generating a pool of nucleosomes uniformly trimethylated at H3K4.
Fig. S1.
Producing site-specifically modified histone H3. (A) Schematic describing enzyme-mediated ligation with sortase to produce recombinant full-length H3K4me3 histone. (B) SDS/PAGE gel of sortase reaction components and products. (C) Western blot on an SDS/PAGE gel of recombinant histone H3 and H3K4me3 made with sortase to verify the presence of the methyl modification.
Fig. S2.
MALDI-TOF mass spectrometry of sortase reaction to confirm the incorporation of the trimethyl modification. Recombinant Xenopus histone H3 C110A/Q125C (Upper) and the product of the sortase reaction (Lower) were dialyzed into water and subjected to MALDI-TOF mass spectrometry. MALDI samples were prepared by the sandwich method using a matrix consisting of 10 mg/mL sinapinic acid diluted in 50% acetonitrile + 0.05% trifluoroacetic acid. Spectra were collected using a Voyager DE-STR (Applied Biosystems) in reflection mode. The molecular weight of sortase-generated H3K4me3 C110A/Q125C is consistent with the addition of three methyl groups. A.U., arbitrary units.
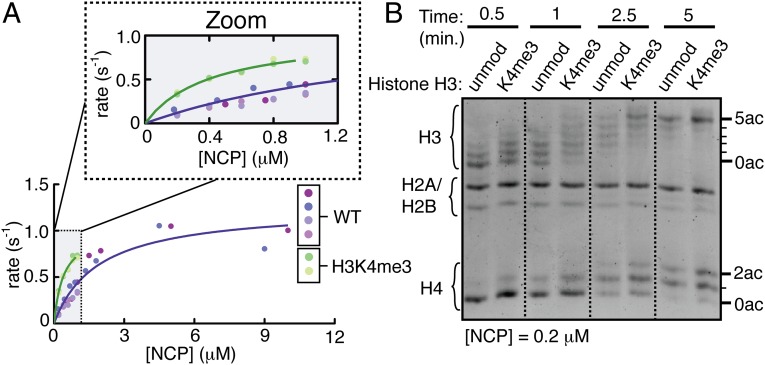
To test whether H3K4 trimethylation affects acetylation by the HAT module in a nucleosomal context, we used a radioactive filter-binding assay to measure steady-state rates of acetylation as a function of nucleosome concentration. We find that the Km for nucleosomes drops from 1.9 ± 0.3 μM for unmodified nucleosomes to 0.41 ± 0.05 μM for H3K4me3 nucleosomes (Fig. 3A and Table 3), whereas kcat stays roughly the same, corresponding to an ∼3.5-fold increase in the specificity constant kcat/Km (Table 3). The Km for nucleosomes is almost two orders of magnitude lower than that for peptides, indicating that the HAT module binds far more tightly to nucleosomes than to peptides. Nonetheless, the 3.5-fold increase in kcat/Km caused by the H3K4me3 modification in a nucleosomal context (Fig. 3A and Table 3) is only modestly greater than the 3.1-fold increase in specificity observed with peptides (Fig. 2B and Table 1).
Fig. 3.
The presence of H3K4me3 increases overall acetylation by the HAT module on nucleosomes. (A) Steady-state kinetic titrations comparing wild-type HAT module activity on unmodified versus H3K4me3 nucleosome core particles (NCPs) using a radioactive filter-binding assay. Replicates are colored with different shades of purple (wild-type) or green (H3K4me3). (B) HAT reaction time course on unmodified versus H3K4me3 NCPs resolved on an acid–urea gel and stained for total protein using SyproRuby stain.
Because radioactive filter-binding assays report on total acetylation and do not distinguish differences among histones, we compared histone acetylation patterns on unmodified versus H3K4me3 nucleosomes using acid–urea gels, which separate histones based on molecular weight as well as the number of acetylated lysine residues (38). At early time points the HAT module acetylates H3K4 trimethylated histone H3 in nucleosomes more quickly than unmodified nucleosomal histone H3 (Fig. 3B). Thirty seconds after the reaction is initiated, bands corresponding to three acetylation sites are evident on the H3K4me3 histone, but only two acetylation sites are populated for unmodified histone H3 (Fig. 3B). Although the HAT module also acetylates histone H4, the rate of histone H4 acetylation is insensitive to H3K4 trimethylation (Fig. 3B). This pattern is consistent with a mechanism in cis, whereby recognition of the methyl mark stimulates acetylation on the same histone tail.
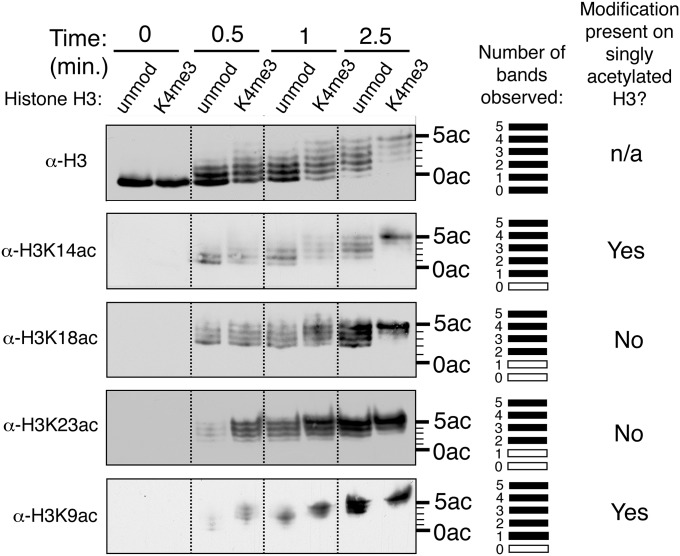
To examine whether recognition of the H3K4me3 modification changes the intrinsic lysine specificity of the Gcn5 subunit, which preferentially catalyzes H3K14 acetylation in vitro (29, 39) and H3K9 acetylation in vivo (20, 40), we probed the reaction products using antibodies that recognize specific acetylated lysine residues. Antibodies to the H3K9ac and H3K14ac modifications recognize five bands on acid–urea gels, corresponding to one through five modification sites per histone, whereas antibodies to H3K18ac and H3K23ac recognize only the top four bands (Fig. S3). This pattern reveals that singly acetylated histones are predominately modified at H3K9 or H3K14 on both unmodified and H3K4me3 substrates. Although H3K4 trimethylation enhances the rate of H3K9 acetylation at early time points, acetylation at all the other lysines tested changes little in the presence of the methyl modification (Fig. S3). Therefore, H3K4me3 recognition does not change the intrinsic specificity of Gcn5 for catalyzing H3K9 and H3K14 acetylation, instead stimulating overall acetylation of histone H3. In agreement with our results, deleting Sgf29 in yeast results in global reductions in acetylation at multiple positions on histone H3 (30).
Fig. S3.
Western blots using acetyl-specific antibodies of HAT reactions resolved on acid–urea gels. HAT reactions using methylated and unmodified nucleosomal substrates were resolved on acid–urea gels and blotted using site-specific acetylation antibodies for total histone H3, H3K14ac, H3K18ac, H3K23ac, and H3K9ac. The number of bands observed with each antibody is listed on the right side, where black lines represent bands that are populated, and empty white boxes represent bands that are not detected by the acetyl-specific antibodies. Of the three acetylation sites tested, only antibodies to H3K9ac and H3K14ac recognize the singly acetylated histone, indicating that the HAT module acetylates these sites first.
Differential Labeling Technique for Studying Mixtures of Posttranslationally Modified Substrates.
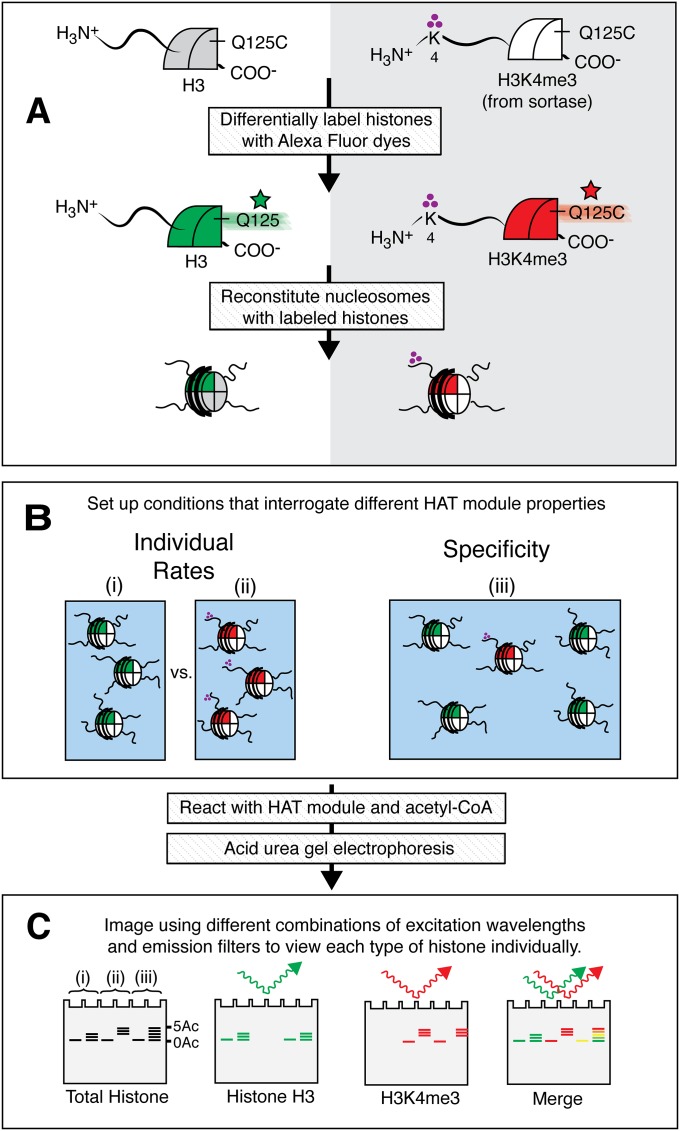
Chromatin in vivo is heterogeneous with respect to the set of posttranslational modifications found on each nucleosome. For an enzyme complex such as the HAT module, which contains multiple chromatin-binding domains (16, 41), the ability to acetylate nucleosomes selectively with particular combinations of histone modifications may be as important as the overall enzymatic activity. Although multiple biochemical assays yield bulk acetylation rates (42), there are no reported assays for selectively monitoring the acetylation kinetics of different types of substrates in the context of a mixture. We therefore devised an approach that combines differential fluorescent labeling of histones with acid–urea gel electrophoresis to quantitate HAT module activity on both modified and unmodified nucleosomes within the same reaction mixture.
To track histone-specific acetylation, we reconstituted nucleosomes whose histone H3 was color-coded according to whether it contained the H3K4me3 modification. Methylated H3 was generated using F40 SrtA as described above; the fluorescent label was incorporated by introducing a Q125C substitution in histone H3 that could be labeled with a dye of choice. Unmodified histone H3 was labeled with Alexa Fluor 647, and H3K4 trimethylated histone H3 was labeled with Alexa Fluor 488 (Fig. S4A). After the SAGA HAT module was incubated with the nucleosomes (Fig. S4B) and the reaction products were resolved on acid–urea gels, the products corresponding to one or the other color-coded histone could be visualized selectively on a laser scanner using excitation wavelengths and emission filters for one or the other fluorophore (Fig. S4C). In this way, acetylation of methylated versus unmethylated H3 can be quantitated within a single mixture.
Fig. S4.
Overview of the differential labeling experiment. (A) Histones containing particular histone posttranslational modifications are labeled with different fluorescent dyes. (B) Substrate composition of HAT reactions for interrogating different kinetic properties. (C) A laser scanner was used to image individual subpopulations of nucleosomes.
Sgf29 Mediates Selective Acetylation of H3K4me3 Nucleosomes in a Heterogeneous Mixture.
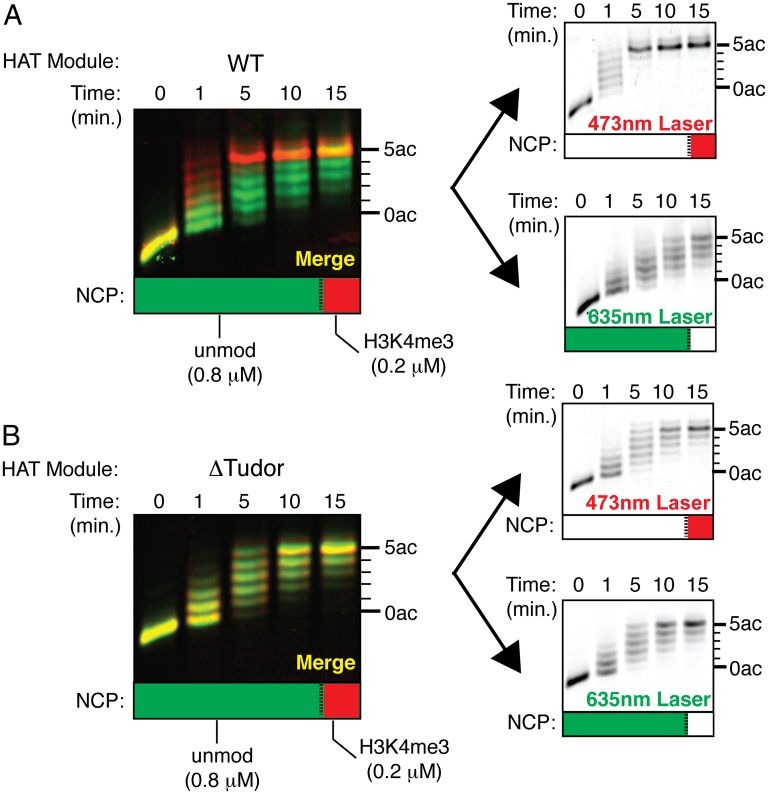
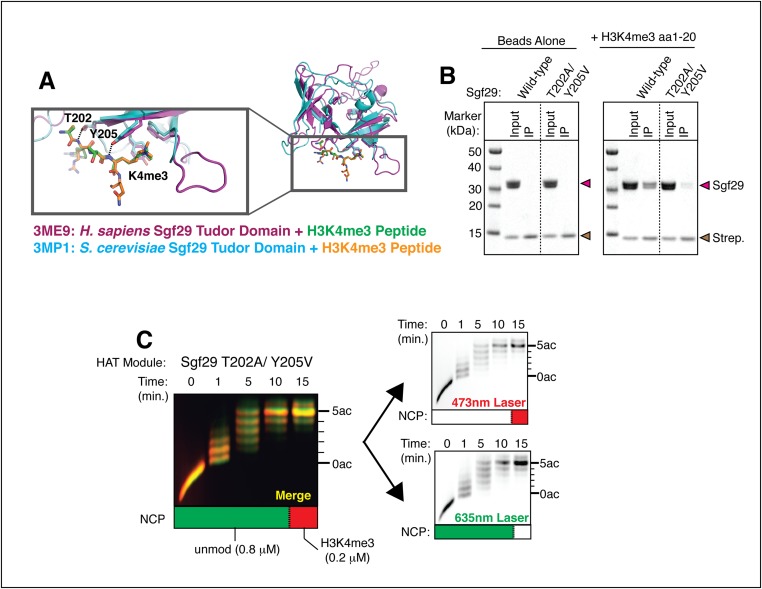
To see whether the HAT module preferentially acetylates nucleosomes containing H3K4 trimethylation, we used the color-coding method to assay histone H3 acetylation in mixtures of methylated and unmodified nucleosomes. Because H3K4 trimethylation increases the kcat/Km for the HAT module by around fourfold (Fig. 3A and Table 3), the HAT module should acetylate H3K4me3 and unmodified nucleosomes at the same rate in mixtures containing a fourfold molar excess of unmodified nucleosomes (43). However, in assays of SAGA HAT module activity using a 4:1 molar ratio of unmodified versus methylated nucleosomes, we unexpectedly found that the HAT module preferentially acetylates the methylated nucleosomes despite the presence of a fourfold molar excess of unmodified nucleosomes (Fig. 4A). An overlay of images corresponding to each labeled substrate reveals that nucleosomes with the H3K4me3 modification are acetylated to completion within the first 5 minutes, whereas unmodified nucleosomes still are not fully acetylated at the last time point (Fig. 4A). For the SAGA HAT module lacking the Tudor domain, the pattern of acetylation is the same for both methylated and unmethylated nucleosomes (Fig. 4B). Because there is fourfold less H3K4me3 substrate in the reaction, this finding indicates that complexes lacking the tandem Tudor domain have lost the ability to distinguish between methylated and unmodified nucleosomes. To verify that deletion of the Sgf29 tandem Tudor domain did not disrupt the overall integrity and activity of the HAT module, we introduced point mutations in the Tudor domain predicted to disrupt binding to a methylated peptide based on structures of the human and yeast Sgf29 tandem Tudor domains bound to H3K4me3 peptides (30). We mutated T202 to alanine and Y205 to valine (T202A/Y205V), thereby eliminating two hydrogen bonds between Sgf29 and the H3 backbone and disrupting the aromatic cage that surrounds the methyl lysine (Fig. S5A). In pull-down assays, we find that isolated Sgf29 containing the T202A/Y205V substitutions binds more weakly to H3K4me3 peptides than does the wild-type protein (Fig. S5B). When incorporated into the HAT module, the effect of mutating Sgf29 mimics that of a full Tudor domain truncation, in which mixtures of methylated and unmodified nucleosomes are acetylated at equal rates (Fig. S5C). Together, these results show that when nucleosomes harboring different histone marks compete for the HAT module active site, the interaction between the Sgf29 tandem Tudor domain and the H3K4me3 modification facilitates preferential acetylation of methylated nucleosomes.
Fig. 4.
The HAT module preferentially acetylates nucleosomes harboring H3K4me3 out of a mixture. (A) Fluorescent images of a HAT reaction containing a 4:1 molar ratio of differentially labeled unmodified versus H3K4me3 nucleosomes resolved on an acid–urea gel. (Left) Merged image with unmodified nucleosomes colored green and H3K4me3 nucleosomes colored red. (Right) Individual images used to generate the merged image. (B) Fluorescent images of a ΔTudor HAT reaction containing a 4:1 molar ratio of differentially labeled unmodified versus H3K4me3 nucleosomes resolved on an acid–urea gel. (Left) Merged image with unmodified nucleosomes colored green and H3K4me3 nucleosomes colored red. (Right) Individual images used to generate the merged image.
Fig. S5.
Mutations that disrupt binding to H3K4me3 by Sgf29 eliminate preferential acetylation of methylated nucleosomes. (A) Superimposed structures of the tandem Tudor domains from human (magenta) and yeast (blue) Sgf29 bound to H3K4me3 peptides (orange and green). T202A and Y205V mutations in Sgf29 eliminate hydrogen-bonding interactions with the histone H3 backbone. Mutating Y205 to valine also disrupts the aromatic cage within the methyl lysine-binding pocket while preserving local hydrophobicity. (B) H3K4me3 peptides pull down T202A/Y205V Sgf29 less efficiently than wild-type Sgf29. (C) Fluorescent images of a HAT reaction using the T202A/Y205V HAT module containing a 4:1 molar ratio of differentially labeled unmodified versus H3K4me3 nucleosomes resolved on an acid–urea gel. (Left) Merged image with unmodified nucleosomes colored green and H3K4me3 nucleosomes colored red. (Right) Individual images used to generate the merged image.
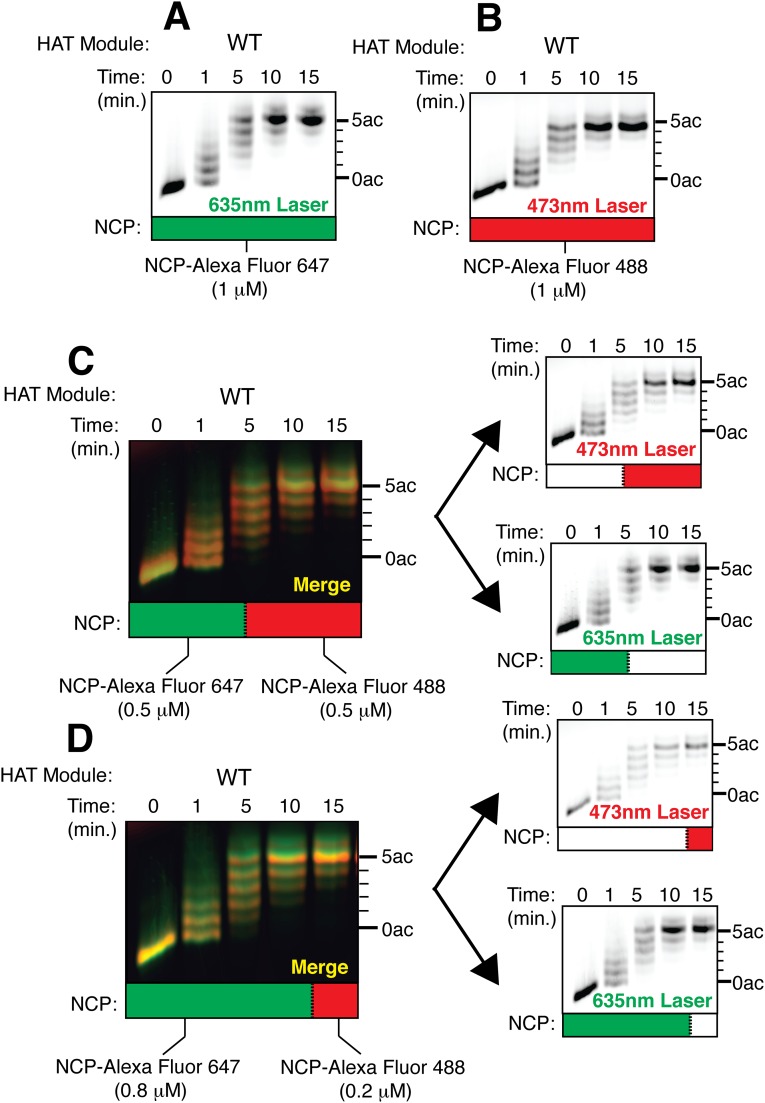
To rule out the possibility that H3K4me3 recognition acts in trans by stimulating overall HAT module activity, we assayed nucleosome acetylation in the presence of an added histone H3 tail peptide containing the H3K4me3 modification. Peptide was added at a concentration 10-fold higher than the reported dissociation constant of methylated peptide for Sgf29 (30) and nucleosomes were held at a concentration matching the amount of H3K4me3 substrate in our experiments using nucleosome mixtures (Fig. 4). As shown in Fig. S6, the added peptide did not increase HAT module activity on unmodified (Fig. S6 A and B) or H3K4me3 nucleosomes (Fig. S6 C and D), ruling out trans stimulation of Gcn5 by H3K4me3 histone tails. To control for fluorophore-specific effects on acetylation by the HAT module, we compared HAT module activity on otherwise unmodified nucleosomes containing histone H3 labeled with either Alexa Fluor 488 or Alexa Fluor 647. The HAT module acetylates these two nucleosomes at the same rate in homogenous preparations (Fig. S7 A and B) and in mixtures containing varying proportions of the Alexa Fluor 488- or Alexa Fluor 647-labeled nucleosomes (Fig. S7 C and D), indicating that the choice of fluorescent label does not influence the observed acetylation patterns.
Fig. S6.
HAT reactions using fluorescently labeled nucleosomes resolved on acid–urea gels to test if H3K4me3 stimulates HAT activity in trans. Reactions contain the wild-type HAT module and the following substrates: 200 nM unmodified nucleosomes (A), 200 nM unmodified nucleosomes supplemented with 50 μM H3K4me3 amino acids 1–8 peptide (B), 200 nM H3K4me3 nucleosomes (C), or 200 nM H3K4me3 nucleosomes supplemented with 50 μM H3K4me3 amino acids 1–8 peptide (D).
Fig. S7.
Alexa Fluor dyes do not bias HAT module activity. (A and B) Fluorescent images of HAT reactions resolved on acid–urea gels containing the wild-type HAT module and 1 μM nucleosomes labeled with Alexa Fluor 647 (A) or Alexa Fluor 488 (B). (C and D) Fluorescent images of HAT reactions resolved on acid–urea gels containing mixtures of differentially labeled but otherwise unmodified nucleosomes at 1:1 (C) or 4:1 (D) molar ratios. (Left) Merged image with Alexa Fluor 488-labeled nucleosomes colored green and Alexa Fluor 647-labeled nucleosomes colored red. (Right) Individual images used to generate the merged image.
Sgf29 Promotes Processive Acetylation by the SAGA HAT Module on H3K4me3 Nucleosomes.
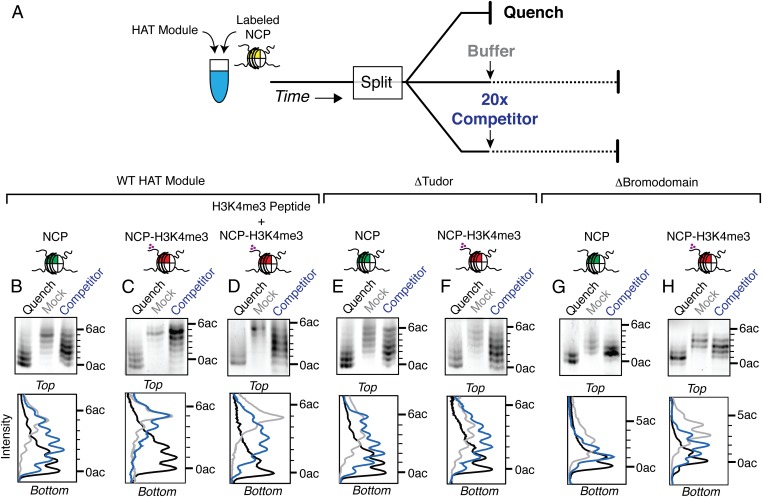
What mechanism links H3K4me3 recognition by Sgf29 at gene promoters to hyperacetylation by Gcn5? When provided with competing substrates, the HAT module clearly modifies H3K4me3 nucleosomes first, even under conditions in which one expects to see equal rates of acetylation (Fig. 4A and Table 3). We hypothesized that H3K4me3 binding by Sgf29 might promote processive acetylation of methylated nucleosomes, with the HAT module acetylating multiple lysines before dissociating from the nucleosome. To test for processive acetylation by Gcn5, we used the reaction scheme diagrammed in Fig. 5A. We incubated the HAT module with labeled nucleosome for a short period and then added a 20-fold molar excess of unlabeled, unmodified competitor nucleosome. As controls, either a portion of the initial reaction was quenched with acetic acid, which halts all enzymatic activity, or buffer alone was added (mock quench), which allows the reaction to proceed (Fig. 5A). The resulting pattern of acetylation on fluorescently labeled histone H3 then was visualized using acid–urea gels. For a distributive (nonprocessive) enzyme, the acetylation pattern in the presence of competitor should mimic the chemical quench, because the enzyme should dissociate readily from the labeled substrate and acetylate the unlabeled competitor instead. A processive enzyme should remain bound to the labeled substrate, even in the presence of added competitor, thus allowing further acetylation of the labeled nucleosome. As a result, the acetylation pattern for a processive enzyme in the presence of competitor should mimic the mock-quench.
Fig. 5.
Sgf29 promotes processive acetylation against H3K4me3 nucleosomes. (A) Schematic of the competition assay to test for processive behavior. (B–H) Results from competition assays containing: the wild-type HAT module and unmodified nucleosomes (B), the wild-type HAT module and H3K4me3 nucleosomes (C), the wild-type HAT module, H3K4me3 nucleosomes, and H3K4me3 peptide (amino acids 1–8) added at the beginning of the reaction (D), the ΔTudor HAT module and unmodified nucleosomes (E), the ΔTudor HAT module and H3K4me3 nucleosomes (F), the Δbromodomain HAT module and unmodified nucleosomes (G), or the Δbromodomain HAT module and H3K4me3 nucleosomes (H). (Upper Row) Acid–urea gel of competition assay visualized using a Typhoon scanner. Background subtraction was performed using a sliding paraboloid in ImageJ. (Lower Row) Quantitation of acid–urea gel in ImageJ.
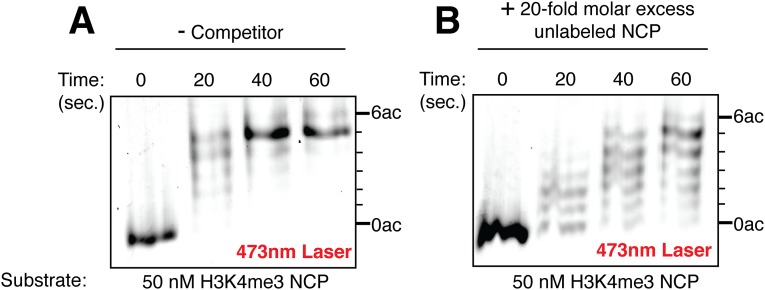
When the SAGA HAT module was incubated with nucleosomes containing unmethylated histone H3 fluorescently labeled with Alexa Fluor 647, the addition of unlabeled nucleosomes competed for HAT module activity and prevented labeled histone H3 from becoming fully acetylated (compare competitor and mock-quenched samples in Fig. 5B). In contrast, the addition of unlabeled competitor nucleosomes had little effect on the acetylation of fluorescently labeled H3K4me3 nucleosomes, as can be seen by the similarity in the intensity profiles of the competitor and mock-quench samples (Fig. 5C). This pattern is characteristic of processive acetylation of H3K4me3 histone H3, presumably mediated by binding of the HAT module subunit, Sgf29, to the methylated N-terminal tail of histone H3 via the Sgf29 tandem Tudor domain (30). Consistent with the role of H3K4me3 binding in processivity, the addition of H3K4me3 peptide at the start of the reaction, at a concentration that should saturate binding to the Sgf29 tandem Tudor domain (30), abrogates processive acetylation by the HAT module (Fig. 5D). Even though the addition of the H3K4me3 peptide has little effect on overall rates of acetylation by the HAT module (Fig. S6D), HAT module processivity is reduced significantly. This behavior indicates that Sgf29 promotes processive acetylation by the SAGA HAT module on H3K4me3 nucleosomes.
To probe whether processive acetylation of H3K4me3 nucleosomes depends on the interaction between the Sgf29 tandem Tudor domain and the H3K4 trimethyl modification, we repeated the experiment using a HAT module lacking the Sgf29 Tudor domain (ΔTudor). Unlike the intact HAT module, the ΔTudor complex can be competed off both unmodified (Fig. 5E) and H3K4me3 nucleosomes (Fig. 5F). These results, along with the fact that the wild-type HAT module is efficiently competed off H3K4me3 nucleosomes in the presence of the H3K4me3 peptide (Fig. 5D), are consistent with a role for the Sgf29 Tudor domain in engaging the methylated histone H3 tail.
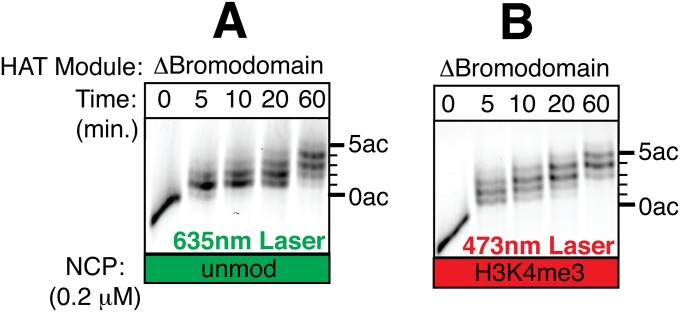
Because the Gcn5 bromodomain recognizes acetyl lysine (44, 45) and regulates the lysine specificity of Gcn5 (39), we asked whether the bromodomain contributes to processive acetylation by the HAT module. Truncating the bromodomain of Gcn5 while leaving the Sgf29 tandem Tudor domain intact (Fig. 1) causes a twofold decrease in kcat/Km for peptide substrates compared with the wild-type HAT module (Table 1). As has been observed previously, deletion of the Gcn5 bromodomain changes the overall pattern of acetylation catalyzed by the HAT module (39), particularly by delaying accumulation of the most highly acetylated states (Fig. S8 A and B). However, processive acetylation is clearly impaired on H3K4me3 nucleosomes (compare competitor and mock-quenched samples in Fig. 5 G and H), although not to the degree seen with the ΔTudor complex (Fig. 5 E and F). These results suggest that, although processivity is governed primarily by binding of the Sgf29 tandem Tudor domain to H3K4me3 marks in nucleosomes, there is some contribution by the Gcn5 bromodomain, presumably through binding to acetylated lysine residues.
Fig. S8.
The bromodomain of Gcn5 facilitates acetylation on nucleosomal substrates. (A) HAT reactions using the Δbromodomain HAT module and 200 nM fluorescently labeled nucleosomes resolved on an acid–urea gel. (B) HAT reactions using the Δbromodomain HAT module and 200 nM fluorescently labeled H3K4me3 nucleosomes resolved on an acid–urea gel.
Under the conditions used to monitor processive acetylation, in which the HAT module and labeled nucleosome are present equimolar ratios, we observe six acetylation events per histone tail (Fig. 5 B–H; also see Fig. S10). However, we only observe five acetylation events per histone in assays containing higher concentrations of nucleosomes (Figs. 3B and 4 and Figs. S3, S5C, S6, and S7). The catalytic efficiency of Gcn5 for the various lysine residues on the H3 tail varies by two orders of magnitude (29). Thus, we attribute the extra acetylation site observed at low concentrations of nucleosomes to the different specificity of Gcn5 for each lysine and would anticipate six acetylation events per histone at long time points in all our assays.
Fig. S10.
The addition of a 20-fold molar excess of unlabeled nucleosomes inhibits acetylation by the HAT module on H3K4me3 nucleosomes. (A) HAT reactions resolved on acid–urea gels containing 50 nM HAT module and 50 nM H3K4me3 nucleosomes labeled with Alexa Fluor 488. (B) As in A but with 1 μM unlabeled nucleosomes added at the start of the reaction.
Discussion
Although crosstalk between histone modifications is a well-documented feature of the histone code (4, 5), the underlying mechanisms have remained obscure. Although studies in vivo have connected the presence of H3K4me3 to transcriptional activation by the SAGA complex (30, 31, 46), the way by which SAGA transduces H3K4me3 recognition into hyperacetylation by Gcn5 was not known. In this study we uncover a mechanism by which the HAT subcomplex of the SAGA transcriptional coactivator couples histone hyperacetylation to H3K4 trimethylation, a universal mark associated with promoter regions of actively transcribed chromatin in organisms ranging from yeast (9, 47) to humans (48). We show that H3K4 trimethylation modestly accelerates acetylation by the HAT module on peptides and nucleosomes (Figs. 2B and 3A and Tables 1 and 3). Using differential histone labeling, we find that the HAT module is highly selective for H3K4me3 histones in mixtures of modified and unmodified nucleosomes (Fig. 4A). This selectivity is a consequence of processive acetylation of nucleosomes containing the H3K4me3 modification (Fig. 5 B and C), with the HAT module acetylating each H3K4me3-containing nucleosome multiple times before dissociating. These data establish a paradigm for Sgf29 function at gene promoters and provide a mechanistic explanation for the observation that acetylation by Gcn5 in vivo is governed by the presence of H3K4 trimethylation (13, 30).
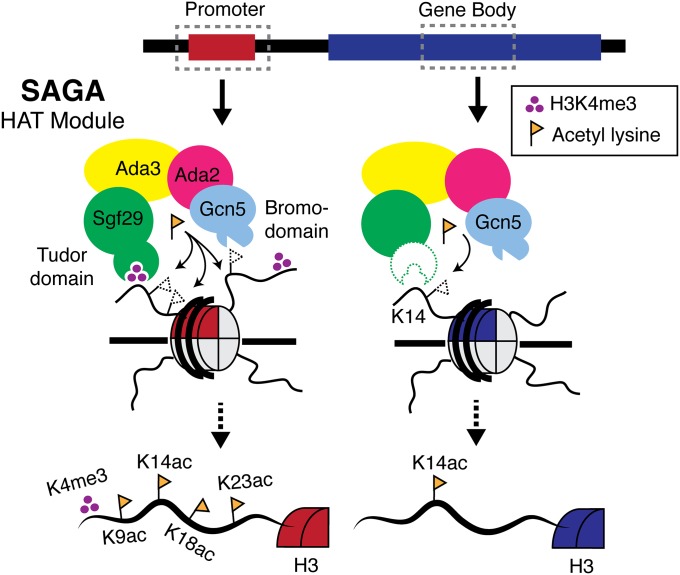
Processive, multisite acetylation on H3K4me3 nucleosomes also explains how the HAT module might establish different acetylation patterns at promoters versus coding regions of transcribed genes. Although Gcn5 is broadly enriched across highly transcribed genes in yeast (49), Gcn5-dependent acetylation patterns differ between gene promoters and coding regions (20, 49). For example, both H3K9 and H3K14 acetylation can be catalyzed by Gcn5 in vivo, but high-resolution ChIP-sequencing data show that H3K9 acetylation is narrowly limited to promoter regions (20), whereas H3K14 acetylation spreads across gene bodies as well (49). How can the same enzyme generate different acetylation patterns? Our results are consistent with a model in which H3K4 trimethylation, which is enriched at gene promoters compared with coding regions (9, 50), promotes a pattern of acetylation in promoter regions different from that in gene bodies (Fig. 6). In the absence of H3K4 trimethylation, the HAT module likely acetylates histones according to the intrinsic lysine specificity of Gcn5, which vastly favors modification at the H3K14 position (29, 39, 51). At promoters, however, recognition of the H3K4me3 mark by Sgf29 promotes processive, multisite acetylation by the HAT module. The bromodomain of Gcn5 can bind to these newly acetylated residues, providing a second feedback mechanism that promotes retention of the complex and, consequently, local hyperacetylation (Fig. 6). Together, the actions of the tandem Tudor domain and the bromodomain provide a mechanism by which the reader functions of SAGA might fine-tune the activity of Gcn5 along different genic regions, thus establishing diffuse patterns of H3K14 acetylation punctuated by narrow regions of hyperacetylation that colocalize with H3K4 trimethylation. These results also explain why depleting Sgf29, a noncatalytic subunit containing a tandem Tudor domain that recognizes H3K4me3, reduces levels of H3K9, H3K14, and H3K18 acetylation in yeast and human cells (30) despite having little effect on overall SAGA activity (30). This model leaves open the possibility that other acetyltransferases, particularly those with H3K4me3-binding domains, also might contribute to acetylation at actively transcribed genes. For example, the yeast HAT complex NuA3 contains a noncatalytic subunit, Yng1, which binds to H3K4 trimethylation and enhances H3K14 acetylation by NuA3 on H3K4me3 peptides in vitro (52).
Fig. 6.
Model depicting posttranslational modification-dependent acetylation by SAGA. (Left) H3K4me3 enrichment at gene promoters stimulates processive, multisite acetylation by the SAGA HAT module, resulting in hyperacetylation of histone H3. (Right) Within gene bodies, Gcn5 catalyzes low levels of acetylation based on its intrinsic lysine specificity, preferentially modifying H3K14.
The effect of H3K4me3 on acetylation by Gcn5 is clearly more complicated than simply providing additional binding energy for methylated substrate. Binding of Sgf29 to methylated peptide is not proportionally reflected in a lower Km, as methylation changes the affinity of isolated Sgf29 for peptides by ∼50-fold (30), whereas we observe only a fourfold change in Km (Tables 1 and 3). We note that, in addition to the Sg29 tandem Tudor domain, the HAT module contains a number of other chromatin-interacting domains that are not sensitive to the H3K4me3 modification. With the potential for multiple histone-binding interactions that may further impact both binding and catalysis, there is not a simple correspondence between Sgf29-H3K4me3 affinity and Km for the HAT complex.
Our results highlight the benefit of using defined, homogeneous chromatin templates containing native modifications to study the mechanistic basis of crosstalk between histone modifications. For many chromatin-modifying complexes, the mechanism by which reader domains translate the histone code into functional outcomes remains an open question that is difficult to address biochemically with recombinant unmodified histones or with heterogeneously modified chromatin isolated from cells. Our histone color-coding assay, combined with established methods for generating uniformly modified histones (36, 53), makes it possible to monitor acetylation on different pools of nucleosomes within the same reaction, thereby better approximating the heterogeneity of chromatin inside the cell. This differential labeling assay also makes it possible to observe substrate competition directly, rather than making inferences from independent measurements of specificity. In the present study, we compared activity on unmodified and H3K4me3 nucleosomes; however, the number of independent subpopulations that can be interrogated simultaneously using color-coding is limited only by the need to avoid significant spectral overlap between the fluorophores used. This technique can be adapted to study tail-specific acetylation on asymmetrically modified nucleosomes as well (54), for example those in which one copy of histone H3 contains the H3K4me3 mark and the other copy is unmodified. By incorporating more than one label into the same octamer, different histones within the same nucleosome could be monitored independently. Our assay using fluorescent histone labeling, along with other recently developed techniques such as fluorescent labeling of nucleosomal DNA (55) and DNA-barcoded nucleosome libraries (56), are particularly suited to addressing the mechanistic basis of histone crosstalk.
Materials and Methods
Cloning, Expression, and Purification of Proteins.
SAGA HAT module subunits were PCR-amplified from S. pombe genomic DNA using KOD polymerase (EMD Millipore) and were cloned into vectors for bacterial expression using the In-Fusion Cloning kit (Clontech). Because S. pombe Sgf29 contains an intron, the two exons were PCR-amplified using primers containing a 15-bp internal overlap and were used together in a single In-Fusion reaction. The HAT module subunits were cloned into three vectors, which were compatible for coexpression in E. coli. Ada3 was cloned into pET32a, The Gcn5/ Gcn5Δbromodomain was cloned into the first multiple cloning site of CDFduet, Ada2 was cloned into the second multiple cloning site of CDFduet, and Sgf29/ Sgf29ΔTudor was cloned into pRSF. Full-length Sgf29 for pull-down assays was cloned into pET32a. Histone H3 from Xenopus laevis containing C110A/Q125C mutations and/or having the first 32 amino acids truncated (H3Δ32) for sortase reactions were generated using QuikChange site-directed mutagenesis (Agilent Technologies).
Plasmids containing the wild-type HAT module and deletion constructs lacking the Sgf29 Tudor domain (ΔTudor corresponding to Sgf29 amino acids 1–95) or the Gcn5 bromodomain (Δbromodomain corresponding to Gcn5 amino acids 1–340) were cotransformed into Rosetta2-(DE3) E. coli cells (EMD Millipore) and coexpressed as intact complexes bearing a hexahistidine tag on the Ada3 subunit. Ada3 was expressed as a thioredoxin fusion in pET32a; Gcn5 and Ada2 were provided on CDFduet; and Sgf29 was supplied on pRSF. Starter cultures cotransformed with all three plasmids were grown overnight at 37 °C in Terrific broth (TB) containing 50 μg/mL carbenicillin, 31 μg/mL chloramphenicol, 50 μg/mL streptomycin, and 25 μg/mL kanamycin. The saturated cultures then were diluted by 100-fold into 4–12 L of TB and grown at 37 °C until reaching an OD600 of 0.6–0.8. The flasks were transferred to an ice bath for 45 min, and the shaker temperature was lowered to 16 °C. The cells were induced with the addition of 0.25 mM isopropyl β-d-1-thiogalactopyranoside overnight (16–18 h), harvested by centrifugation, and resuspended in lysis buffer containing 40 mM Hepes, pH 7.6, 500 mM NaCl, 10% glycerol, 20 mM imidazole, pH 8.0, and 5 mM β-mercaptoethanol (BME).
To purify the HAT module, cell pellets were thawed, lysed using a Microfluidizer (Microfluidics Corp.), and clarified by centrifugation at 32,000 × g. The soluble fraction was loaded onto a HisTrap HP column (GE Life Sciences) equilibrated in lysis buffer. The HAT module was eluted with a 20–400 mM imidazole gradient over 15 column volumes and dialyzed overnight at 4 °C into 20 mM Hepes, pH 7.6, 100 mM NaCl, 10% glycerol, and 5 mM BME in the presence of 1 mg recombinant Tobacco Etch Virus (TEV) protease per 10 mg purified protein to cleave the thioredoxin tag. Following dialysis, imidazole was added to the cleavage reaction at a final concentration of 20 mM, and then the mixture was run over the HisTrap HP column (GE Life Sciences), which retained the hexahistidine-tagged thioredoxin while allowing the HAT module to pass through. Solid ammonium sulfate was added to the flow-through over 10 min with stirring at 4 °C, to a final concentration of 0.7 M. Precipitated protein was removed using a 0.4-μm filter, and the supernatant was applied to a Phenyl HP column (GE Life Sciences) equilibrated in buffer A containing 20 mM Hepes, pH 7.6, 200 mM NaCl, 0.7 M ammonium sulfate, 10% glycerol, and 5 mM BME. The column was stepped to 30% buffer B containing 20 mM Hepes, pH 7.6, 40 mM NaCl, 10% glycerol, and 5 mM BME. The column was developed with a 30–100% gradient over five column volumes. Fractions containing the HAT module were concentrated and loaded onto a HiPrep 26/60 Sephacryl S-400 (GE Life Sciences) gel filtration column equilibrated in 20 mM Hepes, pH 7.6, 100 mM NaCl, and 200 μM Tris(2-carboxyethyl)phosphine (TCEP). The purified HAT module was concentrated to 4–10 mg/mL, flash-frozen in liquid nitrogen, and stored at −80 °C until use. Extinction coefficients were calculated using the ProtParam tool from the ExPASy Bioinformatics Resource Portal (www.expasy.org).
Cells expressing Sgf29 were grown and lysed as described above for the HAT module except that 50 μg/mL carbenicillin and 31 μg/mL chloramphenicol were used as antibiotics. Clarified lysate was loaded onto a HisTrap HP column (GE Life Sciences) equilibrated in buffer containing 20 mM Hepes, pH 7.6, 500 mM NaCl, 20 mM imidazole, pH 8, and 5 mM BME. After washing with 10 column volumes of buffer, the proteins were eluted with 300 mM imidazole and then were dialyzed overnight into 20 mM Hepes, pH 7.5, 100 mM NaCl, and 5 mM BME. The thioredoxin/hexahistidine tags were removed by adding 1 mg of TEV protease per 10 mg recombinant protein during dialysis. The dialyzed samples were loaded onto the HisTrap HP column, and the tag was separated using a gradient of 0–200 mM imidazole over 10 column volumes. Fractions containing pure protein were dialyzed overnight at 4 °C against 20 mM Hepes, pH 7.6, 100 mM NaCl, and 200 μM TCEP. The proteins were concentrated to ∼5 mg/mL, flash-frozen in liquid nitrogen, and stored at −80 °C.
Sortase was purified as previously described (36). Purified protein was dialyzed overnight at 4 °C into 50 mM Hepes, pH 7.5, 150 mM NaCl, and 1 mM DTT, concentrated to 35–48 mg/mL, flash-frozen in liquid nitrogen, and stored at −80 °C.
Recombinant X. laevis histones, including the mutants used for differential labeling and the truncated version of histone H3 for enzyme-mediated ligation in which the first 32 amino acids were deleted (H3Δ32), were purified as previously described (57). DNA for recombinant mononucleosomes was purified from an EcoRV digest of pST55-16xNCP601, a kind gift from Song Tan (Pennsylvania State University, State College, PA) as described previously (58). Nucleosomes were assembled using the salt gradient dialysis method (57) and then were dialyzed into low-salt buffer containing 10 mM Tris⋅HCl, pH 7.5, 5 mM KCl, and 1 mM DTT for storage at 4 °C, where they were used within 6 wk.
Enzyme-Mediated Ligation of Methylated Peptide to Histone H3 with Sortase.
Peptide for enzyme-mediated ligation with the sequence H2N-ART(K-me3)QTARKSTGGKAPRKQLATKAARKSAPATGGK-NH2 was purchased at >85% purity (United Peptide). The peptide was solubilized in MilliQ water at a concentration of 20 mM and was dialyzed against three changes of MilliQ water at 4 °C using cellulose ester dialysis tubing with a molecular mass cutoff of 100–500 kDa (Spectrum Labs). After dialysis, peptide concentrations were determined with a bicinchoninic acid (BCA) assay using BSA as a standard (Thermo Scientific) read in a POLARstar Omega plate reader (BMG Labtech), and then stored at −20 °C. Full-length histone H3 containing trimethylated H3K4 and either the wild-type H3 sequence or a C110A/Q125C double mutant were prepared as previously described (36) with the following modifications: Ligation reactions were run in assay buffer containing 50 mM Hepes, pH 7.6, 150 mM NaCl, 10 mM CaCl2, and 1 mM DTT. Each reaction contained 500 μM 35-mer H3 peptide, 90 μM H3Δ32, and 300 μM sortase enzyme. Because the globular form of histone H3 (missing the first 32 amino acids) has poor solubility in water, lyophilized protein was first dissolved at a concentration of 8–10 mM in solubilization buffer [6 M guanidine⋅HCl, 20 mM Hepes (pH 7.6), and 10 mM DTT] before diluting it into the reaction. The reactions were incubated at 30 °C for 16–18 h, during which the globular and ligated histones gradually precipitated while the sortase enzyme and histone peptide remained soluble. The soluble and insoluble portions of the sortase reaction were separated by spinning at 21,000 × g in a microcentrifuge for 10 min at room temperature. The pellet was resuspended in five reaction volumes of buffer containing 7 M deionized urea, 20 mM Hepes, pH 7.5, 5 mM BME, and 0.5 mM EDTA disodium salt and was loaded onto a MonoS HR 5/5 column (GE Life Sciences) equilibrated in the same buffer. To separate full-length histone from unligated substrate, the column was developed with a 0.1–1 M NaCl gradient over 40 column volumes. Fractions containing the full-length H3K4me3 histone were pooled and dialyzed at 4 °C against three changes of MilliQ water containing 0.1 mM PMSF and 5 mM BME. Dialyzed histone was lyophilized and resuspended in a small amount of MilliQ water, and the concentration was determined spectrophotometrically using the same extinction coefficient (4040 M−1⋅cm−1) as for full-length H3. Equal amounts of purified H3K4me3 histone H3 and full-length bacterially expressed histone H3 ranging from 0.5–10 μg were run on an SDS/PAGE gel and stained with Coomassie Brilliant Blue. To correct the extinction coefficient of purified H3K4me3 for the presence of contaminants, band intensities were compared in ImageJ between the samples to calculate a correction factor. Typically, the extinction coefficient for recombinant histone H3 overestimated the concentration of H3K4me3 by a factor of 1.1–1.5, and this correction factor was applied when calculating the H3K4me3 concentration at the octamer reconstitution step. The protein was lyophilized for long-term storage at −20 °C.
Fluorescent Labeling of Histones and Nucleosomes.
Histone H3 C110A/Q125C was used for fluorescent labeling with cysteine-reactive dyes because the endogenous cysteine in Xenopus histone H3 (C110) points to the interior of the histone octamer and is not in a good position to accommodate a fluorescent dye, whereas Q125 is located on a solvent-exposed helix. Lyophilized histones were resuspended and reduced in 6 M guanidine⋅HCl, 20 mM Hepes, pH 7.5, and 400 μM TCEP for 30–60 min at room temperature at a concentration of 4 mg/mL. Meanwhile, 1 mg of Alexa Fluor 488 C5 maleimide and 1 mg of Alexa Fluor 647 C2 maleimide (Life Technologies) were each dissolved in 100 μL of DMSO at final concentrations of 13.9 mM and 7.7 mM, respectively. Histones bearing different posttranslational modifications were labeled with unique colors; unmodified H3 was labeled with Alexa Fluor 647, and H3K4me3 was labeled with Alexa Fluor 488. Unfolded/reduced histones were diluted to a concentration of 2 mg/mL with labeling buffer and were incubated with a fivefold molar excess dye for 2 h at room temperature with gentle agitation and protection from light. Reactions were quenched with the addition of 1 mM BME and were combined immediately with equimolar amounts of the other three histones to reconstitute histone octamers as previously described (57). Refolded octamers were purified by gel filtration, which also removed excess dye that remained after dialysis. Nucleosomes were reconstituted with differentially labeled octamers as previously described (57). Labeled nucleosomes were dialyzed into nucleosome storage buffer [50 mM KCl, 10 mM Tris⋅HCl (pH 7.5), and 1 mM DTT], concentrated to >5 μM for storage at 4 °C, and were used within 6 wk.
Steady-State Kinetic Assays.
Steady-state kinetic measurements using peptide substrates were done with a continuous spectrophotometric assay as previously described (42), with a few minor modifications. Briefly, each 50-μL reaction contained 5 mM MgCl2, 1 mM DTT, 0.2 mM thiamine pyrophosphate (Sigma), 0.2 mM NAD+, 100 mM Hepes, pH 7.6, 50 mM NaCl, 2.5 mM pyruvate, 1 μL of 0.45 U/mg at 13 mg/mL pyruvate dehydrogenase (Sigma), 0.5–100 μM acetyl-CoA, 25–50 nM HAT module, and 0–1,400 μM histone H3 peptide containing the sequence H2N-ARTKQTARKSTGGKAPRKQLA-COOH (purchased at >90% purity from United Peptide). Peptide concentrations were determined with a BCA assay using BSA as a standard (Thermo Scientific). For titrations in which the peptide concentration was varied, the acetyl-CoA concentration was held at 100 μM. For titrations in which the acetyl-CoA concentration was varied, the peptide concentration was held at 400 μM. All the reaction components, except for acetyl-CoA, were assembled in 384-well plates (Greiner Bio-One) and incubated at 30 °C for 5 min. Reactions were initiated by the addition of acetyl-CoA, and the absorbance at 340 nm was monitored continuously using a POLARstar Omega plate reader (BMG Labtech) for 5–60 min. Absorbance at 340 nm was converted into the molar concentration of NADH using Beer’s Law, assuming ε340nm = 6,220 M−1⋅cm−1. To calculate initial rates, NADH production was plotted as a function of time and fit to a line where initial velocity conditions were satisfied, typically within the first 3 min. A blank reaction containing the HAT module and acetyl-CoA but no substrate was performed for each titration, and the rate was subtracted as background from the other reactions. Initial rates were measured in triplicate, normalized to the enzyme concentration, and plotted as a function of substrate concentration. The resulting curve was fit to the Michaelis–Menten equation using nonlinear least squares regression implemented in GraphPad Prism 5.
Steady-state titrations with nucleosomal substrates were performed using a radioactive filter-binding assay (42). Nucleosome concentrations were determined spectrophotometrically at 260 nm using the extinction coefficient of the 147-bp DNA fragment: ε260nm = 2,346,045 M−1⋅cm−1. Briefly, samples containing 100 mM Hepes, pH 7.6, 1 mM DTT, 0–10 μM unmodified NCP or 0–1 μM H3K4me3 NCP, 50 mM NaCl, and 50 nM HAT module were incubated at 30 °C for 5 min. Reactions were initiated with the addition of acetyl-CoA at 25 μM, containing a 3:1 molar ratio of unlabeled:titrated acetyl-CoA (PerkinElmer) and were quenched by spotting 25 μL onto P81 filter paper. Because proteins and peptides stick to P81 filter but free acetyl-CoA does not, acetylation was monitored by scintillation counting with the filter paper. At first, six time points were collected at 15-s intervals to determine the range in which product formation was linear with time. Under all conditions tested, initial rate conditions were satisfied for the first minute of the reaction. Subsequent experiments were performed by collecting three time points at 15, 30, and 45 s. An unwashed filter was reserved at each substrate concentration to convert counts per minute to a molar concentration of acetyl-CoA. Initial rates were determined by plotting product concentration as a function of time and fitting a line to the data, using the rate of acetyl-transfer in the absence of substrate as a reference. Each experiment was performed in duplicate, and kinetic parameters were fit as described for the enzyme-coupled assay earlier in this paper.
HAT Assays for Analysis by Acid–Urea Gel Electrophoresis.
Nucleosomes were acetylated in buffer containing 20 mM Hepes, pH 7.6, 50 mM NaCl, 1 mM DTT, 50 μM acetyl-CoA, 20 μg/mL BSA, and 0.2 μM or 1 μM nucleosome core particle. Reactions were incubated in buffer at 30 °C for 5 min, initiated by the addition of 50 nM HAT module, quenched at different time points by flash-freezing in liquid nitrogen, and then lyophilized for analysis by acid–urea gel electrophoresis.
Acid–urea gels were assembled and run as previously described (38). Histones were visualized with either SYPRO Ruby protein stain (Life Technologies) or by Western blotting. For Western blotting, proteins were transferred to PVDF membrane as previously described (38). Membranes were blocked overnight in 5% nonfat milk at 4 °C and were washed in TBS. Primary antibodies were diluted in 1% nonfat milk in TBS supplemented with 0.1% Tween 20 (TBST) as follows: anti-H3 (Abcam ab1791, 1:25,000), anti-H3K9ac (Active Motif 39917, 1:5,000), anti-H3K14ac (07-353, EMD Millipore, 1:5,000), anti-H3K18ac (EMD Millipore 07-354, 1:7,500), anti-H3K23ac (EMD Millipore 07-355, 1:5,000), or anti-H3K4me3 (Abcam ab8580, 1:5,000). Each primary antibody was applied for 1 h at room temperature followed by washing in TBST. Goat anti-rabbit IgG-HRP secondary antibody (Amersham Biosciences) was diluted to 1:5,000 in 1% nonfat milk and TBST, applied for 1 h at room temperature, and washed in TBST. Blots were developed using Pierce ECL Western Blotting Substrate (Thermo Scientific) and were exposed using film.
Pull-Down Assays.
To study the interaction between Sgf29 and the H3K4me3 modification, 25 μL of M-280 Streptavidin DynaBeads (Life Technologies) were washed into histone binding buffer [20 mM Hepes (pH 7.6), 50 mM NaCl, 0.02% Tween-20, and 1% BSA] and then were incubated for 1 h at room temperature with 1 μg of biotinylated histone H3 21-mer peptide ± H3K4me3, which was generously provided by S.D.T. The beads were washed twice with histone binding buffer and then twice in complex binding buffer [20 mM Hepes (pH 7.6), 50 mM NaCl, 0.02% Tween 20]. Recombinant Sgf29 was added to the beads at a final concentration of 10 μM and allowed to bind at room temperature for 1 h. The beads were washed three times with complex binding buffer, eluted by boiling with 2× SDS/PAGE loading buffer, run on an SDS/PAGE gel, and stained with Coomassie brilliant blue.
Differential Labeling Assay.
Assays using differentially labeled nucleosomes were run as described in HAT Assays for Analysis by Acid–Urea Gel Electrophoresis. Control reactions containing only one kind of labeled nucleosome were performed at substrate concentrations of 0.2 μM and 1 μM. Nucleosome mixtures were prepared by combining 0.2 μM H3K4me3 nucleosome/Alexa Fluor 488 with 0.8 μM unmodified nucleosome/Alexa Fluor 647 and running the same time-course experiments. Quenched time points were resolved on 28-cm acid–urea gels and visualized using a Typhoon Imager (GE Life Sciences). Reactions containing Alexa Fluor 488 were excited using the 473-nm laser and imaged using the 520BP40 filter, and reactions containing Alexa Fluor 647 were excited using the 635-nm laser and imaged using the 670BP30 filter. Native gels containing mixtures of the labeled nucleosomes were imaged in the same way to ensure that there was no spectral overlap between the two dyes (Fig. S9). Merged images were prepared using Adobe Photoshop.
Fig. S9.
Differentially labeled nucleosomes do not exhibit spectral overlap. Mixtures of fluorescently labeled nucleosomes resolved on a native PAGE gel (6% TBE) and imaged using excitation wavelength/emission filter sets specific for Alexa Fluor 647 (A) or Alexa Fluor 488 (B).
Processivity Assay.
Three reactions were prepared for each combination of nucleosome type/HAT module complex containing 20 mM Hepes, pH 7.6, 50 mM NaCl, 1 mM DTT, 50 μM acetyl-CoA, 20 μg/mL BSA, and 0.05 μM fluorescently labeled nucleosome in a total volume of 25–35 μL. Reactions containing peptide were performed with 50 μM H3K4me3 amino acids 1–8 peptide (ART-Kme3-QTAR), which does not contain any free lysine residues that can be acetylated by Gcn5. Reactions were incubated for 5 min at 30 °C, initiated with the addition of enzyme to a final concentration of 50 nM, and mixed vigorously by pipetting up and down. After the reaction was allowed to proceed for 10–60 s, reactions were quenched by the addition of s one-fifth volume acetic acid, mock-quenched with the addition of one-fifth volume nucleosome storage buffer, or subjected to competition by the addition of a one-fifth volume of unlabeled nucleosomes, bringing the concentration of the unlabeled competitor to 1 μM. After the reactions were incubated for a total of 1–10 min at 30 °C, the samples were flash-frozen in liquid nitrogen, lyophilized, resuspended in 10 μL of acid–urea sample buffer, and run on a 28-cm acid–urea gel (38). Gels were scanned using a Typhoon imager (GE Life Sciences) with the same excitation wavelengths/emission filter combinations as used in the differential labeling assay. ImageJ was used for background subtraction and to quantitate band intensities. To show that the concentration of unlabeled competitor used in the processivity assay acts as an effective chase for methylated nucleosomes, we measured acetylation by the HAT module on reactions containing 50 nM H3K4me3 nucleosomes labeled with Alexa Fluor 488, with 1 μM unlabeled nucleosome added at the beginning of the reaction. Reactions were monitored for the same length of time as the processivity assay using the wild-type HAT module with methylated nucleosomes. The addition of unlabeled nucleosomes effectively slows acetylation by the HAT module (Fig. S10B) compared with acetylation in the absence of competitor (Fig. S10A).
Acknowledgments
We thank Greg Bowman (Johns Hopkins University) for providing the X. laevis histone expression plasmids, Song Tan (Pennsylvania State University) for providing the pST55-16xNCP601 plasmid, and Phil Cole (Johns Hopkins University School of Medicine) for helpful discussions. This work was supported by National Institute of General Medical Sciences Grants R01GM098522 (to C.W.) and R01GM106024 (to S.D.T.) and by National Science Foundation Grant DGE-1232825 (to A.M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508449112/-/DCSupplemental.
References
- 1.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 2.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 3.Turner BM. Histone acetylation and an epigenetic code. BioEssays. 2000;22(9):836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15(2):172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 5.Musselman CA, Lalonde ME, Côté J, Kutateladze TG. Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol. 2012;19(12):1218–1227. doi: 10.1038/nsmb.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suganuma T, Workman JL. Crosstalk among Histone Modifications. Cell. 2008;135(4):604–607. doi: 10.1016/j.cell.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Lalonde ME, Cheng X, Côté J. Histone target selection within chromatin: An exemplary case of teamwork. Genes Dev. 2014;28(10):1029–1041. doi: 10.1101/gad.236331.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: Lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14(11):1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pokholok DK, et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122(4):517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117(6):721–733. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Liu CL, et al. Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol. 2005;3(10):e328. doi: 10.1371/journal.pbio.0030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millar CB, Grunstein M. Genome-wide patterns of histone modifications in yeast. Nat Rev Mol Cell Biol. 2006;7(9):657–666. doi: 10.1038/nrm1986. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, et al. Global assessment of combinatorial post-translational modification of core histones in yeast using contemporary mass spectrometry. LYS4 trimethylation correlates with degree of acetylation on the same H3 tail. J Biol Chem. 2007;282(38):27923–27934. doi: 10.1074/jbc.M704194200. [DOI] [PubMed] [Google Scholar]
- 14.Taverna SD, et al. Long-distance combinatorial linkage between methylation and acetylation on histone H3 N termini. Proc Natl Acad Sci USA. 2007;104(7):2086–2091. doi: 10.1073/pnas.0610993104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noma K, Grewal SIS. Histone H3 lysine 4 methylation is mediated by Set1 and promotes maintenance of active chromatin states in fission yeast. Proc Natl Acad Sci USA. 2002;99(Suppl 4):16438–16445. doi: 10.1073/pnas.182436399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KK, Workman JL. Histone acetyltransferase complexes: One size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8(4):284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen M, et al. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell. 2010;142(6):967–980. doi: 10.1016/j.cell.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Koutelou E, Hirsch CL, Dent SYR. Multiple faces of the SAGA complex. Curr Opin Cell Biol. 2010;22(3):374–382. doi: 10.1016/j.ceb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huisinga KL, Pugh BF. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol Cell. 2004;13(4):573–585. doi: 10.1016/s1097-2765(04)00087-5. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet J, et al. The SAGA coactivator complex acts on the whole transcribed genome and is required for RNA polymerase II transcription. Genes Dev. 2014;28(18):1999–2012. doi: 10.1101/gad.250225.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KK, et al. Combinatorial depletion analysis to assemble the network architecture of the SAGA and ADA chromatin remodeling complexes. Mol Syst Biol. 2011;7:503. doi: 10.1038/msb.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balasubramanian R, Pray-Grant MG, Selleck W, Grant PA, Tan S. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J Biol Chem. 2002;277(10):7989–7995. doi: 10.1074/jbc.M110849200. [DOI] [PubMed] [Google Scholar]
- 23.Grant PA, et al. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: Characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 1997;11(13):1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- 24.Saleh A, Lang V, Cook R, Brandl CJ. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J Biol Chem. 1997;272(9):5571–5578. doi: 10.1074/jbc.272.9.5571. [DOI] [PubMed] [Google Scholar]
- 25.Grant PA, et al. Expanded lysine acetylation specificity of Gcn5 in native complexes. J Biol Chem. 1999;274(9):5895–5900. doi: 10.1074/jbc.274.9.5895. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi J, Silverman N, Marcus GA, Guarente L. ADA3, a putative transcriptional adaptor, consists of two separable domains and interacts with ADA2 and GCN5 in a trimeric complex. Mol Cell Biol. 1995;15(3):1203–1209. doi: 10.1128/mcb.15.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Candau R, Berger SL. Structural and functional analysis of yeast putative adaptors. Evidence for an adaptor complex in vivo. J Biol Chem. 1996;271(9):5237–5245. doi: 10.1074/jbc.271.9.5237. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Luo J, Ranish J, Hahn S. Architecture of the Saccharomyces cerevisiae SAGA transcription coactivator complex. EMBO J. 2014;33(21):2534–2546. doi: 10.15252/embj.201488638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo YM, Andrews AJ. Quantitating the specificity and selectivity of Gcn5-mediated acetylation of histone H3. PLoS One. 2013;8(2):e54896. doi: 10.1371/journal.pone.0054896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bian C, et al. Sgf29 binds histone H3K4me2/3 and is required for SAGA complex recruitment and histone H3 acetylation. EMBO J. 2011;30(14):2829–2842. doi: 10.1038/emboj.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schram AW, et al. A dual role for SAGA-associated factor 29 (SGF29) in ER stress survival by coordination of both histone H3 acetylation and histone H3 lysine-4 trimethylation. PLoS One. 2013;8(7):e70035. doi: 10.1371/journal.pone.0070035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla A, Lahudkar S, Durairaj G, Bhaumik SR. Sgf29p facilitates the recruitment of TATA box binding protein but does not alter SAGA’s global structural integrity in vivo. Biochemistry. 2012;51(2):706–714. doi: 10.1021/bi201708z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clouaire T, Webb S, Bird A. Cfp1 is required for gene expression-dependent H3K4 trimethylation and H3K9 acetylation in embryonic stem cells. Genome Biol. 2014;15(9):451. doi: 10.1186/s13059-014-0451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanner KG, et al. Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator. J Biol Chem. 1999;274(26):18157–18160. doi: 10.1074/jbc.274.26.18157. [DOI] [PubMed] [Google Scholar]
- 35.Tanner KG, Langer MR, Kim Y, Denu JM. Kinetic mechanism of the histone acetyltransferase GCN5 from yeast. J Biol Chem. 2000;275(29):22048–22055. doi: 10.1074/jbc.M002893200. [DOI] [PubMed] [Google Scholar]
- 36.Piotukh K, et al. Directed evolution of sortase A mutants with altered substrate selectivity profiles. J Am Chem Soc. 2011;133(44):17536–17539. doi: 10.1021/ja205630g. [DOI] [PubMed] [Google Scholar]
- 37.Popp MW-L, Ploegh HL. Making and breaking peptide bonds: Protein engineering using sortase. Angew Chem Int Ed Engl. 2011;50(22):5024–5032. doi: 10.1002/anie.201008267. [DOI] [PubMed] [Google Scholar]
- 38.Shechter D, Dormann HL, Allis CD, Hake SB. Extraction, purification and analysis of histones. Nat Protoc. 2007;2(6):1445–1457. doi: 10.1038/nprot.2007.202. [DOI] [PubMed] [Google Scholar]
- 39.Cieniewicz AM, et al. 2014. The bromodomain of Gcn5 regulates site-specificity of lysine acetylation on histone H3. Mol Cell Proteomics 13(11):2896–2910.
- 40.Jin Q, et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30(2):249–262. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spedale G, Timmers HT, Pijnappel WW. ATAC-king the complexity of SAGA during evolution. Genes Dev. 2012;26(6):527–541. doi: 10.1101/gad.184705.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berndsen CE, Denu JM. Assays for mechanistic investigations of protein/histone acetyltransferases. Methods. 2005;36(4):321–331. doi: 10.1016/j.ymeth.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Cornish-Bowden A. Enzyme specificity: Its meaning in the general case. J Theor Biol. 1984;108(3):451–457. doi: 10.1016/s0022-5193(84)80045-4. [DOI] [PubMed] [Google Scholar]
- 44.Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE. Solution structure and acetyl-lysine binding activity of the GCN5 bromodomain. J Mol Biol. 2000;304(3):355–370. doi: 10.1006/jmbi.2000.4207. [DOI] [PubMed] [Google Scholar]
- 45.Owen DJ, et al. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J. 2000;19(22):6141–6149. doi: 10.1093/emboj/19.22.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurabe N, et al. Deregulated expression of a novel component of TFTC/STAGA histone acetyltransferase complexes, rat SGF29, in hepatocellular carcinoma: Possible implication for the oncogenic potential of c-Myc. Oncogene. 2007;26(38):5626–5634. doi: 10.1038/sj.onc.1210349. [DOI] [PubMed] [Google Scholar]
- 47.Santos-Rosa H, et al. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419(6905):407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 48.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Johnsson A, et al. HAT-HDAC interplay modulates global histone H3K14 acetylation in gene-coding regions during stress. EMBO Rep. 2009;10(9):1009–1014. doi: 10.1038/embor.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40(7):897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo MH, et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature. 1996;383(6597):269–272. doi: 10.1038/383269a0. [DOI] [PubMed] [Google Scholar]
- 52.Taverna SD, et al. Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol Cell. 2006;24(5):785–796. doi: 10.1016/j.molcel.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chatterjee C, Muir TW. Chemical approaches for studying histone modifications. J Biol Chem. 2010;285(15):11045–11050. doi: 10.1074/jbc.R109.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S, Shogren-Knaak MA. Cross-talk between histone H3 tails produces cooperative nucleosome acetylation. Proc Natl Acad Sci USA. 2008;105(47):18243–18248. doi: 10.1073/pnas.0804530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranjan A, et al. Nucleosome-free region dominates histone acetylation in targeting SWR1 to promoters for H2A.Z replacement. Cell. 2013;154(6):1232–1245. doi: 10.1016/j.cell.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen UT, et al. Accelerated chromatin biochemistry using DNA-barcoded nucleosome libraries. Nat Methods. 2014;11(8):834–840. doi: 10.1038/nmeth.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luger K, Rechsteiner TJ, Richmond TJ. Preparation of nucleosome core particle from recombinant histones. Methods Enzymol. 1999;304:3–19. doi: 10.1016/s0076-6879(99)04003-3. [DOI] [PubMed] [Google Scholar]
- 58.Dyer PN, et al. Reconstitution of nucleosome core particles from recombinant histones and DNA. Methods Enzymol. 2004;375:23–44. doi: 10.1016/s0076-6879(03)75002-2. [DOI] [PubMed] [Google Scholar]