Significance
The suspensor is a temporary structure that undergoes programmed cell death during seed maturation. It has been suggested that suspensor cells have embryogenic potential that is suppressed by the embryo. Using an established in vivo living cell laser ablation system, we confirmed the embryogenic potential of the Arabidopsis suspensor and the role of the embryo proper in imposing suspensor cell identity. We also showed that auxin redistribution in suspensor cells after laser ablation of embryos may play an essential role in the initiation of suspensor embryos.
Keywords: suspensor, cell-to-cell interaction, embryogenesis, auxin, in vivo laser ablation
Abstract
The suspensor is a temporary supporting structure of proembryos. It has been proposed that suspensor cells also possess embryogenic potential, which is suppressed by the embryo as an effect of the embryo–suspensor interaction. However, data to support this hypothesis are not yet available. In this report, using an in vivo living cell laser ablation technique, we show that Arabidopsis suspensor cells can develop into embryos after removing the embryo proper. The embryo proper plays a critical role in maintaining suspensor cell identity. However, this depends on the developmental stage; after the globular embryo stage, the suspensors no longer possess the potential to develop into embryos. We also reveal that hypophysis formation may be essential for embryo differentiation. Furthermore, we show that, after removing the embryo, auxin gradually accumulates in the top suspensor cell where cell division occurs to produce an embryo. Auxin redistribution likely reprograms the fate of the suspensor cell and triggers embryogenesis in suspensor cells. Thus, we provide direct evidence that the embryo suppresses the embryogenic potential of suspensor cells.
The suspensor is traditionally believed to be a supporting structure during plant embryo development that pushes the embryo proper into the endosperm cavity and connects it to the surrounding maternal and endosperm tissues to facilitate the transfer of nutrients and plant hormones. Therefore, it is supposed to be critical for the early development of the embryo (1–4). The suspensor cells have characteristics of transfer cells (e.g., Phaseolus and Stellaria) (5, 6) and are known to absorb and transport metabolites from the surrounding material tissue to the embryo proper (Phaseolus and Arabidopsis) (7, 8). The cells are characterized by specialized organelles (Phaseolus Pisum, Ipomoea, Stellaria, and Tropaeolum), the polytene chromosome (Phaseolus and Alisma), and higher levels of RNA and protein synthesis compared with the embryo proper during the early development of the embryo, as well as active transcription (Phaseolus and Tropaeolum) (6, 9). Suspensors in some plant species (Phaseolus, Tropaeolum, Cyfisus, and Arabidopsis) can synthesize or transport gibberellin or auxin, which promotes the development of the embryo proper during early embryogenesis (2, 7, 9).
Suspensors are temporary structures that degenerate after the globular embryo stage via programmed cell death; they do not participate in the development of later embryos, excluding hypophysis formation in Arabidopsis (10, 11). Once a suspensor is formed, cells no longer divide and the cell morphology is highly specialized with features that are distinct from those of embryo cells. However, based on experimental data, an hypothesis was developed in the 1970s suggesting that suspensor cells still possesses embryogenic potential and may develop into an embryo if relieved from suppression by the embryo proper (12–15). Based on radiation or acid treatment of the siliques or ovules, some pioneering studies showed that the active dividing embryo is more seriously injured than the highly differentiated suspensor, and a second embryo may be observed after several days of ovule culture. However, the exact origin of the second embryo has remained unclear and whether the radiation or the acid treatment leads to gene mutation in the suspensor cells has remained unknown (16–18).
Phenotypes of some mutants suggest that the embryo proper suppresses the developmental potential of the suspensor. When the embryo proper is abnormal, the suspensor cells can start dividing. Some mutant suspensors can develop into proembryos (e.g., twin, twin2, iyo) or globular embryo-like structures (e.g., SUS, raspberry). These data provide circumstantial evidence for this hypothesis; however, the role of the gene mutation itself or the abnormal embryo in triggering suspensor cell division requires further study (7, 12, 13, 15, 19, 20). In addition, some available data are contradictory. Expressing LTP1::DTA (LTP1, lipid transfer protein 1; DTA, diphtheria toxin A chain) in the protoderm domain of embryos (21) to injure the embryo protoderm can induce excessive divisions in the suspensor (22). However, expression of the LTP1-driven BARNASE (Bacillus amyloliquefaciens ribonuclease) gene in the embryo, which results in defects in the basal tier of the embryo and hypophysis, leads to no signs of deregulation of suspensor development (23). Thus, it remains unclear whether the suspensor–embryo interaction plays a role in determining cell fate transition of the suspensor.
The living cell microdissection system is a powerful approach for studying cell–cell interactions and testing the factors that may determine cell fate (24–28). Separation of the suspensor and embryo using this technique may be used to observe developmental fate in vivo and thus provide direct evidence of the suspensor–embryo interaction. It can also be used to determine whether suspensor cells possess embryogenic potential and whether the embryo suppresses that potential during embryogenesis. However, the embryo is embedded deeply in the ovule tissues, and there is no suitable technique for laser ablation of embryos in vivo at this time. Therefore, we attempted to establish such a technique. We applied this technique to confirm the embryogenic potential of suspensor cells and determine whether the embryo suppresses the potential during embryogenesis.
Results
Establishment of the in Vivo Living Cell Laser Microdissection Technique to Precisely Remove the Connection Between Embryo and Suspensor in Situ in Ovules.
We first constructed the suspensor cell marker lines by transformation using ZC1::NLS:GFP. Several lines were selected with clear GFP signals observed in suspensor cells. In these lines, every cell of the suspensor could be observed in the ovule during the developmental process of the embryo (Fig. S1 A–E1). Based on the marker lines, we established an in vivo laser microdissection technique by optimizing the different conditions and carefully examining cut intensity, radius, and total time for the optimal conditions. This technique allows precise and efficient ablation of any cells of a suspensor to remove the connection between suspensor and the embryo proper at different positions and at different stages of living embryos.
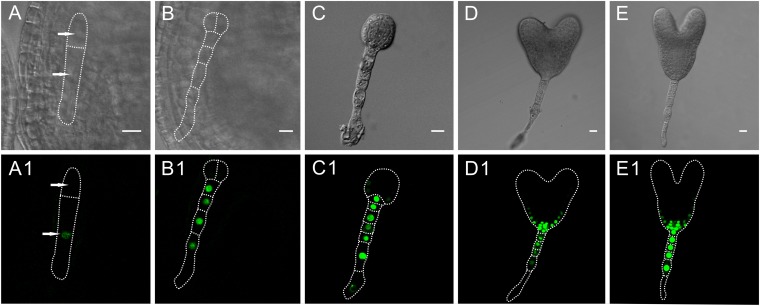
Fig. S1.
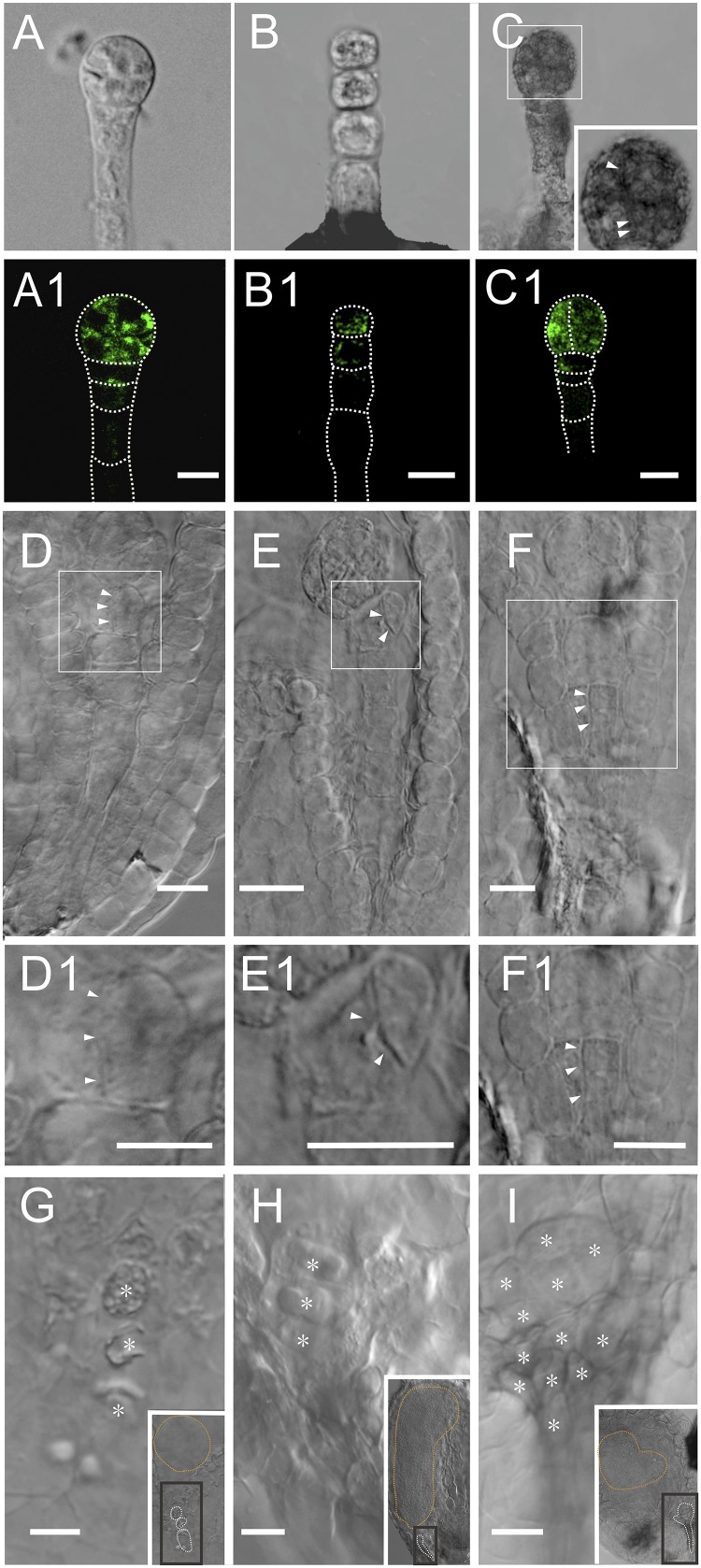
ZC1::NLS:GFP express in the suspensor cells during early embryo development. (A and A1) Two-celled proembryo. The GFP signal is observed in the basal cell. (B and B1) Six-celled embryo. The gene expresses only in the suspensor cells. (C–E1) The gene strongly expresses in the suspensor-derived cells and the lower-tier protoderm. (C and C1) A 32-celled embryo. (D and D1) Heart-stage embryo. (E and E1) Torpedo-stage embryo. (Scale bars, 10 μm.)
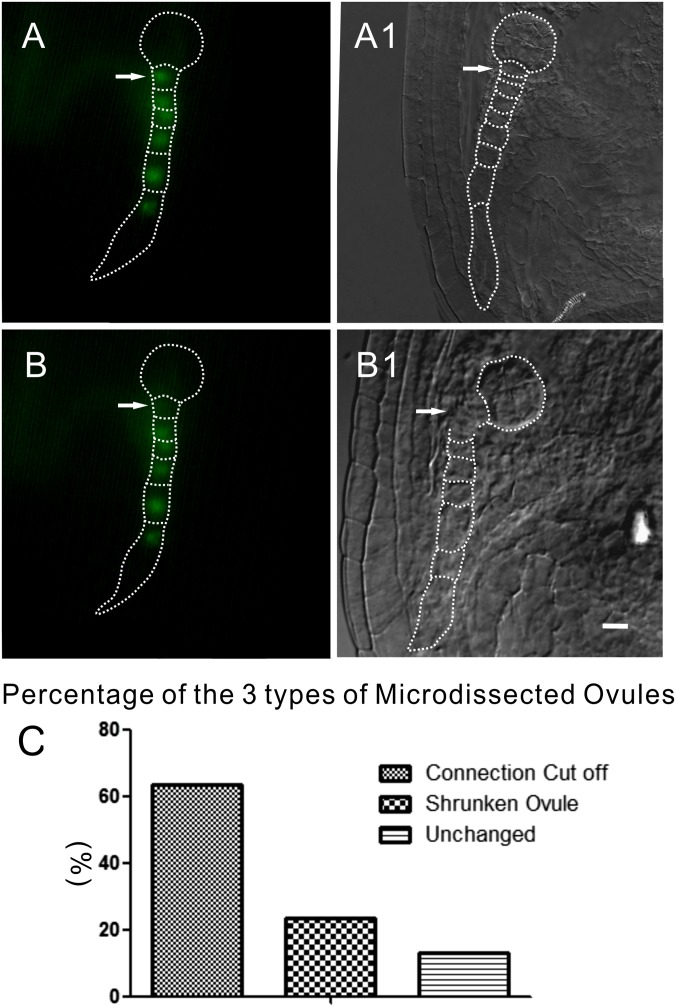
The laser beam usually targets the GFP signal of the uppermost suspensor cell (Fig. S2 A and A1) or the second cell from the top of a suspensor. Ablation of the chosen cell can be traced under a microscope during the manipulation process. To improve the efficiency and propriety of the ablation, microdissection was usually stopped immediately once the fluorescence of the ablated suspensor cell faded (Fig. S2B).
Fig. S2.
In vivo living cell laser microdissection system for removing the connection between suspensors and embryo propers of living embryos. (A and A1) Embryo before laser microdissection. The laser beam usually targets the uppermost suspensor cell (arrows). (B and B1) Embryo after ablation (not the same embryo as in A and A1). The fluorescence in the first cell of the suspensor is eliminated. Arrows indicate the laser-ablated cell. (C) The different percentages of three types of laser-ablated ovule (n = 77). (Scale bars, 20 μm.)
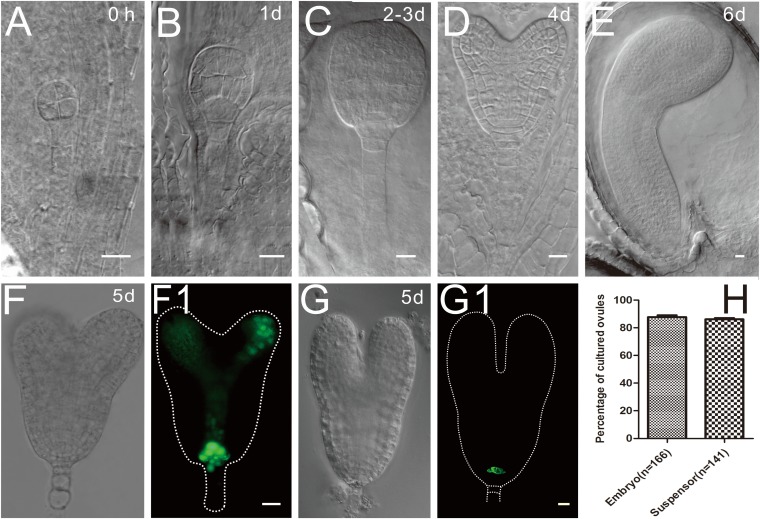
We also established a semi-in vivo embryogenesis system based on previous work in ovule culture (29). The morphology and structure of the embryos in cultured ovules were well developed and morphologically similar to their counterparts in vivo (Fig. S3 A–E). The time course of the embryo development in cultured ovules is also shown in Fig. S3. After a 5-d culture, the embryos developed into torpedo stage (Fig. S3 F and G), and they could further develop into mature embryos in few days. The auxin distribution and the WOX5 (WUSCHEL related homeobox 5) expression in these embryos were identical to that of embryos developed in vivo (Fig. S3 F–G1), indicating their physiological similarity. These experiments proved the reliability of the system for further experiments (Fig. S3H).
Fig. S3.
The Arabidopsis ovule culture system. (A–E) The morphology and the time course of the embryo development in cultured ovules. The numbers indicate the duration of the culture. (A) An eight-celled embryo. (B) A 32-celled embryo. (C) Big globular embryo. (D) Heart-stage embryo. (E) Cotyledon embryo. (F and F1) The auxin distribution indicated by DR5rev::3XVENUS:N7 expression. (G and G1) The expression of WOX5::GFP in hypophysis. (H) The percentage of cultured ovules (young seeds) with normal developed embryos and suspensors.
Based on the culture system, the effect of ablation can be examined after a brief culture of ovules. Under our conditions, the connection between the suspensor and embryo proper was obviously broken in 63.64% (n = 77) of ovules (Fig. S2C). The first suspensor cell that was not obviously ablated or its viability recovered later during culture was also observed in 12.98% of ovules under the same conditions of ablation, probably due to the physiological and structural variation in different ovules. A total of 23.38% ovules shrank during our brief culture due to unknown reasons and were not used for evaluation.
After the microdissection and culture, 11.00% (n = 618) of ovules showed clear cell-division patterns of the suspensor. Compared with results for established Arabidopsis ovule culture systems without laser ablation, this percentage is quite high and sufficient for further analysis.
Suspensor Cells Could Develop into Secondary Embryos After Breaking the Connection Between the Suspensor and Embryo.
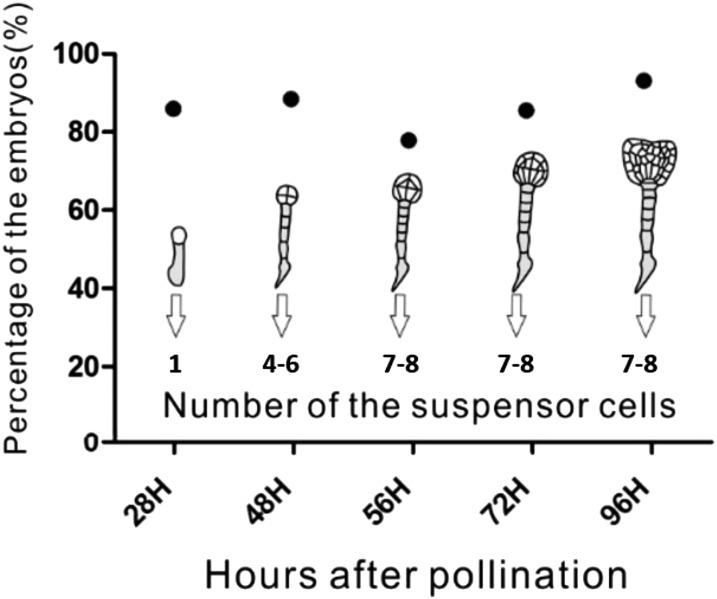
To determine the exact stages of embryonic development, we first investigated the time course of embryogenesis with reference to pollination time. Under our conditions, 28 h after pollination, 85.93% (n = 64) of embryos were at the two-celled embryo stage with an apical cell and a basal cell (Fig. S4). About 48 h after pollination, 88.46% (n = 78) of embryos were at the eight-celled stage with a four- or six-celled suspensor (Fig. S4). About 56 h after pollination, 77.91% (n = 86) of embryos were at the 16-celled embryo stage with a 7- or 8-celled suspensor (Fig. S4). Approximately 72 h after pollination, 85.57% (n = 97) of embryos were at the 32-celled embryo stage, and the suspensors still contained 7–8 cells (Fig. S4). About 96 h after pollination, 93.15% (n = 73) of embryos were at the heart stage, and the suspensor cell number had not changed (Fig. S4). This indicates that the suspensors already formed and the cells did not divide beginning at the 16-celled embryo stage (56 h after pollination).
Fig. S4.
Time course of suspensor development. At 28 h after pollination, 85.93% (n = 64) of proembryos were at two-celled stage. In 48 h after pollination, 88.46% (n = 78) of embryos were at the eight-celled stage with a four- or six-celled suspensor. At 56 h after pollination, 77.91% (n = 86) of embryos were at the 16-celled stage with a 6- or 8-celled suspensor. At 72 h after pollination, 85.57% (n = 97) of embryos were at the 32-celled stage and the suspensors still consisted of 6–8 cells. At 96 h after pollination, 93.15% (n = 73) of embryos were at the heart stage, and the number of suspensor cells remains 6–8.
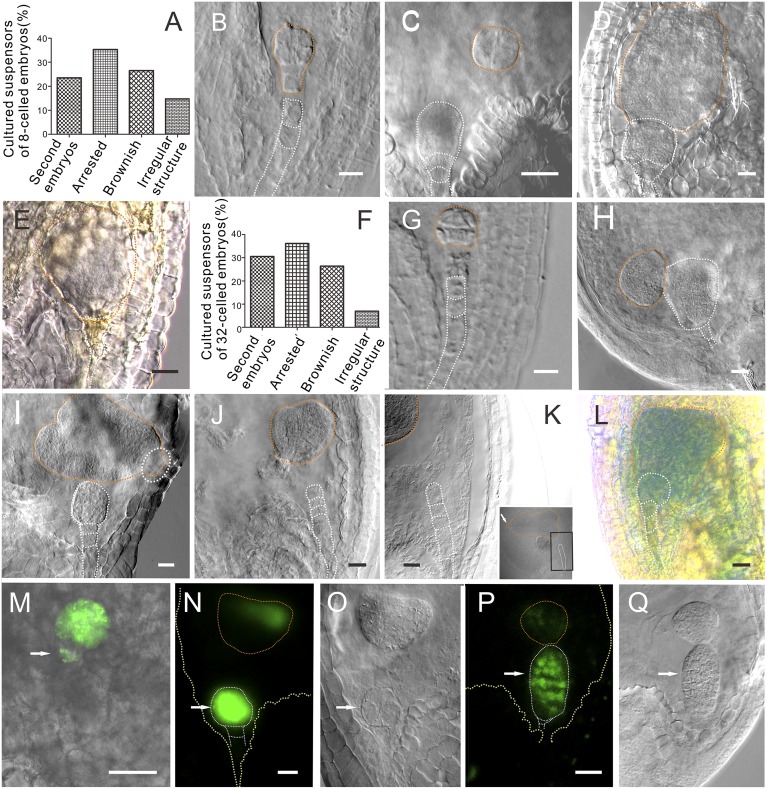
Based on our results, three stages of embryos were chosen for microdissection with the in vivo laser ablation technique to break the connection between suspensor and embryo: 8-celled embryos (48 h after pollination), 32-celled embryos (72 h after the pollination), and heart-stage embryos (96 h after pollination). After the laser ablation (Fig. 1B) and subsequent culture of the eight-celled embryos, 23.53% (n = 68) of suspensors developed into second embryos (Fig. 1 A–D; Fig. S5 A–D), 35.29% (n = 68) stopped developing, 26.47% (n = 68) became brownish and could not be used for the evaluation because it was difficult to clearly observe their morphologies (Fig. 1 A and E; Fig. S5E), and 14.71% (n = 68) divided but did not develop into embryos (Fig. 2A). After the ablation and culture of the 32-celled embryos, 30.56% (n = 72) of suspensors developed into second embryos (Fig. 1 F–I; Fig. S5 F–I), 36.11% (n = 72) arrested their development, 26.39% (n = 72) became brownish, and 6.94% (n = 72) divided but did not form embryos (Fig. 1F). However, after the ablation (Fig. 1J) and subsequent culture of the heart-stage embryos, the suspensors always stayed in the same stage without cell division and ultimately turned brownish (Fig. 1K) (n = 23).
Fig. 1.
The suspensor and embryo proper development after in vivo laser ablation. (A) The statistics of the suspensor development after laser ablation of an eight-celled embryo. (B) The eight-celled embryo after ablation, showing the embryo with two suspensor cells. (C) The laser-ablated eight-celled embryo after a 3-d culture. Note that the suspensor cells were completely removed from the embryo. The top suspensor cell has developed into an embryo (n = 14). (D) The laser-ablated eight-celled embryo developed into a big embryo without further differentiation after a 5-d culture (n = 39). The top suspensor cell has developed into a globular embryo with normal structure and morphology. (E) The brownish suspensor cannot be observed clearly. (F) The statistics of the suspensor development after laser ablation of a 32-celled embryo. (G) The laser-ablated 32-celled embryo after a 3-d culture. The suspensor cells were completely removed. Note the bottom morphology of the embryo. (H) The laser-ablated 32-celled embryo after a 3-d culture (n = 74). (I) The laser-ablated 32-celled embryo after a 5-d culture. The embryo was already differentiated with a clear apical–basal axis. The white dotted-line circle indicates the remnant cells of a suspensor (n = 47). (J) The heart-stage embryo after ablation. (K) The laser-ablated heart-stage embryo after a 3-d culture. The image is an enlargement of the part in the black box. Arrow shows the orientation of embryo with reference to the suspensor (n = 66). (L) The embryo-proper marker ABI3 expressed in the embryo derived from the suspensor cell. (Scale bars, 20 μm.) (M–O) DRN::GFP expressed in both embryos. (O) The differential interference contrast (DIC) image of the same embryos in N. Note that the GFP signal appeared only at the upper part of the heart-shaped embryo. (P) ATML1::H2B:GFP expressed in both embryos. (Q) The DIC image of the same embryos in P. Arrows indicate suspensor-derived embryos in vivo. (Scale bars, 20 μm.)
Fig. S5.
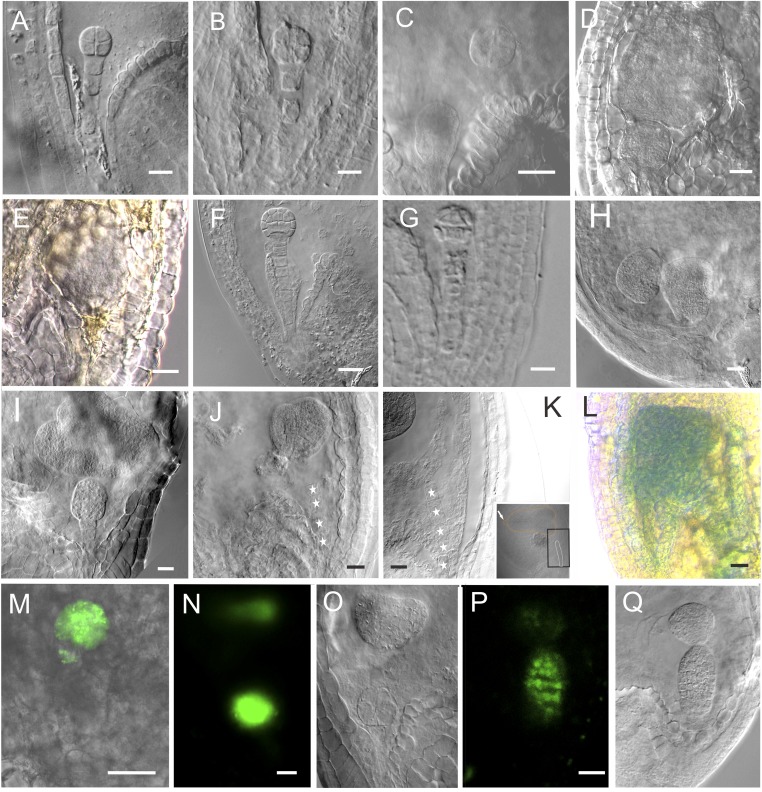
The suspensor and embryo proper development after in vivo laser ablation: the images of embryos in Fig. 1 without outlines. (A) The WT eight-celled embryo. (B) The eight-celled embryo after ablation, showing the embryo with two suspensor cells. (C) The laser-ablated eight-celled embryo after a 3-d culture. Note that the suspensor cells were completely removed from the embryo. The top suspensor cell has developed into an embryo (n = 14). (D) The laser-ablated eight-celled embryo developed into a big embryo without further differentiation after a 5-d culture (n = 39). The top suspensor cell has developed into a globular embryo with normal structure and morphology. (E) The brownish suspensor cannot be observed clearly. (F) The WT 32-celled embryo. (G) The laser-ablated 32-celled embryo after a 3-d culture. The suspensor cells were completely removed. Note the bottom morphology of the embryo. (H) The laser-ablated 32-celled embryo after a 3-d culture (n = 74). (I) The laser-ablated 32-celled embryo after a 5-d culture. The embryo was already differentiated with a clear apical–basal axis (n = 47). (J) The heart-stage embryo after ablation. (K) The laser-ablated heart-stage embryo after a 3-d culture. The image is an enlargement of the part in the black box. Arrow shows the orientation of the embryo with reference to the suspensor. Stars indicate suspensor cells (n = 66). (L) The embryo-proper marker ABI3 expressed in the embryo derived from the suspensor cell. (Scale bars, 20 μm.) (M–O) DRN::GFP expressed in both embryos. (O) The DIC image of the same embryos in N. Note that the GFP signal appeared only at the upper part of the heart-shaped embryo. (P) ATML1::H2B:GFP expressed in both embryos. (Q) The DIC image of the same embryos in P. (Scale bars, 20 μm.)
Fig. 2.
Hypophysis formation after laser ablation of suspensor and its role in embryo development. (A–C) Normal embryogenesis as control, showing hypophysis formation and typical morphology of embryo basal part. (D and E) Embryos with remaining suspensor cells, showing that the hypophysis formation and morphology of the embryo basal part is as normal as control. (F) The expression of WOX5 in globular embryo with suspensor cells. (G–I) Embryo without suspensor cells attached, showing the absence of the hypophysis and unique morphology of the embryo basal part. (J and K) The WOX5 do not express in the embryo without suspensor cells attached. (L and M) The magnification of the black boxes. (L) The hypophysis developed normally in the embryo with remnant suspensor cells (n = 26); note the typical cell organization there (dotted line). (M) The embryo proper without suspensor remnants lacked a hypophysis after 3-d culture. Note the first suspensor cell that started to divide (arrowhead) (n = 56).
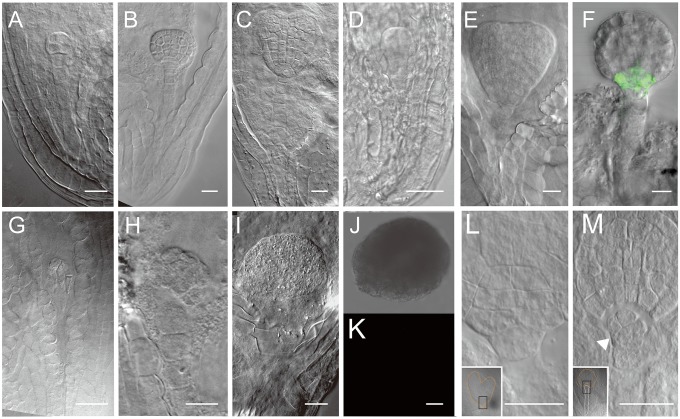
The embryos derived from suspensor cells showed typical embryo morphologies and structures, and the embryos have the distinct proper and suspensor domains (Fig. 1 D and I). The ABI3::GUS (ABI3, abscisic acid insensitive 3), ATML1::H2B:GFP (ATML1, Arabidopsis thaliana MERISTEM LAYER 1), and DRN::GFP (DRN, DORNRÖSCHEN) lines have been used as embryo marker lines to determine the identity of embryos derived from the suspensor (30–32). The results of such studies have shown that suspensor-derived embryos are similar to the first embryo with typical embryo characteristics and clear GUS or GFP signals (Fig. 1 L–P; Fig. S6).
Fig. S6.
DRN::GFP and ATML1::H2B:GFP expression in early embryos. (A–C) DRN::GFP expression in the early embryo proper. (D–F) ATML1::H2B:GFP expression in embryos. (Scale bars, 10 μm.)
These results confirm that, after the embryo–suspensor connection is removed, suspensors at stages before the globular embryo have embryogenic potential, despite the fact that the suspensor cell is still dividing (8-celled embryo) or has stopped division and differentiation (32-celled embryo). Thus, we confirmed the embryogenic potential of suspensor cells (at least in some developmental stages). Suspensors at stages after the heart-embryo stage no longer have embryogenic potential, although the embryo proper is similarly removed, indicating that suspensor cells at only some early stages (not all stages) possess embryogenic potential. Our data also confirm that the embryogenic potential is suppressed by the embryo proper because the second embryo generated from the suspensor occurs only after removing the embryo proper.
Developmental Prospects of Embryos at Different Stages After Disconnection from the Suspensor.
The in vivo embryo laser ablation system allows us to investigate the influence of the suspensor on the development of the embryo at different stages in the actual microenvironment in a seed. We chose to observe embryonic behavior at three stages of development after laser ablation of the suspensor. After breaking the connection between the embryo proper and the suspensor of eight-celled embryos (Fig. 1B) and after a 3-d culture, 11.69% (n = 77) of the embryos arrested (Fig. 1C), about 50.65% (n = 77) divided into globular embryo-like structures (Fig. 1D), 20.78% (n = 77) developed into cell clusters with an irregular shape, and 16.88% (n = 77) became brownish (Fig. 1E). After a 7-d culture, only 3.51% (n = 57) of the embryos developed into heart embryo-like structures. Most of the embryo still arrested at the globular embryo stage (Fig. 1C). After breaking the connection in 32-celled embryos (Fig. 1G), 73.81% (n = 84) of the embryos divided into globular embryos after a 3-d culture (Fig. 1H), 5.95% (n = 84) arrested at this stage, 8.33% (n = 84) developed into irregular structures, and 11.90% (n = 84) became brownish. After a 5-d culture, 57.81% (n = 64) of the embryos developed into heart-stage embryos (Fig. 1I). After cutting off the suspensor (Fig. 1J), 90.91% (n = 43) of the heart-stage embryos developed into torpedo embryos after culture for 3 d (Fig. 1K). Development of the 8- and 32-celled embryos without suspensors was slower than that of embryos with suspensors, especially for the 8-celled embryos. However, the developmental speeds of the heart-stage embryos with or without suspensors were similar.
These results show that the suspensor is critical for the early development of embryos. Embryos older than the 16-celled stage could independently develop in ovules without a suspensor, indicating that nutrient transportation by the suspensor (33) is no longer absolutely required for embryonic development at this stage.
Remaining Suspensor Cells Attached to Embryos After Laser Ablation Cannot Generate New Suspensors but May Ensure Hypophysis Formation.
During normal embryogenesis, the uppermost suspensor cell develops into a hypophysis and finally becomes incorporated into the embryo proper. Signaling between two domains (the suspensor and the embryo proper) connected by the hypophysis is required for precise patterning of the embryo’s apical–basal axis (34). Our laser microdissection technique created embryos with only the uppermost suspensor cell or with the top two suspensor cells. This allowed us to observe the fate of these remaining suspensor cells and to observe their influence on embryonic development.
This manipulation is difficult because we need to ensure that the remaining suspensor cells are intact and living to study their developmental behavior. Based on our conditions, embryos with some intact suspensor cells were obtained in 27.66% (n = 94) of ovules at the eight-celled embryo stage (Fig. 1B), and the uppermost cell of the suspensors were precisely removed in 28.72% (n = 94) of ovules at this stage (Fig. 1C). This allows for a comparison between the two types of material. Globular embryos with one or two suspensor cells were obtained in 34.25% (n = 73) of ovules (Fig. 1I), and precise removal of all suspensor cells was successfully performed in 36.99% (n = 73) of ovules at this stage (Fig. 1G).
When there are some remnant cells of suspensor, the embryo is more likely to develop normally (Fig. 1I; Fig. 2 D–F) and the apical–basal axis can be established, as in nonablated embryos, no matter how the embryos are oriented in the seeds (Fig. 1 I and K). As a reference, the original suspensor represents the original apical–basal orientation (Fig. 1K). Development of the hypophysis in these embryos was similar to embryos without laser ablation (n = 26; Fig. 1 I and K; Fig. 2 A–F and L), indicating that the intact suspensor is not required for hypothesis differentiation, but is influenced by their attachment to embryonic cells. However, when the top cell of the suspensor was ablated, embryos developed further but did not generate a hypophysis from adjacent embryo cells (n = 56; Fig. 2 G–I and M). We typically observed an absence of basal structure in these embryos (Fig. 2 G–I). Thus, unlike that in embryos with suspensor cells (Fig. 2F), in these embryos, the WOX5::GFP signal representing hypophysis (Fig. 2 J and K) was never observed. Consistent with this result, cell organization in the basal end of the embryos differed from that of normally developed embryos, and only cells derived from the embryo proper were observed around the absent hypophysis region (Fig. 2M). These embryos lacking the primary embryonic root structure were usually arrested in the globular stage (n = 34; Fig. 1D; Fig. 2 I and J), suggesting that the top suspensor cell may play a critical role in embryo differentiation.
When one or two suspensor cells remained attached to the embryos after laser ablation, the suspensor never regenerated into a new and intact suspensor (n = 56) during culture under the same conditions.
Auxin Accumulates at the Free End of the Suspensor After Laser Ablation.
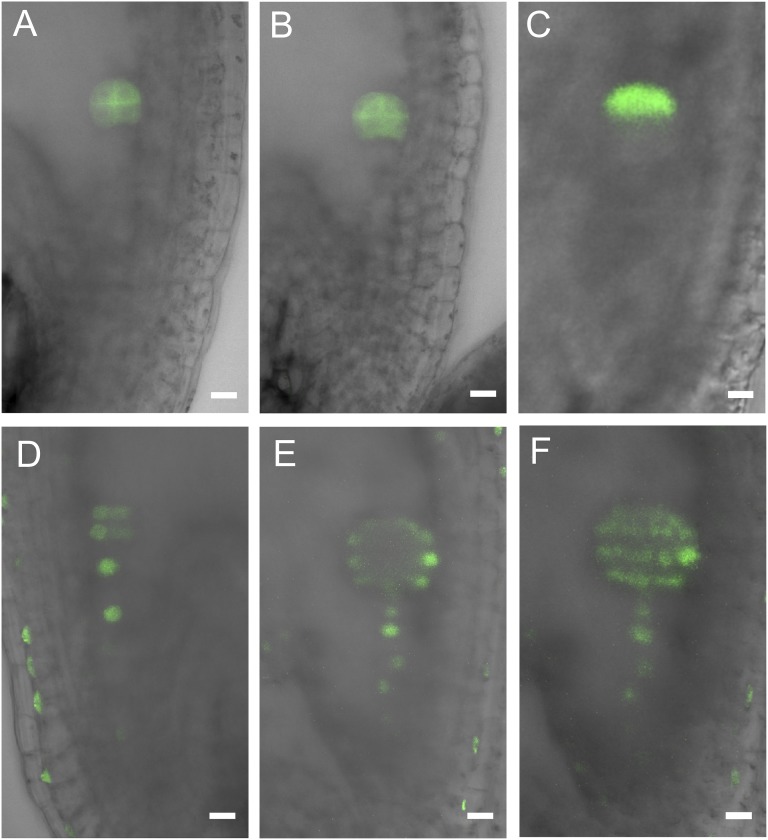
We used DR5rev::GFP as the auxin marker to investigate the distribution of auxin after microdissection and during culture of the suspensors and embryos. Proembryos in vivo showed different levels of auxin distribution in the suspensor and embryo domains. The auxin signal was observed mainly in embryos and rarely in suspensors (Fig. 3 A and A1). After laser ablation of the embryo, the auxin in the suspensor was gradually redistributed and accumulated mainly in the first suspensor cell after a 12-h culture. Later, auxin accumulation showed an obvious polar gradient from the free end of the suspensor to the bottom end after a 24 h culture (Fig. 3 B and B1; Fig. S7), and at the same time the top cell started to divide, which functioned as a novel apical cell. This suggests that auxin redistribution may play a critical role in the cell-fate transition from the suspensor cell to an initial embryo cell.
Fig. 3.
The influence of auxin distribution on triggering the first embryogenic cell division of the suspensor after laser ablation. (A–C1) The auxin distribution indicated by DR5rev::GFP expression. (A and A1) Auxin distribution differs in embryo and suspensor domains. GFP signal is rarely observed in suspensor cells. (B and B1) Auxin polar distribution in the suspensor cells after ablation and a 12-h culture. (C and C1) Auxin accumulates mainly in the uppermost cells of the suspensor after a 24-h culture. The uppermost cell starts to divide (arrowheads). (D and D1) The first longitudinal division occurs in the uppermost cell of the suspensor. D1 is the magnification of the boxed area in D. (E and E1) The division plane is inclined because the top suspensor cell is irregularly shaped. (F and F1) The first longitudinal division occurs in the second cell of the suspensor when the first suspensor cell is a nonembryogenic cell. (G–I) Development of the suspensors in the medium with 20 μM NPA (n = 39). The black box show relative position of suspensor to the first embryo. (G) The brownish and shrank suspensor cells. (H) The arrested suspensor. (I) Suspensor with irregular divisions. Asterisks indicate suspensor cells or suspensor-derived cells. (Scale bars, 20 μm.)
Fig. S7.
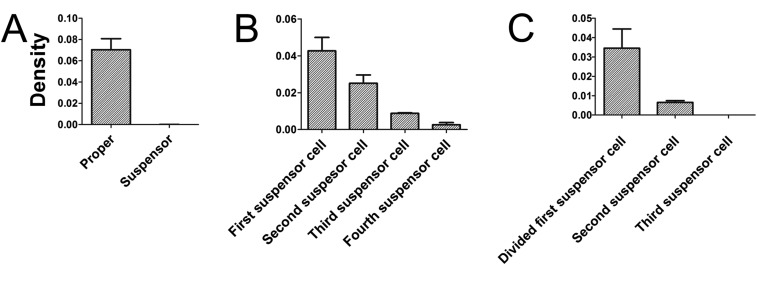
Auxin levels in embryo and suspensor cells after laser ablation. (A) Auxin level in normal embryo proper and suspensor at 32-celled embryo stage (n = 12). (B) Auxin level in different suspensor cells after laser ablation and culture for 12 h (n = 5). (C) Auxin level in different suspensor cells after laser ablation and culture for 24 h (n = 3). All of the analysis is based on partially isolated embryos as shown in Fig. 3.
Early Division Pattern of the Top Suspensor Cell After Laser Ablation May Mimic the Original Apical Cell.
To compare the top suspensor cell and original apical cell during embryo generation, we observed their cell division patterns. During early embryogenesis, one of the most important events is the orientation of the first cell division, which usually determines the developmental fate of the cell, especially in apical or basal cells. In Arabidopsis, the division of apical cells is vertical and the division of basal cells is transversal.
We found that the first vertical cell division usually occurred in the uppermost cell of the suspensor, which marks the cell differentiated into an embryo (Fig. 3 D and D1). The division could be modified because the top suspensor cell was irregularly shaped (Fig. 3 E and E1). When the cytoplasm of the top suspensor cell was thin after the laser ablation and remained undivided, the second suspensor cell acted as the initial embryo cell. In this case, the first vertical cell division was observed in this embryogenic cell (Fig. 3 F and F1), mimicking the behavior of the original apical cell. However, after the first cell division, subsequent cell divisions did not exactly follow the stationary pattern of the first embryo. This may be due to the irregular cell shape compared with the original apical cell of the proembryo.
In Vitro Culture of Suspensors Detached from the Embryo Does Not Generate Embryos.
Next, we further examined whether polar auxin transport plays an important role in triggering embryogenic development of the suspensor by an in vitro culture system. We cultured the isolated suspensors of both Arabidopsis (Fig. 4 A and B) and Brassica napus (Fig. 4 C and D) because we have efficient in vitro embryo culture systems for Brassica, which can be used as a control for our evaluation of the embryogenic potential of suspensor cells. The results showed that isolated suspensors without connections to the embryo proper did not generate new embryos. The suspensors typically shrank and ultimately degenerated after a 7-d culture (Fig. 4 E and F) (n = 87).
Fig. 4.
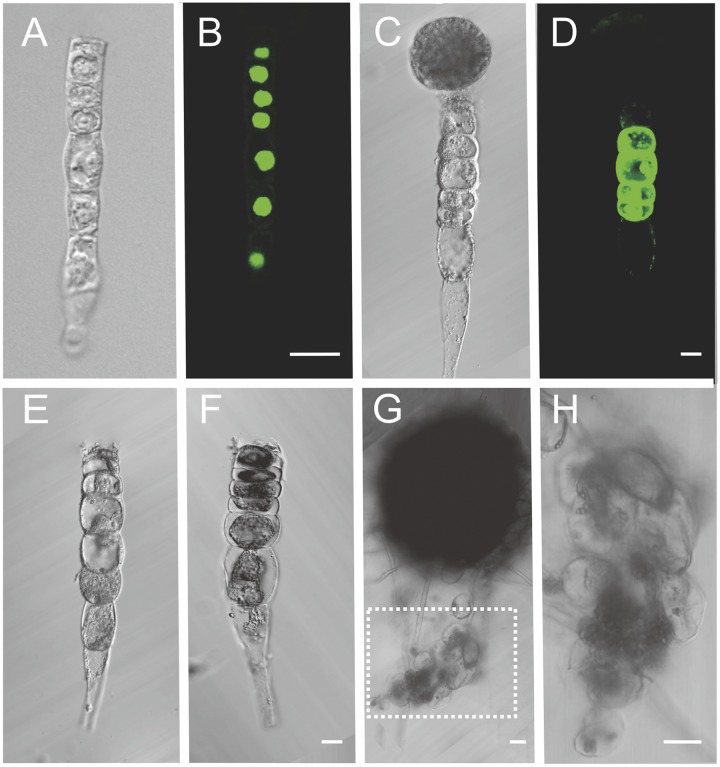
The in vitro culture of isolated suspensors from Arabidopsis and Brassica embryos. (A) The Arabidopsis suspensor of the ZC1::NLS: GFP transgenic lines after laser ablation. (B) The GFP signal of the suspensor in A. (C) The Brassica zygotic embryo after ablation. (D) The FDA (fluorescein diacetate) staining shows the suspensor cell viability. (E) The Brassica suspensor of zygotic embryo after ablation. (F) The suspensor cells of E show cytoplasm shrunken after a 7-d culture (n = 87). (G) The embryo and suspensor development after breaking the connection between them in the medium with hormone. The suspensor cells develop into a cell cluster, but not an embryo. (H) The enlargement of the cell cluster in the box of G. The cells are loosely organized. (Scale bars, 20 μm.)
We also examined different concentrations and combinations of plant hormones in the culture medium. We found that the suspensor cells developed into cell clusters and callus in the presence of 1.0 mg/L 2, 4-D and 0.3 mg/L kinetin (Fig. 4 G and H), but did not develop into embryos in any media that we examined. Hence, the embryogenic potential of suspensor cells was observed only in vivo and not in vitro when the suspensor was detached from both the embryo and the maternal tissue, although auxin was present in the growth environment. Thus, we propose that auxin polar transportation from maternal tissue to the suspensor cell might be essential for the generation of a second embryo.
We then cultured the laser-ablated ovules in the medium containing the auxin polar transport inhibitor NPA (N-1-naphthylphthalamic acid). After a 5-d culture, 61.54% of the suspensors became brownish and shrank, 30.77% arrested their development, and only 7.69% showed irregular cell division (n = 39). None of the suspensor cells differentiated into an embryo. This result again suggests that the polar auxin transport plays a critical role in triggering embryogenic potential of the suspensor cells.
Discussion
In Vivo Living Cell Laser Ablation Technique Confirms the Embryogenic Potential of Suspensor Cells, Which Is Suppressed by the Embryo During Normal Embryogenesis.
After fertilization, the zygote asymmetrically divides into two cells: the apical cell, which develops into an embryo proper, and the basal cell, which mainly forms a suspensor. Suspensor cells soon differentiate into specific cell types and no longer divide. The suspensor was traditionally considered a bridge between maternal tissue and the embryo for nutrient transport and a support structure for spatial positioning of the embryo in a seed. In some early reports, suspensor cells were shown to form an embryo after certain physical or chemical treatments of the embryo proper (16–18). This was also observed in some embryo-defective mutants (7, 12, 13, 15, 19, 20). Thus, it was proposed that the suspensor cells maintain embryogenic potential that is suppressed by the embryo during normal embryogenesis. Based on available data, a two-way communication model has been proposed to explain the possible interactions between the embryo proper and the suspensor. The suspensor provides nutrients and growth regulators to the embryo proper. Conversely, the embryo releases negative regulators that suppress the embryogenic potential of suspensor cells, thereby maintaining suspensor cell identity (3). Because traditional methods are unable to eliminate the effects of the remaining embryo proper and gene mutations in suspensors on suspensor cell identity, more direct evidence supporting the hypothesis is required. Fortunately, the in vivo living cell laser ablation system can be used to explore this hypothesis. Precise laser ablation allows exact microdissection and ensures that cells after laser ablation can grow in the same microenvironment to minimize the effects of manipulation and thus offers a unique opportunity for observing the in vivo behavior of embryo or suspensors during development. Using this approach, we confirmed that Arabidopsis suspensor cells have embryogenic potential. After detaching from the embryo proper, the top suspensor cell may develop into a second embryo with the same morphology as the original embryo. This suggests that, during normal embryogenesis in a seed, the embryogenic potential of suspensor cells is suppressed by the embryo proper. Once this suppression is removed, the top suspensor cell can develop into an embryo. This seems to be a “back-up system” for seed formation established during plant evolution to ensure embryonic generation if the apical lineage fails to form an embryo.
As shown in Fig. S8, the embryogenic potential of the suspensor cell depends on the developmental stage. The suspensors of 8- and 32-celled embryos developed into second embryos, whereas those at or beyond the heart stage did not. This may be because suspensors at this stage are already entering the process of programmed cell death (35) and are unable to reprogram their fate, although the cells are still living.
Fig. S8.
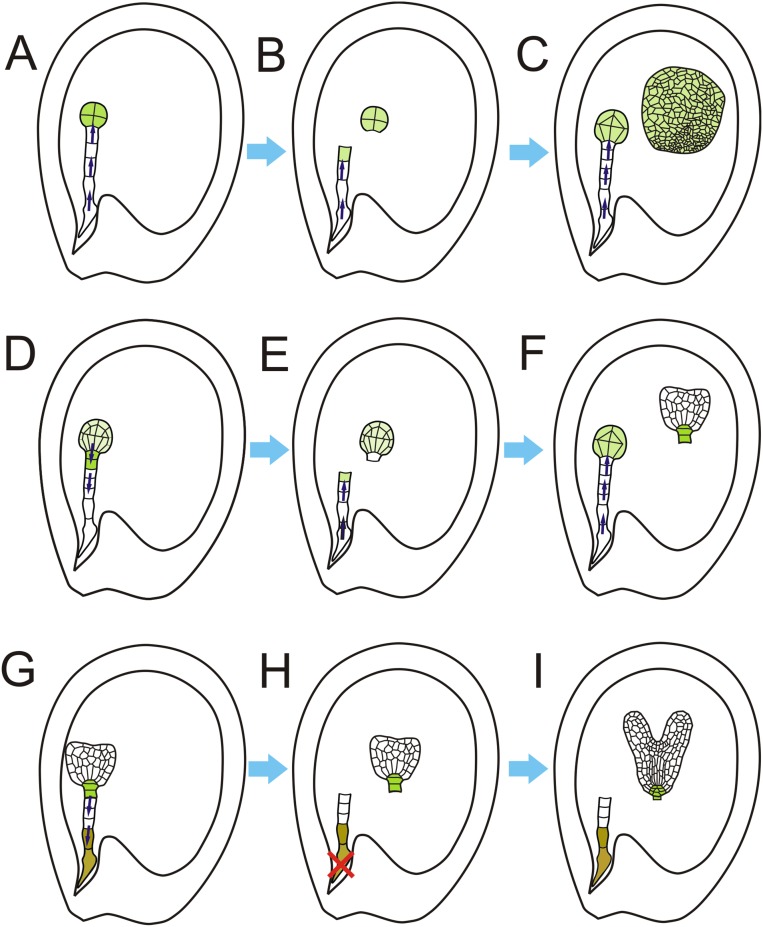
Main stages of suspensor development after laser ablation and the putative mechanism for suspensor cell-fate transition. (A) The eight-celled embryo stage. The auxin accumulates in the embryo proper during this stage (the green area). (B) The eight-celled embryo after ablation. The auxin accumulates in the uppermost cell of the suspensor. The arrows indicate the direction of auxin transport from the suspensor to the top of the suspensor. (C) The ablated eight-celled embryo after culture. The suspensor cell generates the second embryo with similar auxin distribution. (D) The 32-celled embryo stage. Auxin start to transport downward. (E) The 32-celled embryo stage after microdissection. The arrows show the orientation of auxin transport; the auxin accumulates in the uppermost cell of the suspensor. (F) The suspensor cell develops into a second embryo after ablation. (G) The heart-stage embryo. (H) The heart-stage embryo after microdissection. (I) The suspensor does not develop any further after microdissection, but the embryo develops well.
Auxin Accumulation in the Top Suspensor Cell Likely Results in Cell-Fate Transition and the Generation of a Second Embryo.
During normal embryogenesis, auxin polar transportation is from maternal tissue to the embryo via the suspensor, and, after the 32-cell embryo stage, the embryo proper synthesizes sufficient auxin and then transports the auxin downward to the hypophysis (33). Our previous work in roots revealed that auxin distribution in specific cells decided the cell fate and that changes in the auxin level triggered a cell-fate transition from a differentiated cell to an initial cell of a lateral primordial cell (36).
In this study, after removing the connection between the suspensor and early embryo, auxin transport was completely abrogated. In this case, auxin transportation from maternal tissue to the embryo is interrupted at the cutting end of the suspensor, and auxin accumulates in the uppermost cells of the suspensor instead of the embryo proper. The suspensor cell accumulating auxin then starts to divide and ultimately develops into a second embryo, indicating that redistribution of auxin likely resulted in the developmental fate transition of the top suspensor cell. With the low level of auxin exiting the cells, the suspensor tends to become differentiated and functionally specialized. Once the embryo is removed and auxin transportation is interrupted, the suspensor cell-fate transition occurs when auxin concentrations increase in the cell.
Our in vitro culture of isolated suspensors and NPA-treated ovules supports the concept that upward, polar transport of auxin is critical for the cell-fate transition of top suspensor cells. Embryogenesis was never observed in isolated suspensors in the presence or absence of auxin. In the presence of plant hormones including auxin, the media triggered suspensor cell division and the formation of cell clusters but did not generate embryos. In addition, when laser-ablated ovules were cultured in the medium with NPA, suspensor cells never generated the second embryos. These evidences indicate that proper levels of plant hormones, and their polar transport, are critical to the cell-fate transition of suspensor cells for embryogenesis.
In fact, it has been reported that the inhibition of auxin responsive process in the suspensor caused excessive divisions in suspensor cells (37). It was also reported that some cell-autonomous and lineage-specific ARFs (auxin response factor) and their inhibitor IAA10 were involved in preventing suspensor cell-fate transition (37). In the normal embryogenesis process, auxin is obviously accumulated in the top suspensor cell or hypophysis initial cell (33). Interestingly, it is just this cell that changes its cell fate and finally develops into hypophysis. These works actually have already implied the involvement of auxin in suspensor cell-fate determination. Our work reveals that auxin accumulation in the newly formed top suspensor cell after laser ablation is coupled with its cell-fate transition to the embryogenic cell identity and thus provides experimental evidence for the above deductions. However, the auxin-related regulatory mechanism in this process still remains to be elucidated. The previous work on the cell-type dependent auxin response may offer a valuable clue to investigate the cell-fate transition of the suspensor cells. As proposed previously, in addition to auxin, the upper suspensor cell may receive other signals that could modify its auxin-dependent gene expression and regulate its cell-fate transition (37). Thus, the suspensor ARF and IAA10 activity and auxin-activated gene expression will be interesting to analyze after laser ablation.
Materials and Methods
Detailed information on material and methods is provided in SI Material and Methods, which includes detailed description on the plant material and growth conditions; in vivo living cell laser ablation (Fig. S9); culture of isolated suspensors, embryos, and ovules; ovule clearing and GUS staining; and the auxin-level indication and evaluation.
Fig. S9.
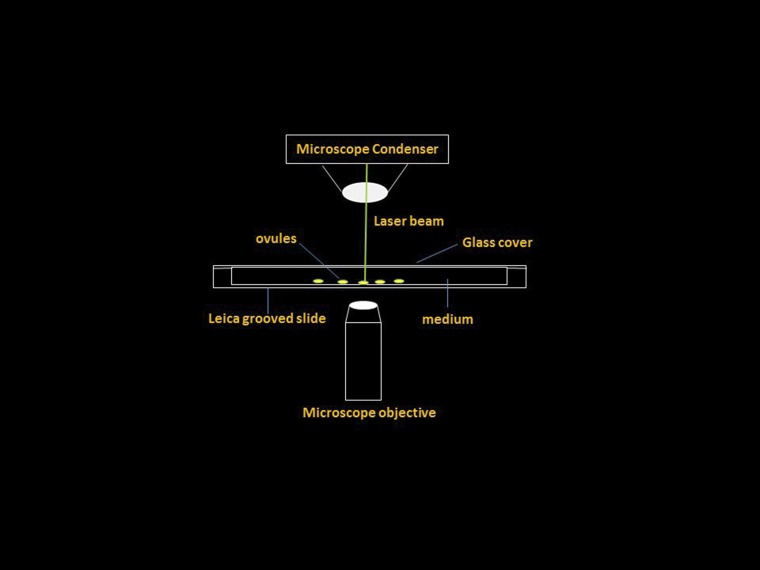
Schematic diagram of laser ablation. The detailed parameters are described in Materials and Methods.
SI Materials and Methods
Plant Material and Growth Conditions.
Arabidopsis thaliana were planted in 9-cm pots containing moistened vermiculite in a greenhouse at 22 ± 1 °C, 16/8 h (light/dark). The seeds of ATML1::H2B:GFP, DR5rev::GFP, DR5rev::3XVENUS-N7, and WOX5::GFP marker lines were grown under the same condition.
For ZC1::NLS:GFP transformation, the constructs were introduced into Agrobacterium tumefaciens strain LBA4404. The promoter sequence of ZC1 is the same as that used in ZC1::PA:GFP (38). Arabidopsis plants were transformed by using the floral dip method (39).
The embryo proper marker line with ABI3::GUS was crossed with the ZC1::NLS:GFP line. The double homozygous lines were used for laser ablation and GUS staining.
In Vivo Living Cell Laser Ablation.
The experiment preparation was performed on an ultra-clean bench. The siliques were first sterilized with 4% Naclo and 1% Triton for 10 min and then were washed three times by sterilized water, 3 min each time. Ovules were carefully isolated from the siliques with ophthalmic knives under a stereomicroscope (SUGUANG XTB-1B) and were put in a 3.5-cm glass petri dish containing 1.5 mL B5 medium with 7% sucrose.
For laser ablation, a small drop of B5 medium with 7% sucrose was made in the Leica grooved slide, which was bottomed by 1.5 μm PET film. The ovules were transferred into the drop by a glass micropipette. Then the ovules in the droplets were adjusted to the best position and orientation convenient for observation and laser ablation. Finally, the slide was covered with a 24- × 50-mm coverslip and sealed with sterilized water.
The laser beam usually targeted the nuclear GFP signal of the uppermost suspensor cell (Fig. S2 A and A1) or other suspensor cells according to experimental requirement. Ablation of the chosen cell was traced under the 60× objective lens of the microscope during the manipulation process. For ablation application, the density was adjusted between 35 and 42, and the aperture was adjusted between 5 and 9 to ensure proper and precise laser ablation. The procedure of the laser ablation is illustrated in Fig. S9. After the ablation, the ovules were transferred onto the B5 medium containing 10% sucrose, 400 mg/L glutamine, and 0.3% Phyta gel in a petri dish. The petri dishes were put in a dark incubator at 25 °C for ovule culture.
Culture of Isolated Suspensors, Embryos, and Ovules.
The Arabidopsis embryo was separated with steel needles in the enzyme solution with 1% cellulose (Cellulase R–10), 0.8% Macerozyme (Macrozyme R–100), and 13% mannitol at pH 5.4 under a microscope. The Brassica globular embryos were isolated with a flat-top glass rod by gently squeezing the ovule in the B5 medium with 7% mannitol under the stereomicroscope. The separation of the embryos proper and suspensors was performed using AS LMD (Lecia). The Arabidopsis and Brassica embryos and suspensors were cultured together in the same plate, as described previously (40).
The ovules were cultured according to previous method (29) with some modifications. The double concentration of B5 solution containing 20% sucrose and 800 mg/L glutamine was prepared and filter-sterilized. Meanwhile, the equal volume of 0.6% Phyta gel was prepared and autoclaved. The solutions were adjusted with KOH to pH 5.9. The two solutions were then mixed when the temperature of the 0.6% Phyta gel was at 50 °C. About 1.5 mL of the medium was added in each of the 3.5-cm diameter petri dishes, which were then sealed with Parafilm. The petri dishes were put in a refrigerator at 4 °C for medium solidification. The laser-ablated and nonablated ovules were cultured on the medium in a dark incubator at 25 °C.
Ovule Clearing and GUS Staining.
Ovule clearing was performed as described previously (41). GUS staining was performed according to a previous method (42). The samples were observed under an inverted microscope (Olympus IMT-2). The images were collected using a cooled charge-coupled device camera (Cool SNAP HQ cooled CCD).
Auxin-Level Indication and Evaluation.
The auxin level in suspensor cells was determined by the DR5rev:GFP signal density, which is indicated by the integrated optical density area (43). The density was analyzed with the Image Pro Plus 6.0. The negative control is the wild-type embryo with no GFP signal. The positive control is the DR5rev:GFP expression in globular embryo. The images were collected by laser scanning confocal microscopy (Leica TCS-SP5) under the same condition. The samples for statistical analysis were cultured in the same petri dish in each of the measurements.
Acknowledgments
We thank Professor Jérôme Giraudat of the Centre National de la Recherche Scientifique for the ABI3::GUS Arabidopsis seeds; Professor Wolfgang Werr (Institute of Developmental Biology, Cologne Biocenter) for the seeds with DRN::GFP; and Professor Jian Xu for the seeds with DR5::GFP (Singapore National University). This work was supported by the “Major Research Plan from the Ministry of Science and Technology of China” (2014CB943400) and the National Science Foundation of China (31430007; 31170297).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508651112/-/DCSupplemental.
References
- 1.Babu Y, Musielak T, Henschen A, Bayer M. Suspensor length determines developmental progression of the embryo in Arabidopsis. Plant Physiol. 2013;162(3):1448–1458. doi: 10.1104/pp.113.217166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umehara M, Kamada H. Development of the embryo proper and the suspensor during plant embryogenesis. Plant Biotechnol. 2005;22(4):253–260. [Google Scholar]
- 3.Schwartz BW, Vernon DM, Meinke DW. Development of the suspensor: Differentiation, communication, and programmed cell death during plant embryogenesis. In: Larkins BA, Vasil IK, editors. Advances in Cellular and Molecular Biology of Plants. Springer; Heidelberg, Germany: 1997. pp. 53–72. [Google Scholar]
- 4.Kawashima T, Goldberg RB. The suspensor: Not just suspending the embryo. Trends Plant Sci. 2010;15(1):23–30. doi: 10.1016/j.tplants.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Raghavan V. Double Fertilization: Embryo and Endosperm Development in Flowering Plants. Springer; Heidelberg, Germany: 2006. Life and times of the suspensor cell signaling between the embryo and suspensor; pp. 81–100. [Google Scholar]
- 6.Zhukova GY. Ultrastructural characteristics of suspensor. Embryol Flowering Plants. 2006;2:202–208. [Google Scholar]
- 7.Yeung EC, Meinke DW. Embryogenesis in angiosperms: Development of the suspensor. Plant Cell. 1993;5(10):1371–1381. doi: 10.1105/tpc.5.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stadler R, Lauterbach C, Sauer N. Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol. 2005;139(2):701–712. doi: 10.1104/pp.105.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piaggesi A, Picciarelli P, Lorenzi R, Alpi A. Gibberellins in embryo-suspensor of Phaseolus coccineus seeds at the heart stage of embryo development. Plant Physiol. 1989;91(1):362–366. doi: 10.1104/pp.91.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozhkov PV, Filonova LH, Suarez MF. Programmed cell death in plant embryogenesis. Curr Top Dev Biol. 2005;67:135–179. doi: 10.1016/S0070-2153(05)67004-4. [DOI] [PubMed] [Google Scholar]
- 11.Bozhkov PV, et al. Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proc Natl Acad Sci USA. 2005;102(40):14463–14468. doi: 10.1073/pnas.0506948102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsden MP, Meinke DW. Abnormal development of the suspensor in an embryo-lethal mutant of Arabidopsis thaliana. Am J Bot. 1985;72(11):1801–1812. [Google Scholar]
- 13.Schwartz BW, Yeung EC, Meinke DW. Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development. 1994;120(11):3235–3245. doi: 10.1242/dev.120.11.3235. [DOI] [PubMed] [Google Scholar]
- 14.Vernon DM, Meinke DW. Embryogenic transformation of the suspensor in twin, a polyembryonic mutant of Arabidopsis. Dev Biol. 1994;165(2):566–573. doi: 10.1006/dbio.1994.1276. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JZ, Somerville CR. Suspensor-derived polyembryony caused by altered expression of valyl-tRNA synthetase in the twn2 mutant of Arabidopsis. Proc Natl Acad Sci USA. 1997;94(14):7349–7355. doi: 10.1073/pnas.94.14.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhundova GG, Grinikh LI, Shevchenko VV. Development of Arabidopsis thaliana embryos after gamma irradiation of plants in the generative phase. Sov J Dev Biol. 1979;9(5):33–45. [PubMed] [Google Scholar]
- 17.Haccius B. Restitution in acidity-damaged plant embryos regeneration or regulation. Phytomorph. 1963;13:107–115. [Google Scholar]
- 18.Haccius B, Reichert H. Restitutionserscheinungen an pflanzlichen meristemen nach röntgenbestrahlung [Restitution phenomena of plant meristems after X-ray irradiation] Planta. 1964;62(4):355–372. German. [Google Scholar]
- 19.Yadegari R, et al. Cell differentiation and morphogenesis are uncoupled in Arabidopsis raspberry embryos. Plant Cell. 1994;6(12):1713–1729. doi: 10.1105/tpc.6.12.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanmartín M, et al. A molecular switch for initiating cell differentiation in Arabidopsis. Curr Biol. 2011;21(12):999–1008. doi: 10.1016/j.cub.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 21.Thoma S, et al. Tissue-specific expression of a gene encoding a cell wall-localized lipid transfer protein from Arabidopsis. Plant Physiol. 1994;105(1):35–45. doi: 10.1104/pp.105.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weijers D, Van Hamburg JP, Van Rijn E, Hooykaas PJ, Offringa R. Diphtheria toxin-mediated cell ablation reveals interregional communication during Arabidopsis seed development. Plant Physiol. 2003;133(4):1882–1892. doi: 10.1104/pp.103.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baroux C, Blanvillain R, Moore IR, Gallois P. Transactivation of BARNASE under the AtLTP1 promoter affects the basal pole of the embryo and shoot development of the adult plant in Arabidopsis. Plant J. 2001;28(5):503–515. doi: 10.1046/j.1365-313x.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 24.Stich M, et al. Live cell catapulting and recultivation. Pathol Res Pract. 2003;199(6):405–409. doi: 10.1078/0344-0338-00437. [DOI] [PubMed] [Google Scholar]
- 25.Berger F, Taylor A, Brownlee C. Cell fate determination by the cell wall in early fucus development. Science. 1994;263(5152):1421–1423. doi: 10.1126/science.263.5152.1421. [DOI] [PubMed] [Google Scholar]
- 26.Bouget FY, Berger F, Brownlee C. Position dependent control of cell fate in the Fucus embryo: Role of intercellular communication. Development. 1998;125(11):1999–2008. doi: 10.1242/dev.125.11.1999. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg C, Willemsen V, Hage W, Weisbeek P, Scheres B. Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature. 1995;378(6552):62–65. doi: 10.1038/378062a0. [DOI] [PubMed] [Google Scholar]
- 28.Reinhardt D, Frenz M, Mandel T, Kuhlemeier C. Microsurgical and laser ablation analysis of interactions between the zones and layers of the tomato shoot apical meristem. Development. 2003;130(17):4073–4083. doi: 10.1242/dev.00596. [DOI] [PubMed] [Google Scholar]
- 29.Sauer M, Friml J. In vitro culture of Arabidopsis embryos within their ovules. Plant J. 2004;40(5):835–843. doi: 10.1111/j.1365-313X.2004.02248.x. [DOI] [PubMed] [Google Scholar]
- 30.Parcy F, et al. Regulation of gene expression programs during Arabidopsis seed development: Roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell. 1994;6(11):1567–1582. doi: 10.1105/tpc.6.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.To A, et al. A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell. 2006;18(7):1642–1651. doi: 10.1105/tpc.105.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada S, Jürgens G. Transcriptional regulation of epidermal cell fate in the Arabidopsis embryo. Development. 2007;134(6):1141–1150. doi: 10.1242/dev.02803. [DOI] [PubMed] [Google Scholar]
- 33.Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426(6963):147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 34.Harada JJ. Signaling in plant embryogenesis. Curr Opin Plant Biol. 1999;2(1):23–27. doi: 10.1016/s1369-5266(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 35.Zhao P, et al. A bipartite molecular module controls cell death activation in the basal cell lineage of plant embryos. PLoS Biol. 2013;11(9):e1001655. doi: 10.1371/journal.pbio.1001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J, Wei J, Xu J, Sun MX. Inducible knock-down of GNOM during root formation reveals tissue-specific response to auxin transport and its modulation of local auxin biosynthesis. J Exp Bot. 2014;65(4):1165–1179. doi: 10.1093/jxb/ert475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rademacher EH, et al. Different auxin response machineries control distinct cell fates in the early plant embryo. Dev Cell. 2012;22(1):211–222. doi: 10.1016/j.devcel.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Li S, He Y, Zhao J, Zhang L, Sun MX. Polar protein transport between apical and basal cells during tobacco early embryogenesis. Plant Cell Rep. 2013;32(2):285–291. doi: 10.1007/s00299-012-1362-5. [DOI] [PubMed] [Google Scholar]
- 39.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 40.Tang XC, He YQ, Wang Y, Sun M-X. The role of arabinogalactan proteins binding to Yariv reagents in the initiation, cell developmental fate, and maintenance of microspore embryogenesis in Brassica napus L. cv. Topas. J Exp Bot. 2006;57(11):2639–2650. doi: 10.1093/jxb/erl027. [DOI] [PubMed] [Google Scholar]
- 41.Schneitz K, Hülskamp M, Pruitt RE. Wild‐type ovule development in Arabidopsis thaliana: A light microscope study of cleared whole‐mount tissue. Plant J. 1995;7(5):731–749. [Google Scholar]
- 42.Stangeland B, Salehian Z. An improved clearing method for GUS assay in Arabidopsis endosperm and seeds. Plant Mol Biol Rep. 2002;20(2):107–114. [Google Scholar]
- 43.Wang CJ, et al. Survivin expression quantified by Image Pro-Plus compared with visual assessment. Appl Immunohistochem Mol Morphol. 2009;17(6):530–535. doi: 10.1097/PAI.0b013e3181a13bf2. [DOI] [PubMed] [Google Scholar]