Significance
Invariant natural killer T (iNKT) cells can facilitate B-cell responses by enhancing helper signals from protein-specific T cells or independently induce a B-cell developmental program; however, key differences in humoral memory after iNKT-cell help remain unclear. We determined that, unlike protein-specific T-cell help, cognate iNKT-cell help expands a large number of IL-10–producing B10 regulatory cells. These findings have broad implications for the types of B cells that may be generated when synthetic iNKT glycolipid ligands are used as vaccine adjuvants and also, outline a direct means to elicit B regulatory cells, which are increasingly being pursued as immunotherapeutic targets for protection against autoimmune disease.
Keywords: iNKT cells, marginal zone B cells, B regulatory cells, iNKT follicular helper cells, IL-10
Abstract
Successful induction of B-cell activation and memory depends on help from CD4+ T cells. Invariant natural killer T (iNKT) cells (glycolipid-specific, CD1d-restricted innate lymphocytes) provide both cognate (direct) and noncognate (indirect) helper signals to enhance B-cell responses. Both forms of iNKT-cell help induce primary humoral immune responses, but only noncognate iNKT-cell help drives humoral memory and plasma cells. Here, we show that iNKT cognate help for B cells is fundamentally different from the help provided by conventional CD4+ T cells. Cognate iNKT-cell help drives an early, unsustained germinal center B-cell expansion, less reduction of T follicular regulatory cells, an expansion of marginal zone B cells, and early increases in regulatory IL-10–producing B-cell numbers compared with noncognate activation. These results are consistent with a mechanism whereby iNKT cells preferentially provide an innate form of help that does not generate humoral memory and has important implications for the application of glycolipid molecules as vaccine adjuvants.
Most successful human vaccines are effective because they induce long-term humoral memory. Establishing B-cell memory depends on a nuanced interplay between B, T, stromal, and antigen-presenting cells (APCs) with antigen in specialized germinal center structures within the spleen (1). We and others have recently found that, in addition to conventional CD4+ T helper cells, invariant natural killer T (iNKT) cells can also provide help to B cells (2–4). iNKT cells are innate, memory-like T cells with a limited T-cell receptor (TcR) repertoire that recognizes CD1d-presented glycolipids. iNKT cells are rapidly activated by the marine sponge-derived glycolipid α-galactosylceramide (αGalCer) (5, 6). iNKT cells that specifically provide help to B cells, termed iNKT T follicular helper (iNKTFH) cells (7), express many of the same surface proteins and transcription factors that identify CD4+ T follicular helper (TFH) cells, including CXCR5, PD-1, ICOS, and BCL6, and are found in germinal centers (7). iNKT cells providing either cognate or noncognate help to B cells enhance primary, antigen-specific antibody production (8) through production of IFN-γ, IL-21, and B cell-activating factor (BAFF), but only during cognate help do the cytokines come exclusively from iNKT cells (2, 9, 10). iNKT cells activated by nitrophenyl (NP)-haptenated αGalCer are induced to provide cognate help to NP-specific B cells (2). iNKT cells activated by αGalCer plus a haptenated protein [NP-keyhole limpet hemocyanin (KLH)] induce noncognate help by licensing dendritic cells (DCs), recruiting conventional TFH cells, and indirectly enhancing NP-specific B cells (2–4).
Germinal center B cells rely on cognate interactions with TFH cells, including binding of MHC class II, CD40L, ICOS, and B7.1 or B7.2 on B cells with their counterpart receptors on TFH cells, to progress through germinal centers and differentiate into antibody-secreting plasma cells (PCs) or memory cells (11–13). TFH cells secrete IL-4, IFN-γ, and IL-21, which contribute to isotype class switching, germinal center maturation (14–17), and reciprocal regulation of TFH cells (18). However, what specifically steers B cells to differentiate into PCs, germinal center cells, or memory B cells remains unknown (19). Immune regulators PD-L1, PD-L2, and PD-1 were recently found to control germinal center B-cell death and PC development (20). PD-L1 is constitutively expressed on T and B cells, but PD-L2 is exclusively and inducibly expressed on APCs, such as macrophages, DCs, and B cells (21). PD-1 is expressed on T and iNKT cells (22) and key for moderating TFH cell development and function, including selection and survival in the germinal center (23). PD-1 is a negative regulator that dampens TcR signals (24–26). PD-1 up-regulation is evidence of continuous TcR triggering by cognate antigen (27) and can be a sign of T-cell exhaustion (28), but the many roles of PD-1 in germinal center responses remain to be completely defined. PD-1–deficient mice have increased TFH cells, and interrupting the interaction between PD-1 and its ligand(s) has led to increased humoral immune responses (29, 30). However, others found that inhibiting PD-1 signaling reduces DC and PC responses (20, 31). Both of these conclusions are complicated by the fact that PD-1 is highly expressed by CD4+CXCR5+ICOS+ T follicular regulatory (TFR) cells, which (32) express Foxp3+ and inhibit germinal center responses (33–35). At the same time, IL-2 has been found to signal through CD25 to negatively regulate bcl-6 expression and inhibit TFH cell development (36). This effect was mediated independently of Treg cells, relying instead on indirect up-regulation of CD25 on T cells.
It remains to be investigated how many of these mechanisms apply to B cells receiving iNKT-cell help. We have found that the primary IgG and IgM antibody titers are similarly induced by cognate and noncognate iNKT-cell help and depend on IL-21, BAFF, IFN-γ, CD40L, and ICOS engagement. However, only noncognate iNKT-cell help induces antigen-specific humoral memory (7, 9). These results are relevant to human vaccine development given the increasing interest in αGalCer as an adjuvant. We found that iNKTFH cells expand with similar kinetics when providing cognate or noncognate help, but conventional TFH cells only expand when protein is present during noncognate help. Clearly, TFH cells provide unique humoral memory signals that are not provided by iNKTFH cells, but the nature of these signals remains unknown. We show here that germinal center B cells multiply more rapidly after cognate iNKT-cell help than after noncognate help, but they are not sustained and undergo death before completing productive maturation beyond the germinal center. At the same time, we detected more protein-specific TFR cells after cognate iNKT-cell help than after noncognate iNKT-cell help, consistent with a regulatory milieu. Cognate iNKT-cell help also preferentially expands marginal zone (MZ) B cells and drives greater numbers of IL-10+ regulatory B10 cells than noncognate activation. These data support the notion that innate, cognate iNKT-cell help induces a rapid, primary antibody response by innate B cells with an increased presence of TFR. However, this innate response can be overridden by inflammatory responses provided by adaptive peptide-specific T cells to drive a B memory response. These results will inform future efforts to develop αGalCer as a vaccine adjuvant.
Results
Noncognate iNKT Cell Help Induces Higher Numbers of TFH Cells than Cognate iNKT-Cell Help.
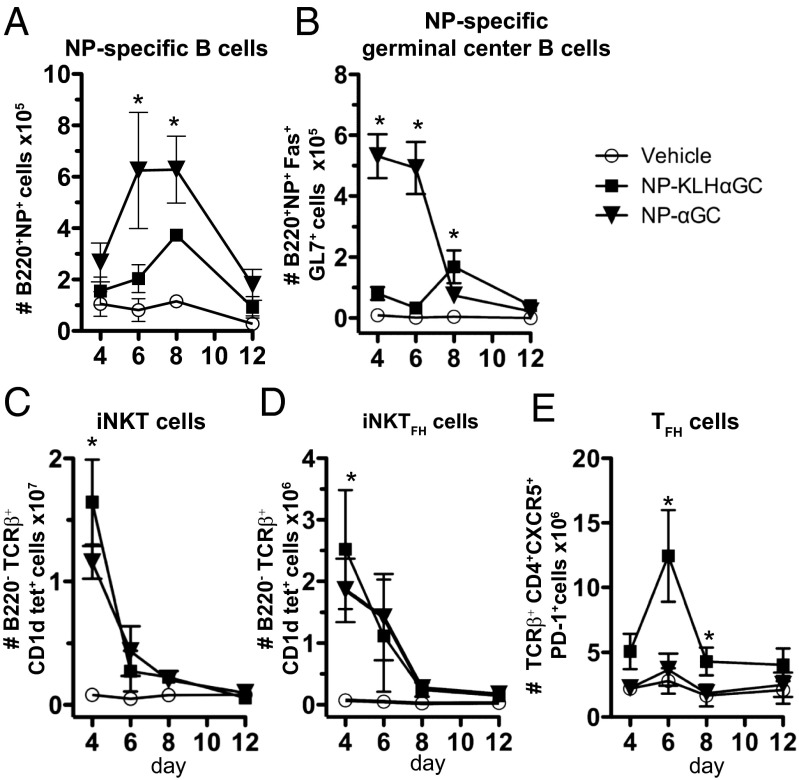
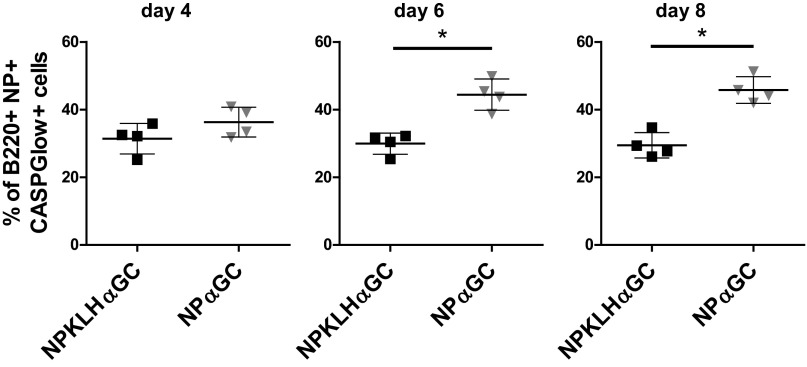
Noncognate iNKT-cell help for B cells drives a greater humoral memory response than cognate iNKT-cell help (9). To determine the relative contribution of cellular subsets to these two types of responses, we immunized mice with NP-KLH plus lipid (αGalCer) or NPαGalCer and monitored expansion of germinal center B, iNKT, iNKTFH, or TFH cells. The number of NP-specific B cells gradually increased from day 4 to day 8, but NP-specific B-cell numbers were significantly higher after immunization with NPαGalCer compared with NP-KLHαGalCer at day 6 and 8 (Fig. 1A). Cognate iNKT-cell help induced a dramatic early expansion of NP-specific germinal center B cells (B220+NP+Fas+GL7+) at days 4 and 6, whereas noncognate help resulted in a later, modest response with increases in germinal center B-cell numbers evident at days 8 and 12 (Fig. 1B). Cognate iNKT cell antigen does not induce B-cell memory, and therefore, higher numbers of NP-specific B and early germinal center B cells were unexpected. The precipitous drop in germinal center B-cell numbers 6 d after cognate antigen immunization coincides with an increase in apoptotic NP-specific B cells (Fig. S1).
Fig. 1.
Noncognate iNKT-cell help induces more TFH cells and later development of germinal center B cells than cognate help. Splenocytes from C57BL/6 mice immunized with vehicle [PBS/0.1% (wt/vol) BSA], 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), or 0.5 μg NPαGalCer (NPαGC) were assessed by FACS analysis for numbers of (A) NP-specific B cells, (B) NP-specific germinal center B cells, (C) iNKT cells, (D) iNKTFH cells, or (E) TFH cells. All cells were measured at days 4, 6, 8, and 12 after immunization. Each data point is a representative of two to four experiments (n = 8–21 mice per group for days 4–8) or one experiment (n = 4 mice per group for day 12). *P ≤ 0.05.
Fig. S1.
NPαGalCer (NP αGC) immunization induces more apoptotic NP-specific B cells than NP-KLH plus αGalCer (αGC). C57BL/6 mice were immunized i.v. with 0.5 μg NPαGC or 100 μg NP-KLH plus 0.5 μg αGC. NP-specific apoptotic B cells in the spleen were identified 4, 6, and 8 d after immunization as B220+NP+ CASPGlow+ using FACS analysis and the CASPGlow Active Caspase Staining Kit [BioVision; representative of two to three experiments for days 4 and 8 (n = 7–11 mice) and one experiment for day 6 (n = 4 mice)]. *P ≤ 0.05.
NP-KLHαGalCer immunization induced slightly more iNKT (B220−TCRβ+CD1dtet+) and iNKTFH (B220−TCRβ+CD1dtet+CXCR5+PD-1+) cells than NPαGalCer at day 4 (Fig. 1 C and D). The most dramatic difference was the significant expansion of TFH cells (B220−TCRβ+CD4+CXCR5+PD-1+) over the entire NP-KLHαGalCer kinetic course, with a peak at day 6, whereas NPαGalCer induced no detectable increase in TFH cells during the entire monitoring period (Fig. 1E). Thus, TFH cells only expanded in the presence of protein antigen (plus αGalCer). To consider other factors that influence the survival and function of antigen-specific B cells, we measured expression of BAFF by iNKT, iNKTFH, and TFH cells after immunization. NP-KLHαGalCer and NPαGalCer induced equal BAFF expression by iNKT, iNKTFH, and TFH cells at day 4, 6, or 8 (Fig. S2 A–C). As shown for TFH cells (37), BAFF was preferentially expressed by the iNKTFH cell subset but was not different between the two immunization groups (Fig. S2 D and E). These results indicate that cognate help drives an early, abortive germinal center B-cell response, but only noncognate iNKT-cell help leads to expansion of TFH cells and a mature germinal center B-cell response.
Fig. S2.
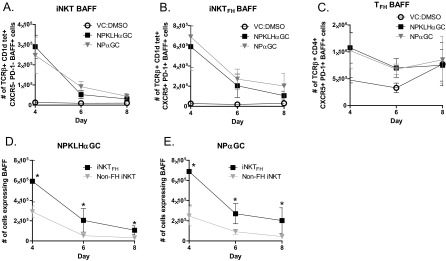
BAFF is preferentially expressed by the iNKTFH cell subset. Splenocytes harvested from C57BL/6 mice immunized i.v. with 0.5 μg NPαGalCer (NPαGC), 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), or vehicle [VC; PBS/0.1% (wt/vol) BSA] were labeled for FACS analysis to measure BAFF expression on the surface of (A) iNKT, (B) iNKTFH, or (C) TFH cells. FACS was also used to determine the number of TCRβ+CD1dtet+ CXCR5−PD1− iNKT or TCRβ+CD1dtet+CXCR5+PD1+ iNKTFH cells expressing BAFF after (D) NP-KLHαGC or (E) NPαGC immunization as in A and B (representative of two experiments; n = 2–5 mice per group). *P ≤ 0.05.
Cognate iNKT-Cell Help Retains More TFR Cells.
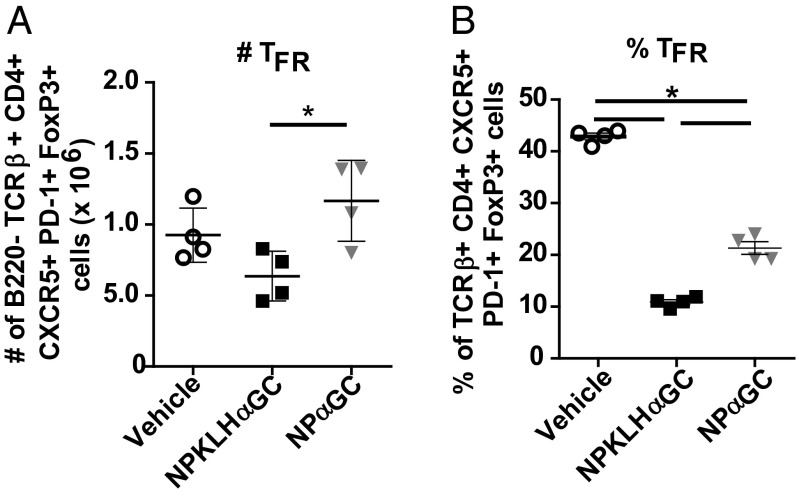
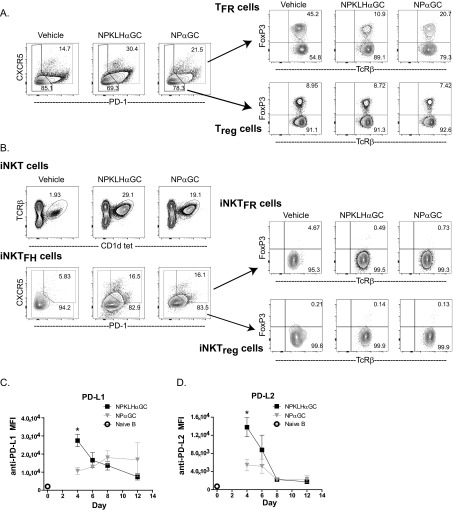
We next investigated the possibility that humoral memory fails to develop after NPαGalCer immunization because of the actions of a regulatory population of cells. TFR cells are CD4+CXCR5+PD-1+; express Foxp3, Bcl-6, and Blimp-1; and function to inhibit the germinal center response (33–35). We detected a significant decrease in percentage and number of FoxP3+CXCR5+PD-1+ TFR cells at 6 d after NP-KLHαGalCer compared with the vehicle control; however, NPαGalCer induced a significantly smaller decrease in percentage and no decrease in the number of TFR cells after NPαGalCer immunization (Fig. 2 and Fig. S3A). There were also no differences in Treg, iNKTFR (natural killer T follicular regulatory cells), or iNKTreg cell numbers after both immunizations (Fig. S3 A and B). PD-1–PD-L1 interactions negatively regulate TFR cell numbers, and as a result, PD-1–deficient mice have more TFR cells (32). We evaluated B-cell PD-L1 and PD-L2 expression and found that cognate iNKT-cell help for NPαGalCer fails to up-regulate PD-L1 and PD-L2 on germinal center B cells at day 4, whereas noncognate help does up-regulate early germinal center B-cell PD-L1 and PD-L2 expression after NP-KLHαGalCer immunization (Fig. S3 C and D). PD-L1 and PD-L2 up-regulation during noncognate iNKT help likely inhibits TFR cells and facilitates expansion of NP-specific germinal center B-cell numbers, which support the humoral memory induced by noncognate iNKT-cell help.
Fig. 2.
Cognate iNKT-cell help induces more TFR cells than noncognate iNKT-cell help. Splenocytes from C57BL/6 mice immunized with vehicle [PBS/0.1% (wt/vol) BSA], 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), or 0.5 μg NPαGalCer (NPαGC) were assessed at day 6 by FACS analysis for (A) numbers or (B) percentages of B220−TcRβ+CD4+CXCR5+PD-1+FoxP3+ TFR cells (representative of three experiments; n = 4 mice per group). *P ≤ 0.05.
Fig. S3.
Noncognate immunizations decrease TFR cells and up-regulate early expression of PD-L1 and PD-L2. Splenocytes harvested from C57BL/6 mice immunized i.v. with 0.5 μg NPαGalCer (NPαGC), 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), or vehicle [PBS/0.1% (wt/vol) BSA] were labeled for FACS analysis to measure (A) TFR (B220−TcRβ+CD4+CXCR5+PD-1+FoxP3+) and Treg (B220−TcRβ+CD4+CXCR5−PD-1−FoxP3+) cells and (B) iNKTFR (B220−TcRβ+CD1dtet+CXCR5+PD-1+FoxP3+) and iNKTreg (B220−TcRβ+CD1dtet+CXCR5−PD-1−FoxP3+) cells (representative of two to three experiments; n = 4 mice per group). Splenic B cells were monitored for expression of (C) PD-L1 and (D) PD-L2 by mean fluorescence intensity (MFI). FACS analysis revealed numbers of B220+ naïve B cells that are not specific for NP (○) and B220+NP+Fas+GL7+ NP-specific germinal center B cells (■ and ▼) at days 4, 6, 8, and 12 after immunization [data represent two to four experiments (n = 8–16 mice) for days 4–8 and one experiment (n = 4 mice) for day 12]. *P ≤ 0.05.
Selective Increases in iNKTFH Cells Do Not Rescue Humoral Memory.
NPαGalCer immunization induces iNKTFH cells but not TFH cells, and retains TFR cells, a combination that may not support memory B-cell development. To determine if one critical aspect of this unsupportive environment is the limiting number of iNKT helper cells, we used mixed bone marrow (BM) chimeras to artificially increase the number of available splenic iNKTFH cells.
The precise role of PD-1 on TFH cells is complex, but at least one report found that PD-1–deficient mice have more TFH cells and enhanced humoral immunity (29). We hypothesized that this mechanism may also regulate iNKTFH cell numbers. To increase iNKTFH cells, we generated mixed BM chimeras, in which PD-1 was selectively missing on iNKT cells. Irradiated Jα18-deficient (iNKT cell-deficient) hosts were reconstituted with a mixture of 25% PD-1–deficient BM and 75% Jα18-deficient BM (iNKT PD-1KO chimeras). Control chimeras with PD-1–sufficient iNKT cells were created by reconstituting Jα18-deficient hosts with 25% C57BL/6J WT BM mixed with 75% Jα18-deficient BM (iNKT WT chimeras). To control for inconsistencies caused by irradiation, we immunized unmanipulated C57BL/6 mice in parallel and noted that the WT BM chimeras generated at least as many iNKTFH cells as the unmanipulated WT B6 mice (Fig. 3C). iNKT PD-1KO chimeras showed no detectable PD-1 on their iNKTFH cells (Fig. 3A), but as expected, significantly more CXCR5+Bcl6+ iNKTFH cells were generated than in the iNKT WT chimeras (Fig. 3 B and C). These data confirm that PD-1 plays the same inhibitory role in iNKTFH cell differentiation as it does for TFH cells (38). Unexpectedly, the number of NP-specific germinal center B and TFH cells after both cognate and noncognate immunization is reduced compared with that in WT chimeras (Fig. 3 D–F). Furthermore, despite increased iNKTFH cell numbers, iNKT PD-1KO chimeras showed no increase in primary or memory anti-NP IgG antibody over WT chimeras (Fig. 3G). Thus, PD-1 signaling on iNKT cells negatively regulates iNKTFH cell numbers.
Fig. 3.
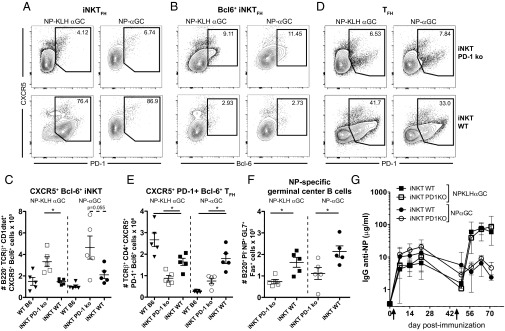
Selective elimination of PD-1 on iNKT cells increases CXCR5+Bcl6+ iNKTFH cells but does not rescue humoral memory. Eight days after i.v. immunization of iNKT PD-1 KO or iNKT WT mixed BM chimeras with 0.5 μg NPαGalCer (NPαGC) or 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), splenocytes were labeled for FACS to determine numbers of (A) CXCR5+PD-1+ iNKT cells, (B and C) CXCR5+Bcl6+ iNKT cells, (D and E) CXCR5+PD-1+Bcl6+ T cells, and (F) NP-specific germinal center B cells (representative of three experiments; n = 5 mice per group). *P ≤ 0.05. (G) ELISA also detected 4-hydroxy-5-iodo-3-NP (NIP) -specific IgG in sera from iNKT PD-1 KO or iNKT WT mixed BM chimeras immunized i.v. with 100 μg NP-KLH plus 0.5 μg αGC or 0.5 μg NPαGC on day 0 followed by an i.v. boost of 100 μg NP-KLH or 0.5 μg NPαGC on day 50 (representative of two experiments; n = 4–6 mice per group). *P ≤ 0.05.
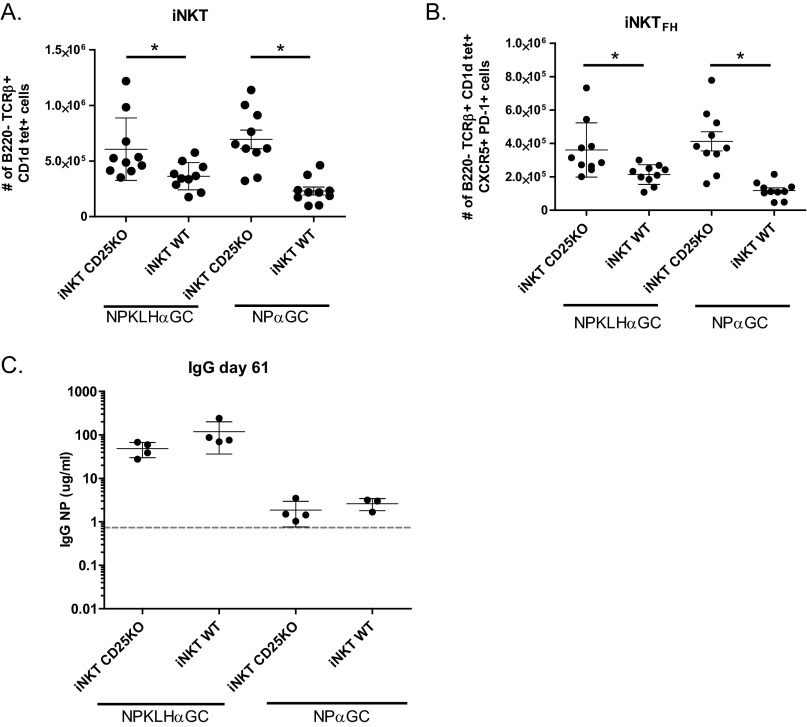
To verify that these results were not a caveat of the PD-1 system, we altered iNKTFH cell numbers using a complementary approach. PD-1 impairs IL-2 signaling through the CD25 receptor (39), which normally favors blimp-1 over bcl-6 expression and T effector over TFH cell development (36). In this context, CD25 negatively regulates TFH cell development, and therefore, we hypothesized that CD25 would negatively regulate iNKTFH cell development. We created mixed BM chimeras as described above, in which CD25 was selectively absent from iNKT cells to increase iNKTFH cell numbers during the antigen response. As predicted, iNKTFH cells expanded preferentially in iNKT CD25KO BM chimeras compared with controls (Fig. S4 A and B); however, humoral memory remained undetectable after cognate immunization (Fig. S4C). The iNKT CD25KO chimera results are consistent with the iNKT PD-1KO chimera data and confirm that increasing iNKTFH cell numbers does not enhance humoral memory. Thus, the lack of humoral memory after NPαGalCer is not a result of insufficient iNKTFH cells but instead, suggests that iNKTFH cells provide fundamentally different help from conventional TFH cells.
Fig. S4.
Selective elimination of CD25 on iNKT cells increases iNKTFH cells but does not rescue humoral memory. Twelve days after i.v. immunization of iNKT CD25KO or iNKT WT mixed BM chimeras with 0.5 μg NPαGalCer (NPαGC) or 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), splenocytes were labeled for FACS to determine numbers of (A) iNKT and (B) iNKTFH cells (representative of two experiments; n = 5 mice per group in duplicate). *P ≤ 0.05. (C) ELISA also detected NIP-specific IgG in sera from iNKT CD25KO or iNKT WT mixed BM chimeras immunized i.v. with 100 μg NP-KLH plus 0.5 μg αGC or 0.5 μg NPαGC on day 0 followed by an i.v. boost of 100 μg NP-KLH or 0.5 μg NPαGC on day 47. Sera was collected 14 d after the secondary boost (representative of two experiments; n = 4 mice per group).
Cognate iNKT-Cell Help Expands Innate B-Cell Populations.
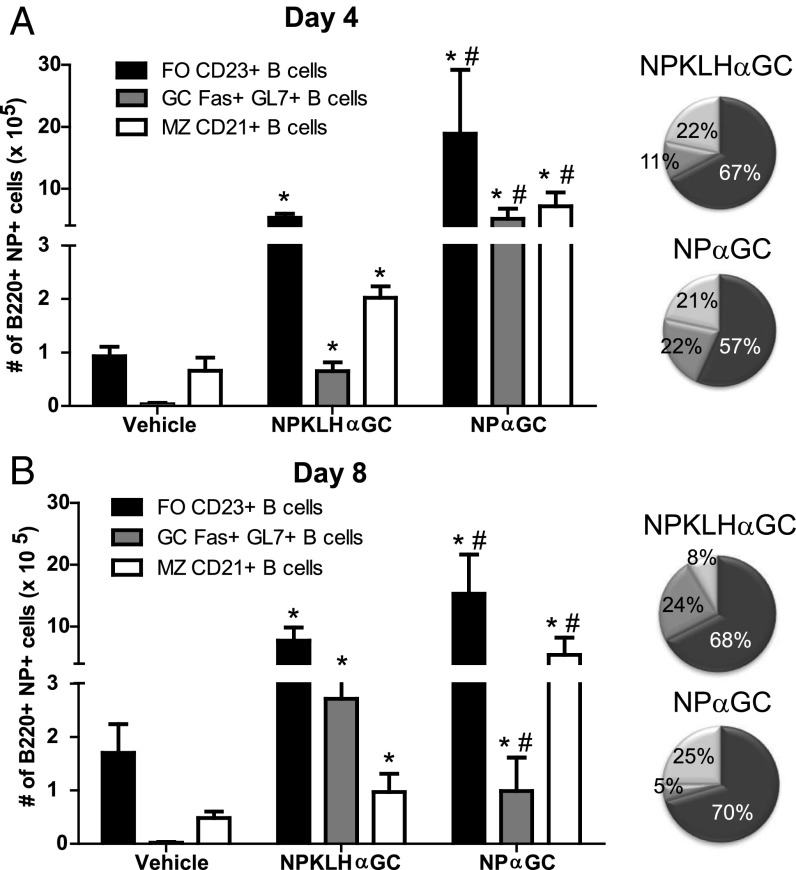
We next considered that iNKT helper cells may partner with innate B cells that do not support humoral memory. Cognate antigen immunization localizes iNKT cells in the MZ (40), and MZ B cells express the highest levels of CD1d of any immune cell (41); therefore, we examined the influence of iNKT-cell help on MZ B cells. We found that mice receiving either NPαGalCer or NP-KLHαGalCer had expanded MZ B-cell populations at day 4, but only mice receiving NPαGalCer still had an expanded MZ B-cell population at day 8 (Fig. 4). Thus, cognate iNKT-cell help sustains expansion of MZ B cells, but noncognate iNKT-cell help favors a more transient MZ B-cell expansion and a later germinal center B-cell response.
Fig. 4.
Cognate iNKT-cell help preferentially expands innate B-cell populations. Splenocytes from C57BL/6 mice immunized with vehicle [PBS/0.1% (wt/vol) BSA], 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), or 0.5 μg NPαGalCer (NPαGC) were assessed by FACS analysis for numbers of follicular (FO) B220+NP+CD21−CD23+ B cells, B220+NP+Fas+GL7+ NP-specific germinal center (GC Fas+GL7+) B cells, and MZ B220+NP+CD23loCD21+ B cells at days (A) 4 and (B) 8 postimmunization. Pie charts show B-cell subsets as percentages of total B cells in the spleen (A) 4 and (B) 8 d after immunization with NPαGC or NP-KLH plus αGC (representative of two experiments; n = 8–10 mice per group). *P ≤ 0.05 to vehicle control; #P ≤ 0.05 to NP-KLHαGC.
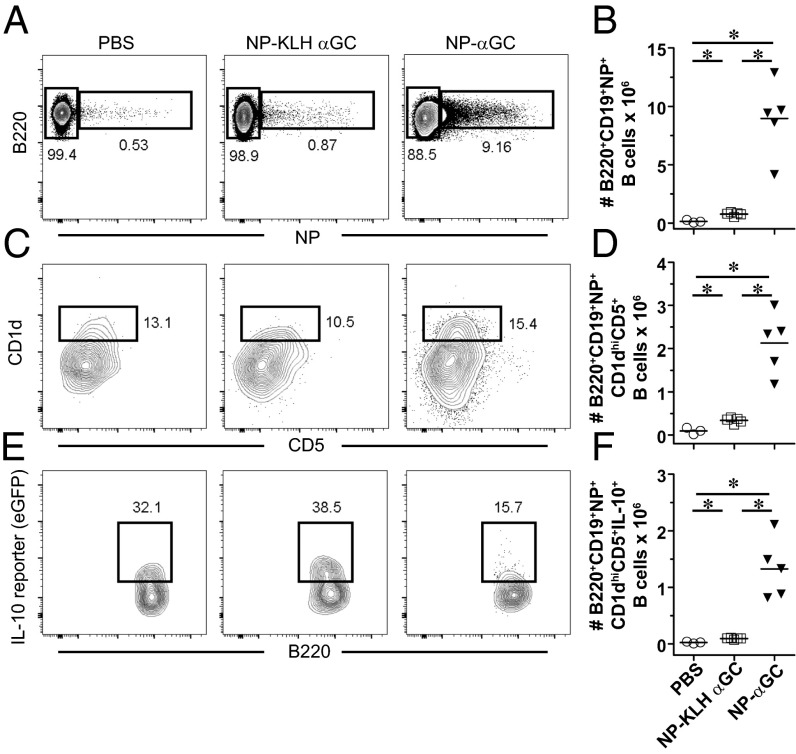
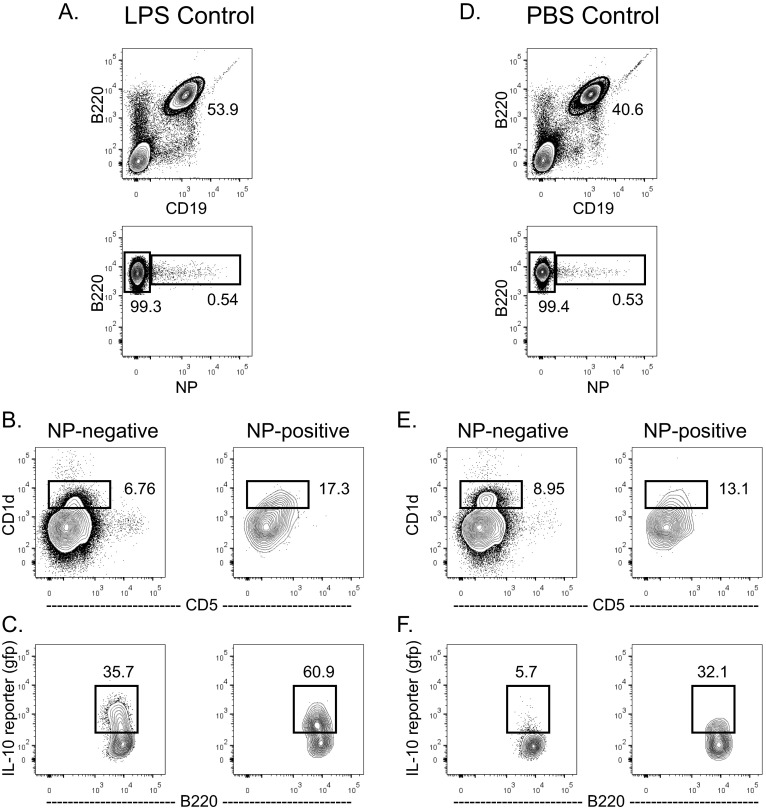
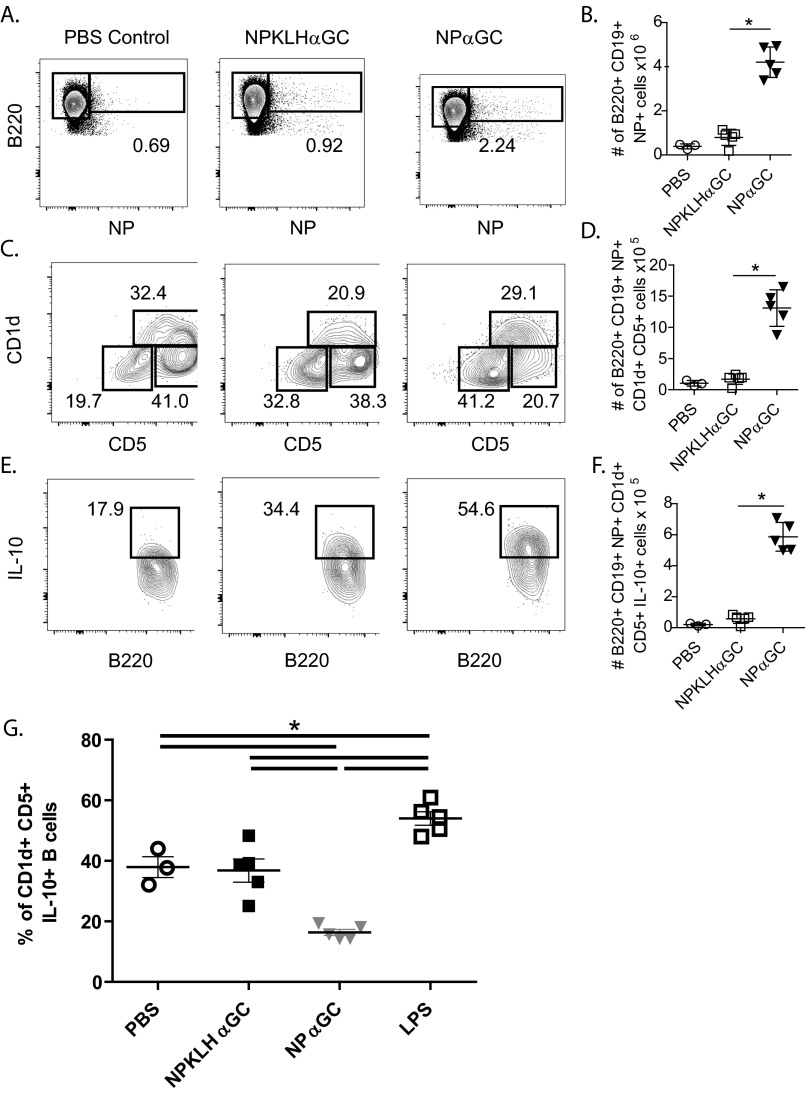
Because cognate iNKT-cell help favors expansion of CD1dhi MZ B cells in the spleen, we evaluated the effect of iNKT activation on a related splenic innate, regulatory B-cell population (IL-10–producing B10 cells), which is also CD1dhi. NPαGalCer induced more NP-specific B10 cells, and higher numbers of those cells were IL-10+ 4 d after immunization than after NP-KLHαGalCer (Fig. 5). We obtained these results using VertX IL-10 reporter mice, which accurately report IL-10 production using a bicistronic GFP reporter downstream of the IL-10 gene (42). LPS immunization confirms the reliability of these mice, inducing significantly more IL-10–producing B cells over vehicle-immunized mice after 2 days (Fig. S5). NPαGalCer immunization induced a higher number of NP-specific B cells (Fig. 5 A and B), a higher number of CD5+CD1d+ B cells (Fig. 5 C and D), and a higher number of IL-10–producing B10 cells in VertX mice than NP-KLHαGalCer (Fig. 5 E and F). We also confirmed these results using intracellular FACS staining for IL-10 protein (Fig. S6 A–F). However, cognate iNKT-cell help does not preferentially induce IL-10–secreting B cells, because the percentage of IL-10+ B cells expanded in the antigen-specific CD5+CD1d+ B regulatory cell populations is actually slightly lower in NPαGalCer-immunized mice than in NPKLHαGalCer immunized mice (Fig. S6G). Because of the large expansion of antigen-specific B cells, the consequence of cognate iNKT-cell help is still an expanded regulatory B-cell population in the spleen. The innate B10 cells engaged by cognate interactions with iNKT cells may go on to become PCs, but whether they go on to develop into long-term memory B cells is unknown (43). This innate B-cell trait may explain the fundamentally different memory outcomes after cognate and noncognate iNKT-cell help.
Fig. 5.
Cognate iNKT-cell help expands antigen-specific IL-10+ B10 B cells. FACS labeling identified expanded populations of B220+CD19+CD1d+CD5+IL-10+ cells using VertX IL-10 reporter mice 4 d after immunization with vehicle [PBS/0.1% (wt/vol) BSA], 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), or 0.5 μg NPαGalCer (NPαGC). FACS analysis revealed (A and B) B220+NP+, (C and D) B220+NP+CD1d+CD5+, and (E and F) B220+NP+CD1d+CD5+IL-10+ subsets of cells. CD5 vs. CD1d gates were selected based on B220+NP− control samples as published (53) (representative of two experiments; n = 8–10 mice per group). *P ≤ 0.05.
Fig. S5.
VertX mice faithfully report IL-10 production by B cells after LPS immunization. VertX mice were immunized i.v. with (A–C) LPS or (D–F) vehicle [PBS/0.1% (wt/vol) BSA], and (A and D) their splenocytes were assessed 2 d later by FACS analysis for presence of B220+NP+ NP-specific B cells. (B and E) Additional gating of NP-specific B220+ B cells revealed the B10 subset cells (B220+CD19+CD1d+CD5+IL-10+). (C and F) Percentage of B10 cells producing IL-10 is identified by expression of GFP in the B10 subset (representative of nine experiments; n = 20+ mice).
Fig. S6.
Noncognate iNKT cells increase in number but do not preferentially expand a greater percentage of IL-10+ B cells. FACS analysis was used to identify NP-specific B10 (B220+CD19+CD1d+CD5+IL-10+) cell numbers 4 d after immunization of C57BL/6 mice with vehicle (PBS/0.1% BSA), 100 μg NP-KLH plus 0.5 μg αGalCer (αGC), or 0.5 μg NPαGalCer (NPαGC). FACS analysis specifically revealed (A and B) B220+NP+, (C and D) B220+NP+CD1d+CD5+, and (E and F) B220+NP+CD1d+CD5+IL-10+ (one experiment; n = 3–5 mice per group;). *P ≤ 0.05. (G) VertX IL-10 reporter mice were immunized i.v. with vehicle (PBS/0.1% BSA), 100 μg NP-KLH plus 0.5 μg αGC, 0.5 μg NPαGC, or LPS, and their splenocytes were assessed 4 d later by FACS analysis to determine the percentage of B10 cells (B220+CD19+CD1d+CD5+IL-10+) within the expanded antigen-specific CD5+ CD1d+ B regulatory cell population (representative of three experiments; n = 3–5 mice per group).
Discussion
We set out to understand the differences in B-cell activation outcome after cognate and noncognate iNKT help. Clarifying the fundamental biology of iNKT and B-cell cooperation may allow us to use this type of help for vaccine development. We examined iNKT and B-cell kinetics after immunization with cognate or noncognate antigen. These studies revealed parallel increases in iNKT cell numbers but early differences in antigen-specific germinal center B cells. It seems that cognate glycolipid antigen induces a marked but unsustained germinal center B-cell expansion. Numbers of germinal center B cells induced by cognate glycolipid antigen drop precipitously at day 6, the same kinetics that de Vinuesa et al. (44) described for spontaneous involution of germinal centers after T-independent antigen immunization. Physiological germinal center maturation may depend on T-cell signals to prevent centrocytes from undergoing apoptosis (44). T-cell signals may rescue germinal center B cells from apoptosis and induce a proportion of germinal center B cells to differentiate into centroblasts or memory B cells. In the absence of appropriate T-cell signals, germinal center B cells fail to become centroblasts and will not produce memory B cells or PCs. Thus, premature involution of germinal centers and lack of memory B cells or PCs after cognate iNKT-cell help are consistent with a lack of T-cell signals to sustain germinal centers. Wermeling et al. (45) have reported that antigen-activated B cells significantly down-modulate CD1d expression on entry into the germinal center, and therefore, these signals may also need to be provided before entry into the germinal center.
In contrast, noncognate antigen induces a later, modestly sustained germinal center B-cell expansion that is consistent with germinal center B-cell expansion patterns after protein-specific TFH-cell help. Furthermore, only noncognate immunization is able to induce expansion of peptide-specific TFH cells. These results are consistent with the findings of others suggesting that TFH cells are critical for iNKTFH-helped long-term humoral memory. For example, when CD4+ T cell-deficient MHC class II−/− mice are immunized with peptide plus αGalCer, they form germinal centers and initiate a primary antibody response but do not maintain long-lasting secondary antibody (46). Thus, CD4+ TFH cells are the cells primarily responsible for noncognate humoral memory. However, these findings still beg the question of why iNKT cells are ineffective at driving B-cell memory on their own.
We first considered that cognate iNKT-cell help may fail to induce humoral memory because of a dominant regulatory population. We compared Treg, TFR, iNKTreg, and iNKTFR cells and found that cognate iNKT-cell help does not reduce TFR cells as much as noncognate iNKT-cell help does. TFR cells suppress germinal center B-cell responses, including affinity maturation, IgG production, and PC differentiation by limiting the number of TFH cells (32–34). The higher levels of TFR cells remaining after cognate iNKT cell activation may contribute to a generally suppressive environment but may not be the only cellular mechanism responsible for the lack of humoral memory.
We next considered that the number of iNKTFH cells generated during cognate iNKT cell activation was inadequate to sustain germinal center B cells through memory B-cell development. To address this possibility, we assessed humoral memory in two sets of mixed BM chimeras with selectively increased iNKTFH cell numbers. This approach revealed that PD-1 and CD25 have the same inhibitory roles in iNKTFH cell development as have been reported for TFH cells (38), because iNKTFH cell numbers were increased in mice selectively missing PD-1 or CD25 on only iNKT cells (23, 29, 38). However, increasing iNKTFH cell numbers did not improve the B-cell humoral memory outcome after cognate iNKT antigen immunization. It is also possible that removing PD-1 from iNKT cells has increased numbers of iNKTFR cells in the same way that TFR cell numbers are enhanced in PD-1–deficient mice (32). However, this caveat is not a concern in the CD25 BM chimeras, which impart their effect independent of PD-1, and they produce the same humoral outcome as the PD-1 chimeras in our studies. These two complementary BM chimeras suggest that the lack of humoral memory after cognate antigen immunization cannot be overcome by increasing iNKTFH cell numbers but rather, that B-cell memory is a unique product of TFH-cell help.
iNKT cells consolidate in the MZ and favor interactions with MZ B cells (40) which express a higher level of CD1d than all other APCs in the spleen (41), and iNKT cells cultured with MZ B cells proliferate more vigorously than iNKT cells mixed with follicular B cells (2). Given this previous work, we evaluated the possibility that activated iNKT cells preferentially expand innate MZ B cells. The MZ is comprised of innate B cells, such as MZ B, MZ-T2 B, and B10 cells, which have restricted repertoires, reduced activation thresholds, and alternative activation responses (47). These B cells all produce IL-10, an immunoregulatory cytokine, which contributes to development of Treg cells, induces expansion of more B regulatory cells, and inhibits activity of effector B cells (48). We found that cognate iNKT-cell help favored more sustained expansion of MZ B cells than noncognate iNKT-cell help, and it yields larger numbers of expanded antigen-specific CD1d+CD5+IL-10+ B10 cells than noncognate iNKT-cell help. Thus, iNKT cells favor interaction with innate MZ-localized B-cell subsets, which may be unlikely or unable to develop into memory B cells. The ability or inability of antigen-specific IL-10–producing B cells to develop into memory B cells in this system will need to be more precisely addressed using mice engineered to visualize IL-10+ cell fate mapping.
It is intriguing to speculate that exclusive iNKT-cell help favors an innate B-cell response, because the two cells cooperate to generate an innate humoral response in advance of a more precise and evolved adaptive response. Recent data from Wingender and coworkers (49) have suggested that iNKT cells previously activated by αGalCer become IL-10–producing iNKT10 cells. Thus, IL-10 from iNKT cells may dominate the response to expand MZB cells and maintain TFR cells under conditions where there are no inflammatory cytokines from TFH cells to override this regulatory environment and expand B effector cells and inhibit TFR cells. It would also follow that innate iNKT cells then preferentially help innate B cells make an early plasmablast antibody response using primarily MZ B cells, which do not develop into a memory population unless accompanied by confirmatory signals from a conventional protein-specific helper response. These results may be one mechanism that allows for an extremely rapid but innate humoral response to bridge the gap between more traditional innate and adaptive responses.
Materials and Methods
Mice.
C57BL/6J WT mice, C57BL/6 Pdcd1 KO mice (gift from Dario Vignali, St. Jude Children’s Research Hospital, Memphis, TN) lacking programmed cell death-1 (50), VertX mice (gift from Markus Mohrs, Trudeau Institute, Saranac Lake, NY) (42), B6.129S4-Il2ratm1Dw/J mice lacking CD25 (51), and Jα18-deficient mice lacking Jα18+ iNKT cells (52) (and a subset of CD4+ T cells) were housed and bred at the Trudeau Institute and the Karolinska Institutet. All live animal experimental protocols were approved by the Trudeau Institute or Karolinska Institutet Animal Care and Use Committee.
Flow Cytometry.
Single-spleen cell suspensions were stained with mAbs for flow cytometry as listed in SI Materials and Methods. Antigen-specific B cells were identified with NP-allophycocyanin as published (9). iNKT cells were identified with mouse CD1d-αGalCer tetramers (PBS57; NIH Tetramer Core Facility) conjugated to allophycocyanin or phycoerythrin. The Foxp3/Transcription Factor Staining Set (eBioscience) was used for intracellular staining of FoxP3, Bcl6, and IL-10. Samples were acquired on an FACSCanto II (BD) and analyzed with FlowJo software (TreeStar).
Bone Marrow Chimeras.
Recipient C57BL/6 Jα18-deficient mice were irradiated two times with 500 rad, with a 3- to 4-h rest between doses. Recipient mice were reconstituted with 5–10 × 106 BM cells. Donor BM included 75% Jα18-deficient BM mixed with either 25% Pdcd1 KO BM or 25% CD25 KO BM. Controls were reconstituted with 75% Jα18-deficient BM mixed with 25% C57BL/6 WT BM. These reconstitution BM mixtures resulted in mice in which predominantly iNKT cells were deficient in PD-1 or CD25 or all cells were PD-1– or CD25-sufficient. Reconstitution of iNKT, T, and B cells in the spleen was confirmed by flow cytometry after 8–10 wk.
Antigens, Immunizations, and Serum Collections.
NP(25)-KLH (Biosearch Technologies), NP(27)-KLH (Biosearch Technologies), NP(30)-KLH (Biosearch Technologies), NP(33)-KLH (Biosearch Technologies), NP-αGalCer, or αGalCer were administered i.v. Immunizations included 100 μg protein suspended in PBS plus 0.1% (wt/vol) BSA or 0.5 μg glycolipid antigen solubilized in ≤0.1% (vol/vol) DMSO and then, resuspended in PBS plus 0.1% (wt/vol) BSA. Serum was collected submandibularly and stored at −20 °C until ELISA assessment.
ELISA.
NP-specific IgG and IgM were detected in serum by heteroclitic 4-hydroxy-5-iodo-3-NP–specific ELISA as published (2) and are described in SI Materials and Methods.
Statistics.
GraphPad PRISM5 software was used for two-tailed t tests for normally distributed datasets.
SI Materials and Methods
ELISA.
NP-specific IgG and IgM were detected in serum by heteroclitic 4-hydroxy-5-iodo-3-NP (NIP) -specific ELISA. In short, Easy Wash COSTAR 96-Well ELISA Plates were coated with NIP(5)-ovalbumin (OVAL) or NIP(11)-OVAL overnight and then blocked with PBS/5% (wt/vol) BSA for 2 h. ELISA plates were washed three times with PBS/0.05% (vol/vol) Tween, and eight half-log dilutions of serum, IgG anti-NP (clone B1-8), or IgM anti-NP (clone J558) standards were applied. After a 2-h incubation, plates were washed, and HRP anti-mouse IgG or HRP anti-mouse IgM-detecting antibody (Southern Biotech) was applied and incubated for 45 min. ELISA plates were washed again, developed with 550 nM ABTS [(2,2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt)] in 0.1 M citric acid (pH 4.35) and 0.03% (vol/vol) H2O2, and read on a Molecular Devices SPECTRAmax Plus 384 at 405 nm.
Flow Cytometry.
Single-cell suspensions were prepared from spleen and stained with the following mAbs for flow cytometry: anti-CD19 (1D3), anti-CD25 (PC61), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-CD95 (Fas; Jo2), anti-TCRβ (H57-597), antistreptavidin (BD), anti-Bcl6 (K112-91), anti-CXCR5 (2G8), anti–B- plus T-cell activation antigen (GL7), anti-CD4 (GK1.5), and anti-CD5 (53-7.3) from BD Biosciences; anti-CD45R/B220 (RA3-6B2), anti-CD69 (H1.2F3), anti-CD273 (PD-L2; TY25), anti-CD274 (PD-L1; 10.F9G2), anti-CD279 (PD-1; RMP1-30), anti-CD40 (3/23), anti-CD154 (CD40L; MR1), anti-CD86 (GL-1), CD1d (1B1), and anti–IL-10 (JES5-16E3) from Biolegend; and anti-Foxp3 (FJK-16s) from eBioscience.
Acknowledgments
The authors thank Markus Mohrs for valuable feedback. Research was supported by NIH Grants T32 AI49823 (to E.E.V.-D., J.Y., and E.A.) and R56 AI104788 (to E.A.L.), a Personal Research Chair from Mr. James Bardrick (to G.S.B.), Medical Research Council Grant MR/K012118/1 (to G.S.B.), Karolinska Institutet (T.H.), the Swedish Research Council (M.C.I.K.), Gustav V’s 80 Years Foundation (M.C.I.K.), the Cardiovascular Research Program (M.C.I.K.), and the Trudeau Institute (E.A.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504790112/-/DCSupplemental.
References
- 1.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 2.Leadbetter EA, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105(24):8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barral P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008;105(24):8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli G, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104(10):3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol. 2010;22(2):79–86. doi: 10.1016/j.smim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 7.Chang PP, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2012;13(1):35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 8.Vomhof-DeKrey EE, Yates J, Leadbetter EA. Invariant NKT cells provide innate and adaptive help for B cells. Curr Opin Immunol. 2014;28:12–17. doi: 10.1016/j.coi.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King IL, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2012;13(1):44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah HB, et al. BAFF- and APRIL-dependent maintenance of antibody titers after immunization with T-dependent antigen and CD1d-binding ligand. J Immunol. 2013;191(3):1154–1163. doi: 10.4049/jimmunol.1300263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11(8):681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 12.Han S, et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155(2):556–567. [PubMed] [Google Scholar]
- 13.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant VL, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: Predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179(12):8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 15.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207(2):353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 18.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206(5):1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zotos D, Tarlinton DM. Determining germinal centre B cell fate. Trends Immunol. 2012;33(6):281–288. doi: 10.1016/j.it.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: The influence of germinal center interactions and dynamics. J Immunol. 2010;185(6):3117–3125. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 21.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 22.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 23.Good-Jacobson KL, et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11(6):535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 25.Sheppard KA, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1-3):37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 26.Yokosuka T, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol. 2009;83(9):4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol. 2013;25(3):381–388. doi: 10.1016/j.coi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hams E, et al. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol. 2011;186(10):5648–5655. doi: 10.4049/jimmunol.1003161. [DOI] [PubMed] [Google Scholar]
- 30.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13(2):188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamel KM, et al. B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis. Eur J Immunol. 2010;40(11):3117–3127. doi: 10.1002/eji.201040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14(2):152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wollenberg I, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187(9):4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 36.Ballesteros-Tato A, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36(5):847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goenka R, et al. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med. 2014;211(1):45–56. doi: 10.1084/jem.20130505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13(6):412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 39.Chikuma S, et al. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182(11):6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- 40.King IL, et al. The mechanism of splenic invariant NKT cell activation dictates localization in vivo. J Immunol. 2013;191(2):572–582. doi: 10.4049/jimmunol.1300299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roark JH, et al. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol. 1998;160(7):3121–3127. [PubMed] [Google Scholar]
- 42.Madan R, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183(4):2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedder TF. B10 cells: A functionally defined regulatory B cell subset. J Immunol. 2015;194(4):1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 44.de Vinuesa CG, et al. Germinal centers without T cells. J Exp Med. 2000;191(3):485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wermeling F, Lind SM, Jordö ED, Cardell SL, Karlsson MC. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J Exp Med. 2010;207(5):943–952. doi: 10.1084/jem.20091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonti E, et al. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J Immunol. 2012;188(7):3217–3222. doi: 10.4049/jimmunol.1103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2(5):323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 48.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 49.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124(9):3725–3740. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10(10):1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 51.Willerford DM, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3(4):521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 52.Cui J, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 53.Yanaba K, et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]