Abstract
Free-living amoebae (FLA) occupy a wide range of freshwater, marine, and soil habitats, and are opportunistic pathogens in human beings. While Acanthamoeba spp., Naegleria fowleri, and Balamuthia mandrillaris are well-known opportunistic organisms, Vannella epipetala is nonpathogenic. Sediments were collected from a freshwater source from a park in Jamaica to investigate the presence of FLA. Acanthamoeba and Naegleria spp. were not recovered; however, a Vannellid species identified by microscopy and PCR analysis as V. epipetala was isolated. These nonpathogens pose a threat to human beings as they may act as Trojan horses for microsporidian parasites and other pathogens, thereby facilitating their transmission to human beings.
Keywords: Free-living amoebae (FLA), Vannella epipetala, microsporidian parasites, Jamaica, West Indies
Introduction
Free-living amoebae (FLA) are widely distributed in the environment and are normally harmless to human beings; however, Acanthamoeba spp., Naegleria fowleri, Sappinia spp., and Balamuthia mandrillaris are opportunistic pathogens causing severe CNS, eye, skin, colon, and liver diseases.1 Vannella spp. are commonly found in fresh and salt water bodies and are more recently found in tap water, soil, gills and organs of fishes, biofilm, sewer outflows, municipal waste water treatment plant release sites, and the leaf surface of plants (Spondias mombin [Anacardiaceae]) found in Costa Rica.2–7 FLA are increasingly recognized as important pathogens with increased reports of human cases and exposure to infection through antibody titer studies.1,8,9 Vannella spp. isolated from soil are not pathogenic but can act as reservoirs for pathogenic microsporidian parasites. While there are over 40 aquatic species of Vannella, only some are pathogenic to vertebrates, in particular fishes.2,7 The SSU rRNA gene of Vannellids are constantly mutating and are linked to increased pathogenicity in fishes. It was established that some members of the genus cause diseases of the gills and organs in fishes, but no data exist on the potential to cause disease in human beings. The fact that this gene is rapidly evolving should raise interests to understand its evolutionary behavior, its pathogenicity in fishes, their ability to transmit other pathogens, and the possibility of causing disease in human beings and other animals.6 Since Vannella epipetala is not only found to inhabit soil and fresh and salt water bodies but also the surface of leaves, the diversity of habitat types highlights its survival versatility.6 V. epipetala was not reported from previous studies of FLA in Jamaica,10,11 and the occurrence of this organism in the Caribbean is unknown. The aim of the study was to investigate the presence of FLA types in river sediments and to characterize these organisms and investigate their pathogenic potential.
Materials and Methods
Soil sediments were collected from the bank of the Castleton River in a 50 mL sterile centrifuge tube (Corning Incorporated). Approximately 10 g of soil was inoculated onto 2% non-nutrient agar (NNA) plates seeded with heat-killed Escherichia coli and incubated at room temperature (~30°C) for seven days. NNA plates were examined using an inverted microscope for the growth of amoebae. Plates containing amoebae were scraped, and the material was centrifuged after addition of phosphate-buffered saline. DNA was extracted by placing 1–2 mL of amoebic cultures directly into the Maxwell® 16 Tissue DNA Purification Kit sample cartridge (Promega Corporation). Amoebic genomic DNA was purified using the Maxwell® 16 Instrument as described in the Maxwell® 16 DNA Purification Kits Technical Manual #TM284 (Promega Corporation). DNA yield and purity were determined using the NanoDrop® 1000 spectrophotometer (Fisher Scientific) as previously described.10 DNA amplification reactions were performed using universal markers for FLA and specific markers for V. epipetala. Amplification products were fractionated using 2% agarose electrophoresis gel stained with a solution of 20,000× of REALSAFE Nucleic Acid Staining Solution (Durviz) and visualized under UV light. PCR products were purified using the QIAquick® PCR Purification kit (QIAGEN), according to the manufacturer’s instructions, and sequenced in both directions. The sequencing was done in a MegaBACE 1000 automatic sequencer (Healthcare Biosciences) using the University of La Laguna sequencing services (Servicio de Secuenciación SEGAI, University of La Laguna). Homology analyses of the obtained DNA sequences were performed using BLAST analysis and compared to the sequences available at the GenBank database.
Results and Discussion
A single isolate of Vannella was recovered from the NNA plate. This was identified as V. epipetala using PCR and DNA sequencing. BLAST analysis revealed a 99% homology with the available sequences of other V. epipetala strains deposited in the GenBank database. This is the first report of V. epipetala from the Caribbean and expands the geographic location from where this species has been recovered. The Castleton River from which the organism was recovered is associated with a botanical garden, which is a heavily used recreational site. The public heath significance cannot be established on a single isolate, although this finding has some significance. Vannella spp. are not harmful to human beings but are hosts and Trojan horses of microsporidian parasites, bacteria, and other pathogens.2,7,12 Furthermore, this study and studies conducted by Amaral-Zettler et al6 reported bacteria other than E. coli growing with the cultured amoebae and it is not known if these were symbiotically associated with V. epipetala.
Hoffmann et al2 reported the isolation of Vannella spp. infected with microsporidian parasites from domestic tap water. Lasjerdi et al7 reported the isolation of Vannella housing microsporidian parasites from biofilms from a hospital, and earlier reports by Scheid13 reported the presence of microsporidian parasites in Vannella spp. isolated from a keratitis patient. The symbiotic relationship favors the growth and proliferation of these pathogens in which they are protected from chlorine and other chemicals used for water treatment and harsh environments that may threaten their survival. The ubiquitous nature of the amoebae allows these pathogens to harbor a wider niche within them and to increase their chances of human contact; therefore, more attention should be given to these FLA.
FLA are commonly found in freshwater and are the main organisms responsible for bacterial population control in soil.4 Vannellidae are most frequently isolated from tissues of marine and fresh water fishes. Vannella, Neoparamoeba, and Platyamoeba spp. are most frequently found on the gills of fishes. Vannella spp. is not reported to have a serious impact on the fish industry; however, Vannellids have been isolated from the gills of asymptomatic and clinically diseased fishes with amoebic gill disease.4 Neoparamoeba is reported to result in gill disease and death in demersal dwellers like Scophthalmus maximus and in anadromous fishes like Salmo salar, negatively impacting the fish industry.4
FLA such as Flabellula and Platyamoeba have similar morphological characteristics to Vannella.14 The similarities between the marine dwellers of the genera Platyamoeba and Vannella, were closer than between freshwater and marine Vannella spp. Also, similarities between the marine dwellers of the genera Platyamoeba and Vannella were closer than between freshwater and marine Platyamoeba spp.6,15 Morphological characteristics and locomotary behavior differentiated Vannella from Flabellula based on a radiant floating form with rounded tips, lack of subpseudopodia or uroidal filaments, and a fan-shaped appearance during locomotion.6,16 Although Sims et al15,17 reported that the SSU ribosomal ribonucleic acid (SS rRNA) gene sequence could be used to differentiate between species of Vannella, the presence of pentagonal glycostyles in this genus was the main characteristic used to separate it from Platyamoeba. Contradictory to this, molecular work established the unreliability of relying on morphological features for the differentiation of organisms at the genus or species level.6 Further, Page18 designated cyst formation as a morphological characteristic that could be used for the identification of Platyamoeba spp.; however, the cystic form was later observed in Vannellids.3,19 Despite the efforts to differentiate the genera Vannella and Playamoeba, morphological, molecular, and phylogenetic studies performed by Sims et al,17 Dyková et al,5 and Amaral-Zettler et al,6 respectively, proved that both genera were very closely related. Similar to the observation noted by Amaral-Zettler et al,6 the floating form remained contracted with short pseudopodia-like protrusions (Fig. 1). Amoebae growth was achieved at room temperature (~30°C), which differed significantly to the findings of Amaral-Zettler et al,6 who reported optimal growth at temperatures of 20°C and 25°C, no growth and a low survival rate at 30°C. The variation in growth patterns might be associated with the isolate type.
Figure 1.
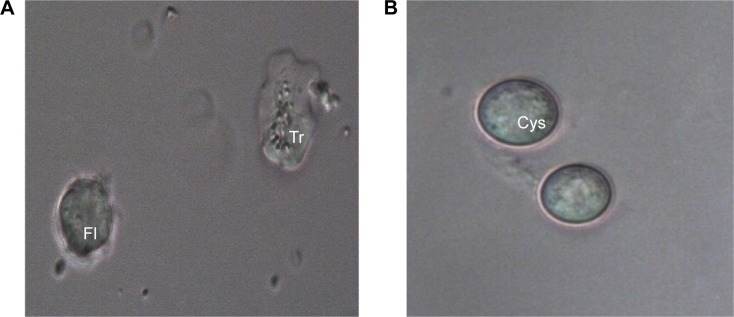
Stages of V. epipetala from river sediment. (A) trophozoite (Tr) and floating form (Fl) and (B) cyst (cys) (40×).
Acanthamoeba spp. and B. mandrillaris have been isolated from soil from recreational sites in Jamaica; however, this was the first report of V. epipetala.10,11 The isolation of V. epipetala from soil in this study is the second report of the isolation of this amoeba from an environmental source and the first report of its isolation in the Caribbean. Further work should be done to investigate the possibility of V. epipetala hosting pathogens that may be potentially harmful to human beings.
Footnotes
ACADEMIC EDITOR: Raul Rivas, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 453 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by the grants RICET (project no. RD12/0018/0012 of the program of Redes Temáticas de Investigación Cooperativa, FIS), Spanish Ministry of Health, Madrid, Spain, and the PI13/00490 “Protozoosis Emergentes por Amebas de Vida Libre: Aislamiento, Caracterización, Nuevas Approximaciones Terapéuticas y Traslación Clínica de los Resultados” from the Instituto de Salud Carlos III and Project ref. AGUA3 “Amebas de Vida Libre como Marcadores de Calidad del Agua” from CajaCanarias Fundación. M.R.B. was funded by Becas Fundación Cajacanarias y Obra Social La Caixa para Postgraduados 2014. J.L.M. was supported by the Ramón y Cajal Subprogramme from the Spanish Ministry of Economy and Competitivity, RYC. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: CDT, and JLM. Analyzed the data: CDT, JLM, MRB, BV and JFL. Wrote the first draft of the manuscript: CDT, JLM, JFL. Contributed to the writing of the manuscript: CDT, MRB, BV, JFL and JLM. Agree with manuscript results and conclusions: CDT, MRB, BV, JFL and JLM. Jointly developed the structure and arguments for the paper: CDT, JLM, and CDT. Made critical revisions and approved final version: CDT, JLM, BV, MRB and JFL. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Gianinazzi C, Schild M, Wüthrich F, Müller N, Schürch N, Gottstein B. Potentially human pathogenic Acanthamoeba isolated from a heated indoor swimming pool in Switzerland. Exp Parasitol. 2009;121(2):180–186. doi: 10.1016/j.exppara.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann R, Michel R, Schmid EN, Müller KD. Natural infection with microsporidian organisms (KW19) in Vannella spp (Gymnamoebia) isolated from domestic tap-water supplies. Parasitol Res. 1998;84(2):164–166. doi: 10.1007/s004360050377. [DOI] [PubMed] [Google Scholar]
- 3.Smirnov AV, Brown S. First isolation of a cyst-forming Vannella species, from soil— Vannella persistens n. sp. (Gymnamoebia, Vannellidae) Protistology. 2000;1(3):120–123. [Google Scholar]
- 4.Dyková I, Lom J. Advances in the knowledge of amphizoic amoebae infecting fish. Folia Parasitol. 2004;51(2–3):81–97. [PubMed] [Google Scholar]
- 5.Dyková I, Boháčová L, Fiala I, Macháčková B, Pecková H, Dvořáková H. Amoebae of the genera Vannella Bovee, 1965 and Platyamoeba Page, 1969 isolated from fish and their phylogeny inferred from SSU rRNA gene and ITS sequences. Eur J Protistol. 2005;41(3):219–230. [Google Scholar]
- 6.Amaral-Zettler LA, Cole J, Laatsch AD, Nerad TA, Anderson OR, Reysenbach AL. Vannella epipetala n. sp. isolated from the leaf surface of Spondias mombin (Anacardiaceae) growing in the dry forest of Costa Rica. J Eukaryot Microbiol. 2006;53(6):522–530. doi: 10.1111/j.1550-7408.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 7.Lasjerdi Z, Niyyati M, Haghighi A, Zaeri F, Nazemalhosseini Mojarad E. First report of Vannellidae amoebae (Vannella spp.) isolated from biofilm source. Iran J Parasitol. 2011;6(4):84–89. [PMC free article] [PubMed] [Google Scholar]
- 8.Khan N. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiology Reviews. 2006;30(4):564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 9.Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic. Int J of Trop Med and Hyg. 2004;34(9):1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Todd CD, Reyes-Batlle M, Martín-Navarro CM, et al. Isolation and genotyping of Acanthamoeba strains from soil sources from Jamaica, West Indies. J Eukaryot Microbiol. 2015;62(3):416–421. doi: 10.1111/jeu.12197. [DOI] [PubMed] [Google Scholar]
- 11.Todd CD, Reyes-Batlle M, Piñero JE, et al. Balamuthia mandrillaris therapeutic mud bath in Jamaica. Epidemiol Infect. 2015;143(10):2245–2248. doi: 10.1017/S0950268814002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nazar M, Haghighi A, Taghipour N, et al. Molecular identification of Hartmannella vermiformis and Vannella persistens from man-made recreational water environments, Tehran, Iran. Parasitol Res. 2012;111:835–839. doi: 10.1007/s00436-012-2906-x. [DOI] [PubMed] [Google Scholar]
- 13.Scheid P. Mechanism of intrusion of a microspordian-like organism into the nucleus of host amoebae (Vannella sp.) isolated from a keratitis patient. Parasitol Res. 2007;101(4):1097–1102. doi: 10.1007/s00436-007-0590-z. [DOI] [PubMed] [Google Scholar]
- 14.Smirnov AV. Redescription of Vannella mira Schaeffer 1926 (Gymnamoebia, Vannellidae), an often mentioned but poorly known amoebae species. Protistology. 2002;2(3):178–184. [Google Scholar]
- 15.Sims GP, Aitken R, Rogerson A. Identification and phylogenetic analysis of morphologically similar naked amoebae using small subunit ribosomal RNA. J Eukaryot Microbiol. 2002;49(6):478–484. doi: 10.1111/j.1550-7408.2002.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 16.Bovee EC. An emendation of the ameba genus Flabellula and a description of Vannella gen. nov. Trans Am Microsc Soc. 1965;84(2):217–227. [Google Scholar]
- 17.Sims GP, Rogerson A, Aitken R. Primary and secondary structure of the small-subunit ribosomal RNA of the naked, marine amoeba Vannella anglica: phylogenetic implications. J Mol Evol. 1999;48(6):740–749. doi: 10.1007/pl00006518. [DOI] [PubMed] [Google Scholar]
- 18.Page FC. A New Key to Freshwater and Soil Gymnamoebae with Instructions for Culture. Cumbria: Freshwater Biological Association; 1988. p. 122. [Google Scholar]
- 19.Smirnov AV. Vannella ebro n. sp. (Lobosea, Gymnamoebia), isolated from cyanobacterial mats in Spain. Eur J Protistol. 2001;37(2):147–153. [Google Scholar]


