Abstract
Objective
To define the expression and function of DNA methyltransferases (DNMTs) in response to decidualizing stimuli in endometriotic cells compared with healthy endometrial stroma.
Design
Basic science.
Setting
University research center.
Patients
Premenopausal women with or without endometriosis.
Interventions
Primary cultures of stromal cells from healthy endometrium (E-IUM) or endometriomas (E-OSIS) were subjected to in vitro decidualization (IVD) using 1 µM medroxyprogesterone acetate, 35 nM 17β-estradiol, and 0.05 mM 8-Br-cAMP.
Main Outcome Measure(s)
DNMT1, DNMT3A, and DNMT3B expression in E-IUM and E-OSIS were assessed by qRT-PCR and immunoblotting. DNMT3B recruitment to the promoters of steroidogenic factor 1 (SF-1) and estrogen receptor α (ESR1) was examined by chromatin immunoprecipitation
Results
IVD treatment reduced DNMT3B mRNA (74%) and protein levels (81%) only in E-IUM. DNMT1 and DNMT3A were unchanged in both cell types. Significantly more DNMT3B bound to the SF-1 promoter in E-IUM compared with E-OSIS, and IVD treatment reduced binding in E-IUM to levels similar to those in E-OSIS. DNMT3B enrichment across three ESR1 promoters was reduced in E-IUM after IVD, although the more distal promoter showed increased DNMT3B enrichment in E-OSIS after IVD.
Conclusions
The inability to downregulate DNMT3B expression in E-OSIS may contribute to an aberrant epigenetic fingerprint that misdirects gene expression in endometriosis and contributes to its altered response to steroid hormones.
Keywords: endometriosis, DNA methyltransferase, decidualization, estrogen receptor, steroidogenic factor-1
INTRODUCTION
The human endometrium consists primarily of epithelial and stromal cells that undergo continued cycles of growth and shedding in order to maintain fertility. This cycle requires remarkable plasticity in the endometrial stroma, which must renew as well as differentiate in response to the sex steroids. The differentiation of estrogen-primed, uterine stromal cells into the stromal cells of pregnancy is termed decidualization, and is a feature common to most placental mammals (1). However, in humans and menstruating primates decidualization is initiated in response to ovarian hormones independently of an implanting blastocyst (2). Full differentiation and maintenance of the decidua is contingent upon pregnancy, since the absence of a conceptus provokes shedding of the pre-decidualized endometrial layer and menstruation, and it has been suggested that the evolution of spontaneous decidualization necessitated menstruation (3). This intriguing observation led us to propose that the gene regulatory pathways that coordinate spontaneous decidualization in menstruating primates are the key pathways dysregulated in endometriosis (4).
Endometriosis affects 5–10% of women of reproductive age. It is a chronic and recurrent disease characterized by the presence of endometrium-like tissue outside the uterine cavity, primarily on the ovaries and pelvic peritoneum (5, 6). The main symptoms are severe dysmenorrhea, chronic pelvic pain, dyspareunia, and it is a common cause of infertility (5, 6). While estrogen is crucial for the establishment and maintenance of endometriosis, the exact origin of the disease remains enigmatic (6–8). Most evidence supports Sampson’s model of endometriosis, suggesting that the disease arises from innate or acquired defects in endometrial tissue that has been shed to distal sites by retrograde menstruation (9–11). However, many cases of the disease cannot be explained by an endometrial origin, and it is difficult reconcile the fact that nearly all women experience retrograde menstruation, while only a fraction develop the disease (12, 13). Recent studies characterizing epigenetic defects in endometriosis may help explain this link. There has been explosive growth in the identification of genes whose expression is correlated with aberrant DNA methylation in endometriotic cells (4, 14–23), including many of the nuclear hormone receptors such as estrogen receptor alpha and beta (ESR1 and ESR2) and steroidogenic factor-1 (SF-1). In addition to supporting a role for epigenetic defects in the pathogenesis of endometriosis, it is remarkable that many of these genes serve key roles during decidualization (4, 24, 25).
DNA methylation is initiated and maintained by three DNA methyltransferases—DNMT1, DNMT3A, and DNMT3B—which catalyze the addition of methyl groups to the 5′ carbon of cytosine in targeted cytosine-phosphate-guanine dinucleotides (CpGs) (26). Unlike cancer, where the frequent hypermethylation of CpG islands (CGIs) are observed against a backdrop of global hypomethylation, experiments comparing global methylation in healthy endometrium and endometriosis identified more focused deviations in methylation (4, 27). Our recent work demonstrated that CpGs near transcriptional start sites but distal to classical CpG islands were significantly altered in endometriotic cells; however, the mechanism by which specific CpGs are altered is unknown. Studies examining the DNMTs and their binding partners in the endometrium have identified highly variable expression levels throughout the menstrual cycle, but there is a lack of consensus on the levels of functional protein in decidualizing stroma (28–32). Additionally, few genome-wide changes in methylation have been observed during decidualization, thus the effects of the DNMTs in the endometrium may be more tightly coordinated across key genes affecting stromal cell function (14, 32). Ovarian steroids have also been reported to alter the expression of DNMTs in both the eutopic endometrium of women with endometriosis, and in endometriotic lesions. There is again no consensus on how the DNMTs are expressed in the diseased stromal cells, but we and others have suggested that these DNMTs might affect key targets associated with disease progression (33).
We hypothesize that hormonally driven changes in DNMTs expression alter their genomic distribution in endometriotic cells, which may underlie the pathologic epigenetic defects observed in diseased tissues. Without prior knowledge of how DNMTs affect early stages of the disease, we sought to characterize DNMTs in stromal cells that were clearly healthy or diseased, and compared normal eutopic endometrium (E-IUM) to established endometriosis (E-OSIS). Herein we characterized the expression of all three DNMTs expression in vitro, identifying altered DNMT3B expression in E-OSIS. The functional outcomes of these findings were explored by defining DNMT3B occupation at known differentially methylated loci in two key genes affecting endometriosis: ESR1 and SF-1.
MATERIALS AND METHODS
Tissue acquisition
The Northwestern Institutional Review Board for Human Research approved this study (1375-005). Written, informed consent from each subject was obtained before surgery. Eutopic endometrium was obtained from subjects without surgically confirmed endometriosis (average age 43.8 ± 4.2 years) undergoing hysterectomy for benign conditions (cervical dysplasia or uterine leiomyoma). Ectopic endometriotic tissue from the cyst walls of surgically diagnosed ovarian endometriomas was obtained immediately after surgery from subjects with an average age of 40.5 ± 2.4 years. No subject received any preoperative hormonal therapy and all surgeries were performed during the proliferative stage of the patients’ menstrual cycle. Endometriosis was confirmed in the E-OSIS samples by histological examination.
Isolation and culture of primary stromal cells
Unless specified, all chemicals and reagents were obtained from Sigma (St. Louis, MO, USA). E-IUM and E-OSIS stromal cells were isolated from normal endometrium and endometriotic lesions as previously described, with minor modifications (34, 35). Tissues were first dissected by a pathologist. Following pathological evaluation of tissues, the stromal and glandular tissues were carefully dissected from surrounding uterine (for E-IUM) or ovarian endometriotic (for E-OSIS) tissues. Tissues were minced and digested first with collagenase and DNase at 37°C for 30 min, then with collagenase, DNase, pronase and hyaluronidase for an additional 30 min. Stromal cells were separated from epithelial cells by progressive filtration through 70- and 20-µm sieves. Cells were then dispensed for adherent growth and maintained in DMEM/F12 1:1 (GIBCO/BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 1,000 units/mL penicillin G, 0.1 mg/mL streptomycin sulfate, and 0.25 µg/mL Amphotericin B. Adherent cells were grown in a humidified atmosphere with 5% CO2 at 37°C.
In vitro decidualization
In vitro decidualization (IVD) treatments were performed as previously described (36). Briefly, both normal and diseased stromal cells were grown to ~75% confluency, and then switched to DMEM/F12 1:1 media containing 2% charcoal-dextran-stripped FBS. Cells were then stimulated with media containing 1 µM medroxyprogesterone acetate (MPA), 35 nM 17β-estradiol (E2) and 0.05 mM 8-Br-cAMP (BIOLOG Life Science Institute, Bremen Germany). The medium was replenished every other day for 14 days.
Cell imaging
Stromal cells were cultured on sterile #1 glass coverslips, and subjected to IVD. Cells were fixed in 4% paraformaldehyde and then stained as described previously (37). Cells were examined with a Zeiss Axiovert 200 with a 20× LDPlan-NEOFLUAR objective and images were acquired using an Axiocam HRc.
Preparation of total RNA and quantitative analysis by qRTPCR
The preparation of cDNA for PCR was performed on 1 µg of total RNA (RNeasy Mini Kit, QIAGEN, Valencia, CA) using Q-script cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA) (38). Real-time qPCR was performed using SYBR green Taq in an ABI 7900 (Applied Biosystems, Foster City, CA, USA). The mRNA levels of target genes were quantified relative to 18S mRNA and beta-actin using the comparative threshold (ΔΔCT) method as described previously (39). Primer documentation is provided in Supplemental table 1.
Preparation of protein and immunoblotting
Whole cell lysates from E-IUM and E-OSIS were prepared by washing the cells with ice-cold PBS and scraping the cells in RIPA buffer (50 mM Tris pH 7.6, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40) supplemented with protease inhibitors. Lysates were cleared by centrifugation at 14,000 × g for 10 min. Equal amounts of protein (20 µg) were resolved on 4–12% Bis-Tris Gels (Invitrogen, Carlsbad, CA, USA) for 90 min at 100 V, transferred to PVDF membranes, and blocked with 4% nonfat milk. The antibodies used are documented in Supplemental table 2. Protein bands were detected by chemiluminescence, and quantified using ImageJ software (http://rsbweb.nih.gov/ij/) (40).
Chromatin immunoprecipitation (ChIP) assay
The in situ binding of DNMT3B to the promoter regions of the ESR1 and SF1 genes was analyzed using ChIP as previously described (41). E-IUM and E-OSIS were cultured in 15-cm plates and subjected to IVD or vehicle treatment for 14 days. The cells were then cross-linked with 1% formaldehyde at 37°C for 10 min. Cross-linking reactions were stopped by the addition of a 1/10 volume of 1.5 M glycine and incubation at room temperature for 10 minutes. The cross-linked chromatin was sheared on ice using a Branson sonifier 250 (G. Heinemann, Schwäbish Gmünd, Germany; average DNA length < 0.5 kb). The soluble chromatin fraction was immunoprecipitated with an equal amount of either mouse IgG or DNMT3B antibody overnight. The immunoprecipitated chromatin was purified (42) and analyzed by qPCR as described above. PCR data was quantified as the fold enrichment of ChIPped DNA relative to IgG. ChIP primers were designed using the Primer3 Software (http://frodo.wi.mit.edu/primer3/input.htm) and documented in Supplemental table 1 (43).
Statistical analysis
Results are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA followed by Tukey’s test. Significance was accepted for P-values < 0.05.
RESULTS
In vitro decidualization of human E-IEM and E-OSIS stromal cells
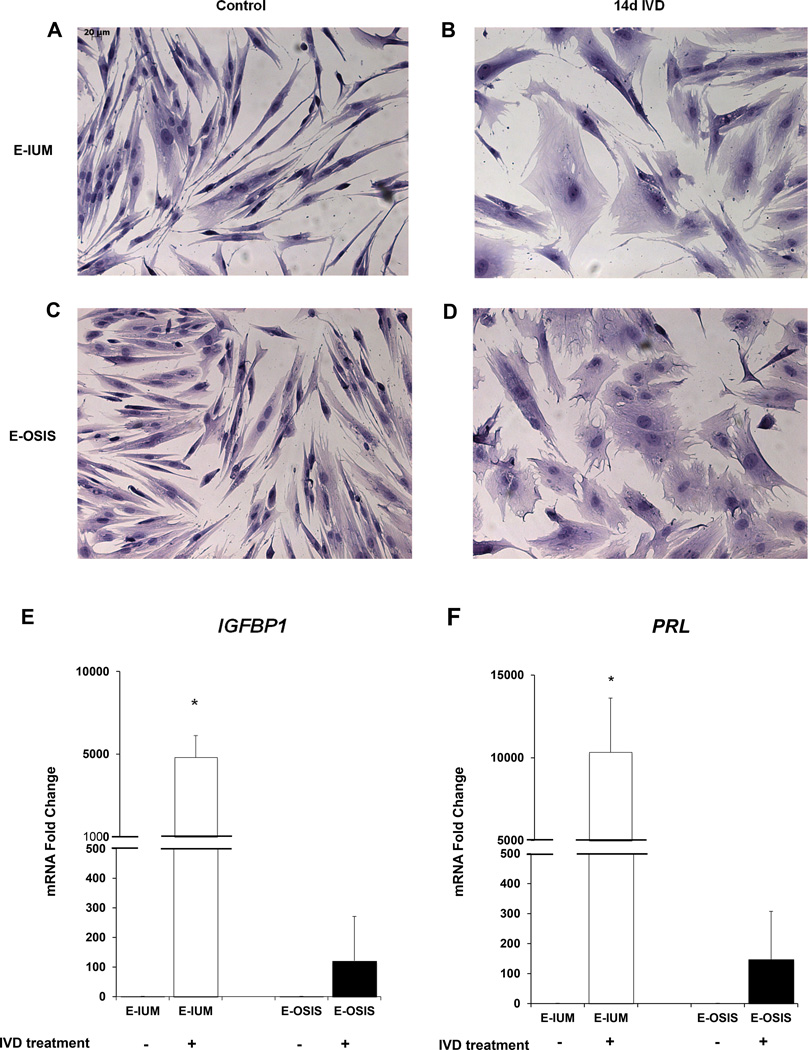
Both groups of untreated cells retained a spindle-like appearance that was lost during IVD. Following IVD, both E-IUM and E-OSIS stromal cells developed into large polygonal cells with increased intracellular spaces (Fig. 1A–D). Morphological differences were apparent between E-IUM and E-OSIS cells after IVD, with E-OSIS displaying irregularly-shaped cell membranes, characterized by more numerous and sharper cellular extensions (Fig. 1B, D). After IVD, the nuclei of E-IUM, but not E-OSIS, showed slightly increased eosin staining. We further characterized E-IUM and E-OSIS by measuring mRNA levels of the decidual stromal markers IGFBP1 and PRL after IVD. Consistent with previous reports, IGFBP1 and PRL were expressed in both cell types after IVD treatment; however, the induction of these markers in E-OSIS cells was significantly lower than in E-IUM cells (Fig. 1E, F) (44). This indicated that our time course and IVD treatment were sufficient to induce the differentiation characteristics of decidualization.
Figure 1.
In vitro decidualization of E-IUM and E-OSIS stromal cells. Changes in cellular morphology were visualized by H&E staining of (A) untreated E-IUM and (B) E-IUM cells following 14-day IVD. Changes were also observed in (C) untreated E-OSIS and (D) E-OSIS cells after 14-day IVD (×20 objective). Spindle-shape morphology was lost in both cell types, but membrane ruffling was more apparent in E-OSIS than E-IUM cells following IVD. Expression of IGFBP1 (E) and PRL (F) mRNA were measured in E-IUM and E-OSIS cells as markers of decidualization. Error bars are SEM, n=6, *P<0.01 compared to untreated controls.
DNMT expression during IVD
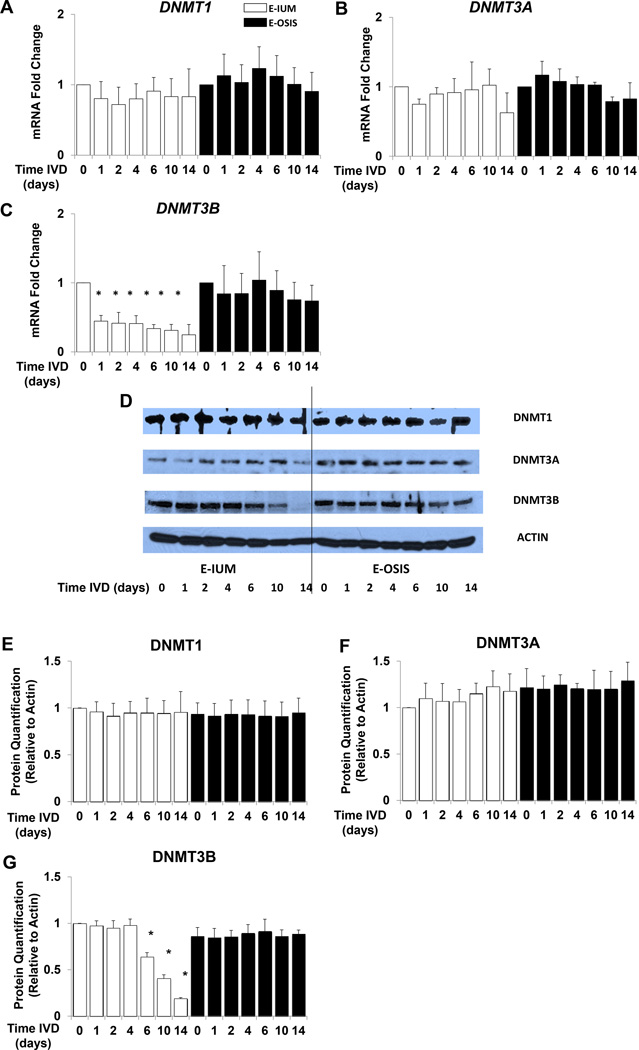
IVD-induced changes in DNMT1, DNMT3A, and DNMT3B mRNA expression in E-IUM and E-OSIS are shown in Fig. 2. Detectable levels of all three genes were observed in untreated E-IUM and E-OSIS cells. While DNMT1 and DNMT3A were unchanged in E-IUM and E-OSIS after IVD (Fig. 2A, B). expression of DNMT3B decreased by 59% (P < 0.05) in E-IUM cells within 24 h of IVD treatment. The levels of DNMT3B progressively fell for the duration of the IVD treatment, and were reduced by 74% on day 14 of IVD relative to controls. In E-OSIS, DNMT3B expression remained unchanged in response to IVD (Fig. 2C).
Figure 2.
IVD changes DNMT1, DNMT3A, and DNMT3B expression in E-IUM and E-OSIS stromal cells. E-IUM and E-OSIS cells underwent IVD treatment for 14 days. Changes in mRNA expression of (A) DNMT1, (B) DNMT3A, and (C) DNMT3B at successive time points were analyzed by qRTPCR. Error bars are SEM, n=6 *P<0.05 compared to E-IUM untreated controls. DNMT immunoblots of matching IVD time courses are shown for (D) E-IUM and E-OSIS. Beta actin was used as a loading control. (E) DNMT1, (F) DNMT3A, and (G) DNMT3B proteins were quantified using ImageJ software. Error bars are SEM, n=4, *P<0.05 compared to E-IUM untreated controls.
Immunoblot analysis was performed to measure DNMT1, DNMT3A, and DNMT3B protein expression in E-IUM and E-OSIS stromal cells in response to IVD (Fig. 2D–G). Similar to the mRNA data, all three isoforms of DNMT were detectable in both E-IUM and E-OSIS. Comparable basal expression was observed with respect to each isoform in both normal and diseased cells. The pattern of change in protein expression for the DNMT isoforms was similar to that seen for mRNA, with DNMT1 and DNMT3A protein levels in E-IUM and E-OSIS remaining unchanged in response to IVD (Fig. 2D–F). While DNMT3B expression decreased in E-IUM, significant differences were not observed until after day 6 of IVD (P < 0.05). By day 14 of IVD, DNMT3B protein levels were 19% of the controls. No change in DNMT3B protein level was observed in E-OSIS (Fig. 2D, G).
ChIP analysis of DNMT3B binding to the SF-1 and ESR1 genes
DNMT3B is conventionally thought to induce de novo DNA methylation. Its downregulation in E-IUM during IVD suggested that DNMT3B might affect gene methylation in normal endometrium throughout decidualization. Similarly, the expression of DNMT3B in E-OSIS independent of steroid signaling during IVD may correlate with the aberrant gene methylation observed in endometriotic tissues. To explore this, we performed DNMT3B ChIP analysis at regions near the promoters of SF-1 and ESR1. Specific regions of these two genes are differentially expressed in E-IUM and E-OSIS, and we have previously characterized the patterns of differential methylated DNA across these genes (4, 6, 15, 45). ChIP was performed with and without IVD.
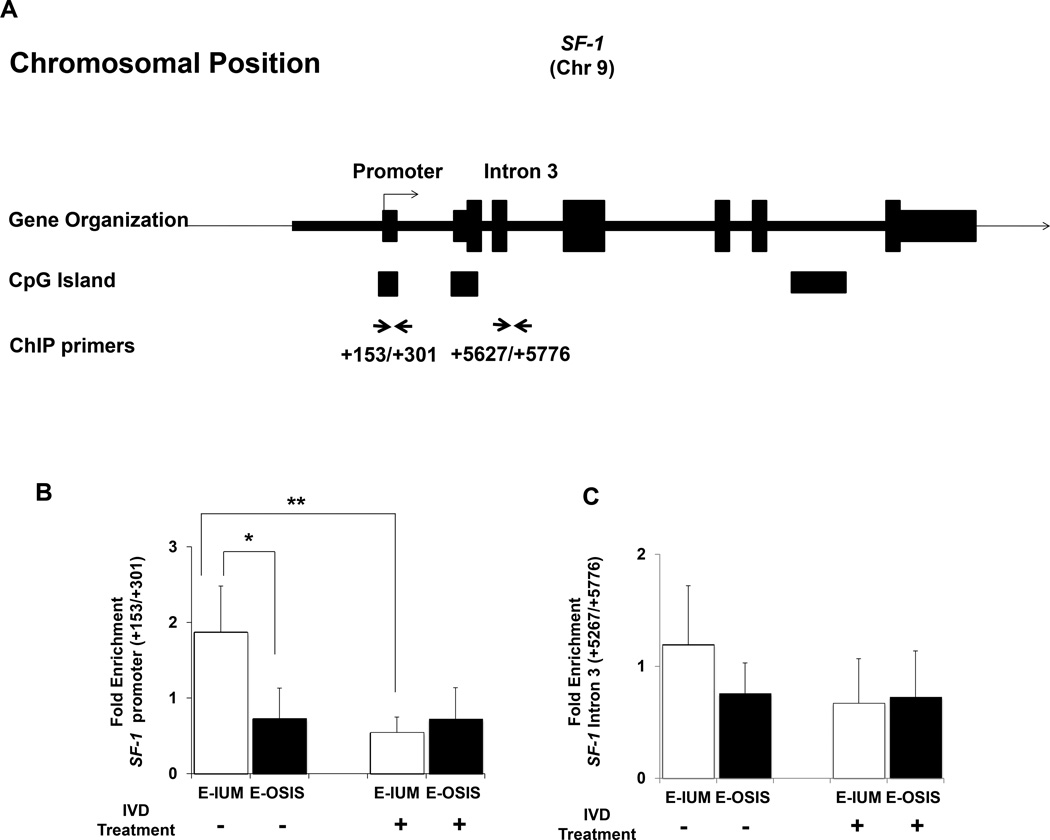
Tissue-specific SF-1 expression is strongly affected by the methylation of both its basal promoter as well as multiple intronic regions (15, 46). Two primer pairs were used for ChIP analysis of the SF-1 gene in untreated and treated stromal cells (Fig. 3A). The first amplicon included CpGs near the transcriptional start site (TSS) of SF-1 that are methylated in E-IUM but not E-OSIS, and which contribute to pathologic SF-1 expression in the diseased cells. The second primer pair amplified the intronic region downstream of exon 3, and is also differentially methylated, being methylated in E-OSIS but not E-IUM. DNMT occupancy near the TSS was reduced by 71% in E-IUM cells following IVD (Fig. 3B, P < 0.01). A lower level of DNMT3B recruitment was seen in untreated E-OSIS cells (E-IUM vs. E-OSIS, P < 0.05), and remained low after IVD. With the second primer pair, while DNMT3B enrichment trended downward relative to untreated E-IUM, there was no statistical difference across the groups (Fig. 3C).
Figure 3.
ChIP assay of DNMT3B enrichment at SF-1 in E-IUM and E-OSIS stromal cells. (A) Organization of the SF-1 gene, showing primer binding sites used for ChIP in relation to the promoter and intron 3. ChIP for DNMT3B was performed on chromatin from E-IUM and E-OSIS cells with or without 14-day IVD treatments. Data shows average fold-enrichment of DNMT3B relative to IgG for four independent ChIP experiments at the SF-1 (B) promoter and (C) intron 3. Error bars are SEM. Statistical differences were detected by ANOVA followed by Tukey’s test. *P<0.05, **P<0.01.
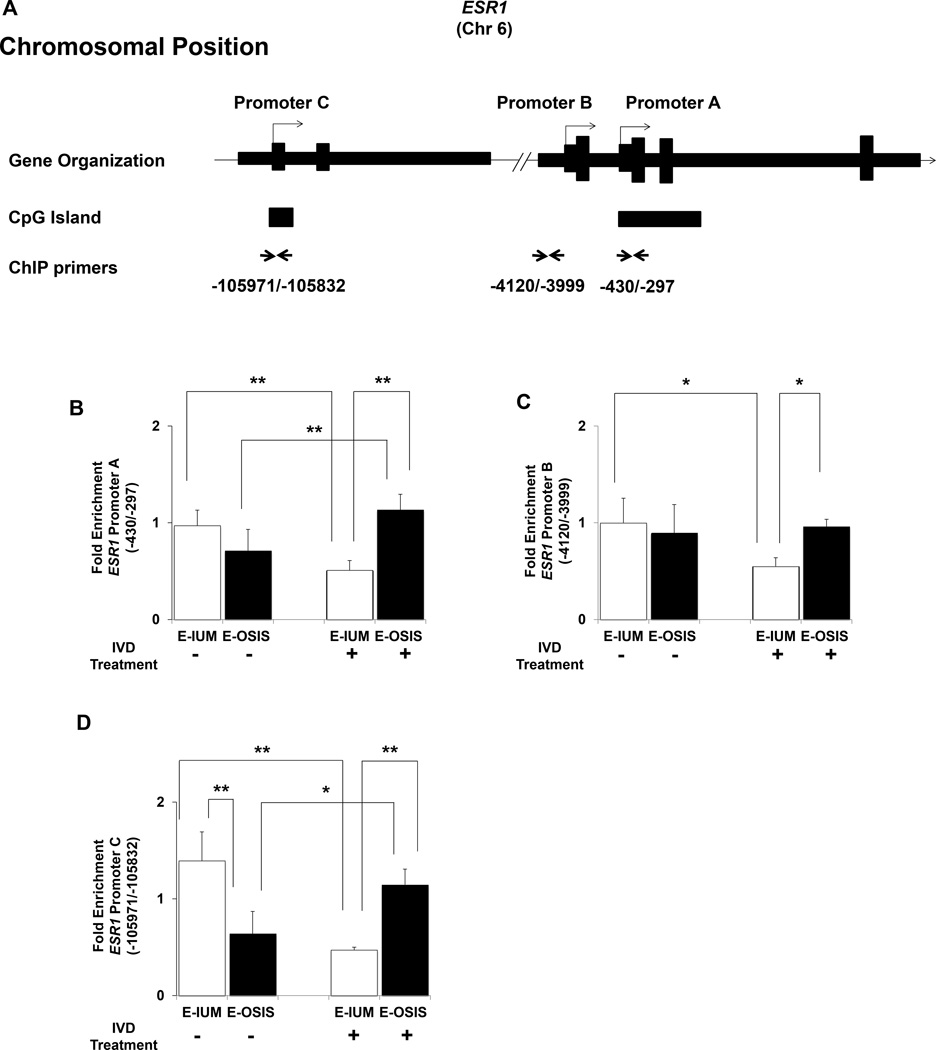
The effect of DNA methylation on ESR1 expression in the endometrium is poorly understood. Hypomethylation of ESR1 in breast cancers correlates with increased expression, and more recently, DNMT3B was hypothesized to target ESR1 in the endometrium during the estrous cycle (47–49). Our previous work characterizing ESR1 expression in the same cell types revealed that promoter A is primarily responsible for steroid-regulated expression in E-IUM. Promoters A and C are utilized in E-OSIS (Fig. 4A), although with significantly less activity compared to healthy cells (43). More recently we discovered several differentially methylated regions clustered near the ESR1 promoters (4). ChIP analysis of these same regions revealed that DNMT3B enrichment was reduced (by approximately 50%, P < 0.05) at all three ESR1 promoters in E-IUM cells after IVD relative to untreated E-IUM cells (Fig. 4B–D). In contrast, DNMT3B enrichment at promoters A and B remained unchanged in E-OSIS cells after IVD, compared to either untreated E-OSIS or untreated E-IUM cells. Promoter C was unique, as DNMT3B enrichment was 54% lower (P < 0.05) in untreated E-OSIS cells relative to untreated E-IUM cells, and then increased to levels equal to those of untreated E-IUM cells after IVD.
Figure 4.
ChIP assay of DNMT3B enrichment across ESR1 promoters in E-IUM and E-OSIS stromal cells. (A) Organization of the ESR1 gene, showing primer binding sites used for ChIP. Promoters A, B, and C are depicted as previously described (43). ChIP for DNMT3B was performed on chromatin from E-IUM and E-OSIS cells with or without 14-day IVD treatments. Data shows average fold-enrichment of DNMT3B relative to IgG for four independent ChIP experiments at ESR1 (B) promoter A, (C) promoter B, and (D) promoter C regions. Error bars are SEM. Statistical differences were detected by ANOVA followed by Tukey’s test. *P<0.05, **P<0.01.
DISCUSSION
The altered phenotype of endometriotic cells has a strong epigenetic component, and variation in DNMT is a putative mechanism affecting DNA methylation in diseased cells (29–32, 50–52). Here we demonstrated that EIUM and E-OSIS express all three DNMT isoforms, but that IVD treatment reduced DNMT3B only in healthy stromal cells. This decrease correlated with reduced DNMT3B occupancy of key regions of ESR1 and SF-1 that are differentially methylated and associated with aberrant expression in diseased cells (4, 6).
Several groups have examined DNMT1 expression in whole tissue isolates of the endometrium, producing conflicting reports (29–31, 53). The greatest discrepancies occur at the mRNA level. Several groups report decreased expression of DNMT1 in whole endometrium during the secretory phase, whereas van Kaam et al found increased DNMT1 at similar time points. Additionally, DNMT1 levels appear reduced in endometrial explants treated with estradiol and progesterone (30, 31), and Grimaldi et al showed DNMT1 is transiently repressed by IVD in isolated healthy stromal cells (32). We observed little change in DNMT1 expression after IVD of isolated stromal cells, consistent with Yamagata et al. While difficult to resolve completely, it is possible that DNMT1 expression in the endometrial epithelium is more sensitive to steroid hormones, and as van Kaam points out, the epithelial response to steroids would be affected by the presence or absence of stromal cells. Notably, the previous reports found little change in DNMT1 protein levels throughout the menstrual cycle by immunohistochemistry (IHC), although staining appeared stronger in the epithelium. This supports our finding that stromal DNMT1 expression is unchanged by IVD. Fewer reports have examined DNMT1 in endometriosis. Wu et al found elevated DNMT1 mRNA levels in endometriosis, but only looked only in epithelium (33). Additionally, DNMT1 appeared to be low in whole tissue taken from endometriotic lesions during the proliferative phase, but little change was observed in protein levels by IHC (30). As before, it seems likely that DNMT1 expression varies in the diseased epithelium more significantly than in the stroma, where detectable protein levels remain unchanged.
Unlike DNMT1, which is thought to maintain existing methylation patterns, the DNMT3 isoforms are thought to coordinate de novo methylation (54, 55). Both endometrial explants and isolated stromal cells have been shown to express decreased levels of DNMT3A and DNMT3B mRNA in response to IVD treatment; however, no change has been previously shown at the protein level (29, 31, 32). Similarly, mRNA levels of DNMT3A and DNMT3B trend downward during the secretory phase of the menstrual cycle, but IHC of the endometrium throughout the cycle does not reveal detectable differences in protein levels (29). In women with endometriosis, Wu et al. showed increased DNMT3A and DNMT3B mRNA in ectopic endometriotic epithelium (33). More recently Szczepanka described elevated DNMT3A mRNA expression in the eutopic endometrium from women with endometriosis, although the other DNMTs remained unchanged (56). In contrast, Van Kaam observed little change in DNMT3B in whole tissue explants (30). Our data helps clarify previous findings, and shows that both DNMT3A and DNMT3B are readily detectable at the protein level in normal and diseased stromal cells. Taken alongside the many observations from other groups, we would generalize this and suggest that decreased DNMT expression is characteristic of secretory phase endometrium; however, the epigenetic machinery in the epithelium and the stroma appear to be regulated through unique hormone-dependent pathways. It is likely that many of the conflicting reports result from the dynamic and variable proportions of cell types making up the endometrium throughout the menstrual cycle. Such inherent heterogeneity commonly cofounds gene expression studies when used to examine whole tissue (57).
Cell differentiation and cell fate decisions are commonly governed by specific changes in DNA methylation, and it is suggested that changes in DNA methylation could underlie the differentiation of stromal cells during decidualization (58). Evidence indicates that global patterns of DNA methylation remain unchanged in response to IVD (4, 32). It is more likely that focused changes in methylation arise in the endometrium as DNMTs target specific CpGs. In contrast, it is striking that endometriotic cells maintain a fixed expression of DNMTs. DNA methylation in endometriotic cells changes very little in response to IVD, and we suspect that aberrant DNA methylation is a defining feature of endometriosis that limits the differentiation potential of the cells once the disease becomes established. The loss of fidelity in the patterns of tissue-specific methylation pattern is now linked to a wide number of diseases (59). In malignancies, the deregulation of DNA methylation leads to hypermethylation of CpG islands, but a loss of methylation elsewhere (60–62). However, endometriosis is characterized by unchanging DNMT expression, and the mechanism by which the DNMTs cooperate to affect gene expression in the endometrium is not fully understood (26). DNMT3B binding has been reported to affect gene expression in instances both dependent and independent of the methylation status of the DNA to which it is bound (63, 64). Consequently, we were very interested in the recruitment pattern of DNMTs in E-IUM and E-OSIS to regions of the chromatin where the methylation pattern is already established to be different between the cell types (4, 15, 45, 65).
The cycling availability of DNMT3B in E-IUM contrasted with its constant expression in OSIS, and this observation was largely recapitulated in our ChIP data. Generally, DNMT3B recruitment to SF-1 was reduced in correspondence with its expression levels in healthy stromal cells. However, DNMT3B was poorly recruited to the promoter of SF-1 in E-OSIS, despite its consistent abundance. This underscores the complex mechanisms that direct DNMT recruitment to the chromatin. Transcription factors, polycomb-group proteins, microRNAs, and histone modifications all interact with the DNMTs in directing DNA methylation (66–69). In this context, unmethylated elements of the SF-1 promoter are inaccessible to DNMT3B in E-OSIS, but not in E-IUM, where DNMT3B recruitment and hypermethylation correlate with gene silencing. In contrast, recruitment of DNMT3B to the intronic region of SF-1 is not significantly affected by availability, yet this region is fully methylated in only the diseased cells. Prolonged occupancy of this region by the DNMTs may be necessary to maintain the continued expression of SF-1.
The role of DNMT3B in regulating ESR1 appears reversed, since increased DNMT3B recruitment was not observed across ESR1 promoters in E-OSIS. One possibility is that other DNMT isoforms are responsible for ESR1 methylation. Notably, DNMT1 is associated with ESR1 silencing in breast cancer (70). Alternatively, the methylation of distal enhancers may be more important in governing the ESR1 expression. A simple interpretation of our findings is that DNMT3B maintains the methylation of SF-1 in healthy tissue while repressing ESR1 in diseased tissue. However, the exact role of DNMT3B in governing these promoters is likely to be more complex, and complimentary studies of DNMT1 and DNMT3A recruitment to these promoters along with bisulfite sequencing of these genes will undoubtedly shed more light on how they are specifically methylated.
We predict the bias for developing endometriosis arises when cells susceptible to aberrant DNA methylation are either shed or develop in the peritoneum. One possibility is that a defective methylation signature in eutopic endometrial cells predisposes them to persisting as endometriosis in the peritoneal environment. Should this defective epigenetic fingerprint arise or develop in other tissues, it is plausible that they too could develop into endometriosis-like lesions. Alternatively, the endometrium may retain a population of epigenetically naïve cells, possibly stem cells, that maintain endometrial regenerative capacity, but when shed to distal sites possess the ability to differentiate aberrantly into endometriosis. Supporting this concept, the success rates for using menstrual tissue to surgically induce endometriosis in non-human primates approaches 100%, suggesting that the endometrium naturally harbors the cells that give rise to endometriosis, but that they are not typically shed in sufficient quantities to initiate the disease (71). Research has yet to fully define the characteristics of endometrial stem cells, but emerging evidence suggests these cells are pivotal for maintaining the endometrium’s ability to regenerate (72). Further work exploring epigenetic controls in the endometrium’s stem/progenitor cell populations will likely provide a deeper understanding of how stromal cell plasticity is maintained through each menstrual cycle.
The present findings set the stage for several key future studies. Our use of homogenous cultured primary stromal cells allowed strongly reduced the confounding effects that arise when examining complex tissues. However, the interpretations of our findings are tempered by this in vitro model, and need to be evaluated in vivo. The next critical experiment will be to determine if altered DNMT recruitment and aberrant DNA methylation are present in the eutopic endometrium of women with endometriosis. This presents an enormous challenge as the cells bearing an aberrant epigenetic fingerprint may not comprise a significant percentage of the cells in the endometrium, and dissecting this signal will be challenging. Moreover, many of the changes in DNA methylation that are observed in ectopic tissue are likely to arise during or after its transplantation to the pelvic peritoneum. Recent studies demonstrated that the altered uterine environment caused by endometriosis potently alters gene expression in both eutopic endometrial tissue and in the developing lesions (73). We anticipate the results presented here along with future studies profiling the DNA methylation in diseased cells will allow us to prioritize the most likely targets underlying the disease, and will provide a foundation for accurately determining how the epigenetic differences defining endometriosis arise.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health, National Institute of Child Health and Human Development grants (R37-HD038691 and U54-HD40093 to S.E.B.), and by funding from the Friends of Prentice Grants Initiative (to M.T.D).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: M.T.D. has nothing to disclose. T.K. has nothing to disclose. M.E.P. has nothing to disclose. D.M. has nothing to disclose. A.N. has nothing to disclose. S.S.M. has nothing to disclose. M.O. has nothing to disclose. S.E.B. has nothing to disclose.
REFERENCES
- 1.Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28:17–26. doi: 10.1055/s-0029-1242989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 3.Emera D, Romero R, Wagner G. The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness. Bioessays. 2012;34:26–35. doi: 10.1002/bies.201100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyson MT, Roqueiro D, Monsivais D, Ercan CM, Pavone ME, Brooks DC, et al. Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis. PLoS Genet. 2014;10:e1004158. doi: 10.1371/journal.pgen.1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 6.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 7.Osteen KG, Bruner-Tran KL, Eisenberg E. Endometrial biology and the etiology of endometriosis. Fertil Steril. 2005;84:33–34. doi: 10.1016/j.fertnstert.2005.01.124. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 8.Gurates B, Bulun SE. Endometriosis: the ultimate hormonal disease. Semin Reprod Med. 2003;21:125–134. doi: 10.1055/s-2003-41319. [DOI] [PubMed] [Google Scholar]
- 9.Dyson MT, Bulun SE. Cutting SRC-1 down to size in endometriosis. Nat Med. 2012;18:1016–1018. doi: 10.1038/nm.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bricou A, Batt RE, Chapron C. Peritoneal fluid flow influences anatomical distribution of endometriotic lesions: why Sampson seems to be right. Eur J Obstet Gynecol Reprod Biol. 2008;138:127–134. doi: 10.1016/j.ejogrb.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Signorile PG, Baldi F, Bussani R, Viceconte R, Bulzomi P, D'Armiento M, et al. Embryologic origin of endometriosis: analysis of 101 human female fetuses. J Cell Physiol. 2012;227:1653–1656. doi: 10.1002/jcp.22888. [DOI] [PubMed] [Google Scholar]
- 13.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- 14.Furst RW, Meyer HH, Schweizer G, Ulbrich SE. Is DNA methylation an epigenetic contribution to transcriptional regulation of the bovine endometrium during the estrous cycle and early pregnancy? Mol Cell Endocrinol. 2012;348:67–77. doi: 10.1016/j.mce.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, et al. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5' CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92:3261–3267. doi: 10.1210/jc.2007-0494. [DOI] [PubMed] [Google Scholar]
- 16.Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77:681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1:106–111. doi: 10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 2005;193:371–380. doi: 10.1016/j.ajog.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Izawa M, Harada T, Taniguchi F, Ohama Y, Takenaka Y, Terakawa N. An epigenetic disorder may cause aberrant expression of aromatase gene in endometriotic stromal cells. Fertil Steril. 2008;89:1390–1396. doi: 10.1016/j.fertnstert.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 20.Nasu K, Kawano Y, Tsukamoto Y, Takano M, Takai N, Li H, et al. Aberrant DNA methylation status of endometriosis: epigenetics as the pathogenesis, biomarker and therapeutic target. J Obstet Gynaecol Res. 2011;37:683–695. doi: 10.1111/j.1447-0756.2011.01663.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Chen Q, Zhang C, Ren F, Li T. DNA hypomethylation of the COX-2 gene promoter is associated with up-regulation of its mRNA expression in eutopic endometrium of endometriosis. Eur J Med Res. 2012;17:12. doi: 10.1186/2047-783X-17-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamagata Y, Nishino K, Takaki E, Sato S, Maekawa R, Nakai A, et al. Genome-wide DNA methylation profiling in cultured eutopic and ectopic endometrial stromal cells. PLoS One. 2014;9:e83612. doi: 10.1371/journal.pone.0083612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi H, Ilagan Y, Krikun G, Taylor HS. Altered Genome-Wide Methylation in Endometriosis. Reprod Sci. 2014 doi: 10.1177/1933719114532841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubel CA, Franco HL, Jeong JW, Lydon JP, DeMayo FJ. GATA2 is expressed at critical times in the mouse uterus during pregnancy. Gene Expr Patterns. 2012;12:196–203. doi: 10.1016/j.gep.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Gao F, Ma X, Rusie A, Hemingway J, Ostmann AB, Chung D, et al. Epigenetic changes through DNA methylation contribute to uterine stromal cell decidualization. Endocrinology. 2012;153:6078–6090. doi: 10.1210/en.2012-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo SW. The endometrial epigenome and its response to steroid hormones. Mol Cell Endocrinol. 2012;358:185–196. doi: 10.1016/j.mce.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Borghese B, Barbaux S, Mondon F, Santulli P, Pierre G, Vinci G, et al. Research resource: genome-wide profiling of methylated promoters in endometriosis reveals a subtelomeric location of hypermethylation. Mol Endocrinol. 2010;24:1872–1885. doi: 10.1210/me.2010-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao X, Siu MK, Chan KY, Wong ES, Ngan HY, Chan QK, et al. Hypermethylation of RAS effector related genes and DNA methyltransferase 1 expression in endometrial carcinogenesis. Int J Cancer. 2008;123:296–302. doi: 10.1002/ijc.23494. [DOI] [PubMed] [Google Scholar]
- 29.Yamagata Y, Asada H, Tamura I, Lee L, Maekawa R, Taniguchi K, et al. DNA methyltransferase expression in the human endometrium: down-regulation by progesterone and estrogen. Hum Reprod. 2009;24:1126–1132. doi: 10.1093/humrep/dep015. [DOI] [PubMed] [Google Scholar]
- 30.van Kaam KJ, Delvoux B, Romano A, D'Hooghe T, Dunselman GA, Groothuis PG. Deoxyribonucleic acid methyltransferases and methyl-CpG-binding domain proteins in human endometrium and endometriosis. Fertil Steril. 2011;95:1421–1427. doi: 10.1016/j.fertnstert.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 31.Vincent ZL, Farquhar CM, Mitchell MD, Ponnampalam AP. Expression and regulation of DNA methyltransferases in human endometrium. Fertil Steril. 2011;95:1522 e1–1525 e1. doi: 10.1016/j.fertnstert.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Grimaldi G, Christian M, Quenby S, Brosens JJ. Expression of epigenetic effectors in decidualizing human endometrial stromal cells. Mol Hum Reprod. 2012 doi: 10.1093/molehr/gas020. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril. 2007;87:24–32. doi: 10.1016/j.fertnstert.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 34.Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78:642–649. doi: 10.1210/jcem.78.3.8126136. [DOI] [PubMed] [Google Scholar]
- 35.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 36.Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13:323–332. doi: 10.1093/molehr/gam005. [DOI] [PubMed] [Google Scholar]
- 37.Schutte SC, Taylor RN. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil Steril. 2012;97:997–1003. doi: 10.1016/j.fertnstert.2012.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro A, Yin P, Monsivais D, Lin SM, Du P, Wei JJ, et al. Genome-wide DNA methylation indicates silencing of tumor suppressor genes in uterine leiomyoma. PLoS One. 2012;7:e33284. doi: 10.1371/journal.pone.0033284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, et al. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26:2157–2164. doi: 10.1093/humrep/der172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee TI, Johnstone SE, Young RA. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc. 2006;1:729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujita PA, Rhead B, Zweig AS, Hinrichs AS, Karolchik D, Cline MS, et al. The UCSC Genome Browser database: update 2011. Nucleic Acids Res. 2011;39:D876–D882. doi: 10.1093/nar/gkq963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94:615–622. doi: 10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue Q, Zhou YF, Zhu SN, Bulun SE. Hypermethylation of the CpG island spanning from exon II to intron III is associated with steroidogenic factor 1 expression in stromal cells of endometriosis. Reprod Sci. 2011;18:1080–1084. doi: 10.1177/1933719111404614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoivik EA, Bjanesoy TE, Mai O, Okamoto S, Minokoshi Y, Shima Y, et al. DNA methylation of intronic enhancers directs tissue-specific expression of steroidogenic factor 1/adrenal 4 binding protein (SF-1/Ad4BP) Endocrinology. 2011;152:2100–2112. doi: 10.1210/en.2010-1305. [DOI] [PubMed] [Google Scholar]
- 47.Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, et al. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furst RW, Kliem H, Meyer HH, Ulbrich SE. A differentially methylated single CpG-site is correlated with estrogen receptor alpha transcription. J Steroid Biochem Mol Biol. 2012;130:96–104. doi: 10.1016/j.jsbmb.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 50.Zhou H, Li J, Podratz KC, Tipton T, Marzolf S, Chen HB, et al. Hypomethylation and activation of syncytin-1 gene in endometriotic tissue. Curr Pharm Des. 2014;20:1786–1795. doi: 10.2174/13816128113199990540. [DOI] [PubMed] [Google Scholar]
- 51.Szczepanska M, Mostowska A, Wirstlein P, Malejczyk J, Ploski R, Skrzypczak J, et al. Polymorphic variants of DNMT3A and the risk of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2013;166:81–85. doi: 10.1016/j.ejogrb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Borghese B, Santulli P, Hequet D, Pierre G, de Ziegler D, Vaiman D, et al. Genetic polymorphisms of DNMT3L involved in hypermethylation of chromosomal ends are associated with greater risk of developing ovarian endometriosis. Am J Pathol. 2012;180:1781–1786. doi: 10.1016/j.ajpath.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Logan PC, Ponnampalam AP, Steiner M, Mitchell MD. Effect of cyclic AMP and estrogen/progesterone on the transcription of DNA methyltransferases during the decidualization of human endometrial stromal cells. Mol Hum Reprod. 2013;19:302–312. doi: 10.1093/molehr/gas062. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Oakeley EJ, Sun L, Jost JP. Multiple domains are involved in the targeting of the mouse DNA methyltransferase to the DNA replication foci. Nucleic Acids Res. 1998;26:1038–1045. doi: 10.1093/nar/26.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh CL. In vivo activity of murine de novo methyltransferases, Dnmt3a and Dnmt3b. Mol Cell Biol. 1999;19:8211–8218. doi: 10.1128/mcb.19.12.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szczepanska M, Wirstlein P, Skrzypczak J, Jagodzinski PP. Expression of HOXA11 in the mid-luteal endometrium from women with endometriosis-associated infertility. Reprod Biol Endocrinol. 2012;10:1. doi: 10.1186/1477-7827-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Repsilber D, Kern S, Telaar A, Walzl G, Black GF, Selbig J, et al. Biomarker discovery in heterogeneous tissue samples -taking the in-silico deconfounding approach. BMC Bioinformatics. 2010;11:27. doi: 10.1186/1471-2105-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munro SK, Farquhar CM, Mitchell MD, Ponnampalam AP. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod. 2010;16:297–310. doi: 10.1093/molehr/gaq010. [DOI] [PubMed] [Google Scholar]
- 59.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 60.McCabe MT, Brandes JC, Vertino PM. Cancer DNA methylation: molecular mechanisms and clinical implications. Clin Cancer Res. 2009;15:3927–3937. doi: 10.1158/1078-0432.CCR-08-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plass C, Smiraglia DJ. Genome-wide analysis of DNA methylation changes in human malignancies. Curr Top Microbiol Immunol. 2006;310:179–198. doi: 10.1007/3-540-31181-5_9. [DOI] [PubMed] [Google Scholar]
- 62.Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, et al. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res. 1999;27:2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghoshal K, Motiwala T, Claus R, Yan P, Kutay H, Datta J, et al. HOXB13, a target of DNMT3B, is methylated at an upstream CpG island, and functions as a tumor suppressor in primary colorectal tumors. PLoS One. 2010;5:e10338. doi: 10.1371/journal.pone.0010338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Bai S, Ghoshal K, Jacob ST. Identification of T-cadherin as a novel target of DNA methyltransferase 3B and its role in the suppression of nerve growth factor-mediated neurite outgrowth in PC12 cells. J Biol Chem. 2006;281:13604–13611. doi: 10.1074/jbc.M513278200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Xue Q, Xu Y, Yang H, Zhang L, Shang J, Zeng C, et al. Methylation of a novel CpG island of intron 1 is associated with steroidogenic factor 1 expression in endometriotic stromal cells. Reprod Sci. 2014;21:395–400. doi: 10.1177/1933719113497283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 67.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 68.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 69.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–7029. [PubMed] [Google Scholar]
- 71.D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–161. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- 72.Deane JA, Gualano RC, Gargett CE. Regenerating endometrium from stem/progenitor cells: is it abnormal in endometriosis, Asherman's syndrome and infertility? Curr Opin Obstet Gynecol. 2013;25:193–200. doi: 10.1097/GCO.0b013e32836024e7. [DOI] [PubMed] [Google Scholar]
- 73.Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod. 2013;88:44. doi: 10.1095/biolreprod.112.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.