Summary
Dietary carbohydrate restriction has been purported to cause endocrine adaptations that promote body fat loss more than dietary fat restriction. We selectively restricted dietary carbohydrate versus fat for 6 days following a 5 day baseline diet in 19 adults with obesity confined to a metabolic ward where they exercised daily. Subjects received both isocaloric diets in random order during each of two inpatient stays. Body fat loss was calculated as the difference between daily fat intake and net fat oxidation measured while residing in a metabolic chamber. Whereas carbohydrate restriction led to sustained increases in fat oxidation and loss of 53±6 g/d of body fat, fat oxidation was unchanged by fat restriction leading to 89±6 g/d of fat loss and was significantly greater than carbohydrate restriction (p=0.002). Mathematical model simulations agreed with these data, but predicted that the body acts to minimize body fat differences with isocaloric diets varying in carbohydrate and fat.
Introduction
Weight loss diets often recommend targeted restriction of either carbohydrates or fat. While low fat diets were popular in the latter part of the 20th century, carbohydrate restriction has regained popularity in recent years with proponents claiming that the resulting decreased insulin secretion causes elevated release of free-fatty acids from adipose tissue, increased fat oxidation and energy expenditure, and greater body fat loss than restriction of dietary fat (Ludwig and Friedman, 2014; Taubes, 2007, 2011; Westman et al., 2007). One influential author concluded that “any diet that succeeds does so because the dieter restricts fattening carbohydrates…Those who lose fat on a diet do so because of what they are not eating – the fattening carbohydrates” (Taubes, 2011). In other words, body fat loss requires reduction of insulinogenic carbohydrates. This extraordinary claim was based on the observation that even diets targeting fat reduction typically also reduce refined carbohydrates. Since the primary regulator of adipose tissue fat storage is insulin, and a reduction in refined carbohydrates reduces insulin, carbohydrate reduction alone may have been responsible for the loss of body fat – even with a low fat diet.
While the first law of thermodynamics requires that all calories are accounted, could it be true that reducing dietary fat without also reducing carbohydrates would have no effect on body fat? Could the metabolic and endocrine adaptations to carbohydrate restriction result in augmented body fat loss compared to an equal calorie reduction of dietary fat? Several randomized controlled trials have demonstrated greater short-term weight loss when advising obese patients to restrict dietary carbohydrates (Foster et al., 2010; Gardner et al., 2007; Shai et al., 2008), but such outpatient studies are difficult to interpret mechanistically because it is not currently possible to accurately measure adherence to the recommended diets since the instruments for assessing food intake rely on self-report and have been demonstrated to be biased (Winkler, 2005). Therefore, outpatient studies cannot determine to what extent any observed differences in weight loss are due to a metabolic advantage of reduced carbohydrate diets versus a greater reduction in overall energy intake.
We performed an in-patient metabolic balance study examining the effect of selective isocaloric reduction of dietary carbohydrate versus fat on body weight, energy expenditure, and fat balance in obese volunteers. A mechanistic mathematical model of human macronutrient metabolism (Hall, 2010) was used to design the study and predict the metabolic response to each diet before the study was conducted (Hall, 2012). Here, we report the results of this experiment and use the mathematical model to quantitatively integrate the data and make in silico predictions about the results of long-term diet studies that are not practical to perform in the real world. In agreement with our model simulations, we found that only the reduced carbohydrate diet led to significant changes in metabolic fuel selection, with sustained reductions of carbohydrate oxidation and increased fat oxidation. Remarkably, fat oxidation on the reduced fat diet remained unchanged and resulted in a greater rate of body fat loss compared to the reduced carbohydrate diet, despite being equivalent in calories.
Results
Baseline data
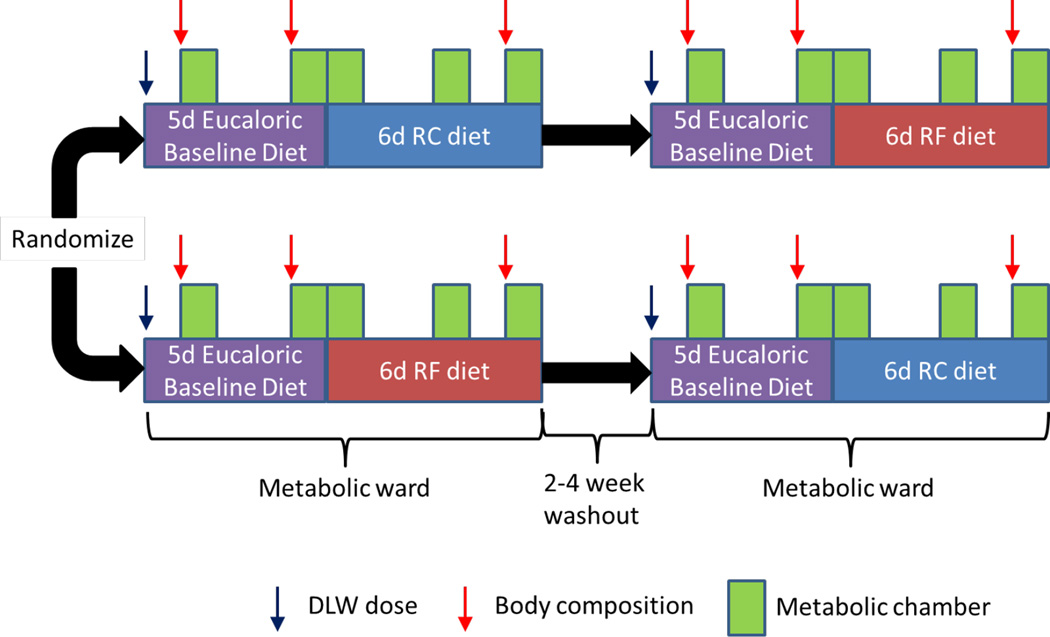
We investigated 10 male and 9 female subjects who all had obesity with a BMI of (mean±SE) 35.9±1.1 kg/m2 (Table 1). While the men and women had similar body weight and BMI, the women had significantly higher body fat and lower rates of energy expenditure and food intake, as expected. All subjects were admitted to the metabolic unit at the NIH Clinical Center where they resided for a pair of two-week inpatient periods separated by a 2–4 week washout period (Figure 1). The subjects exercised on a treadmill for 1 hour each day at a clamped pace and incline to maintain a relatively constant physical activity. For the first 5 days of each visit, they consumed a eucaloric baseline diet composed of 50% carbohydrate, 35% fat, and 15% protein with a total energy content of 2740±100 kcal/d which was not significantly different from their average total energy expenditure (TEE) of 2880±160 kcal/d (p=0.19) as measured by the doubly labeled water method. During the days spent residing in a metabolic chamber, 24hr energy expenditure (24hr EE) was 2560±110 kcal/d which was slightly less than the baseline energy intake (p=0.001) as well as TEE (p=0.008). This was likely due to decreased spontaneous physical activity when confined to the metabolic chamber, as confirmed by an overall 23.4±4% reduction in accelerometer counts during chamber days (not shown, p<0.0001).
Table 1.
Body composition, energy metabolism, and macronutrient intake during the baseline phase. Mean ±SE.
| All Subjects n=19 |
Female n=9 |
Male n=10 |
p-value F vs. M |
|
|---|---|---|---|---|
| Age (y) | 35.4±1.74 | 32.7±2.78 | 37.7±2 | 0.15 |
| BW (kg) | 106±3.8 | 103±6.5 | 110±4.3 | 0.38 |
| BMI (kg/m2) | 35.9±1.1 | 37.9±1.8 | 34.1±1.1 | 0.082 |
| % Body Fat | 39.3±2 | 46.5±1.3 | 32.8±1.8 | <0.0001 |
| Fat Mass (kg) | 42±2.8 | 48.2±3.9 | 36.4±3.1 | 0.029 |
| SMR (kcal/d) | 1770±76 | 1570±100 | 1950±80 | 0.01 |
|
24hr EE (kcal/d) |
2560±110 | 2240±110 | 2840±120 | 0.0017 |
| 24hr RQ | 0.852±0.0077 | 0.859±0.0082 | 0.846±0.013 | 0.41 |
| TEE (kcal/d) | 2880±160 | 2500±260 | 3250±120 | 0.026 |
|
Energy Intake (kcal/d) |
2740±100 | 2460±130 | 2990±110 | 0.0062 |
|
CHO Intake (g/d) |
343±13 | 308±17 | 374±14 | 0.0065 |
|
Fat Intake (g/d) |
105±4 | 95±5.1 | 115±4.6 | 0.0099 |
|
Protein Intake (g/d) |
104±3.9 | 92.1±4.5 | 114±4.2 | 0.0024 |
Fig. 1.
Overview of the study design. Adults with obesity were admitted to the metabolic unit at the NIH Clinical Center where they received a eucaloric baseline diet for 5 days followed by a 30% energy restricted diet achieved either through selective reduction of fat (RF) or carbohydrate (RC) for a period of 6 days. Subjects spent 5 days residing in metabolic chambers and had a dose of doubly labeled water (DLW) administered on the first inpatient day. Body composition was assessed by dual energy x-ray absorptiometry (DXA) during baseline and at the end of the reduced energy diets. Subjects returned after a 2–4 week washout period to undergo the opposite RC or RF diet following the same 5 day baseline phase. The order of the RC and RF diet periods was randomized.
Changes in diet, insulin secretion, and energy metabolism
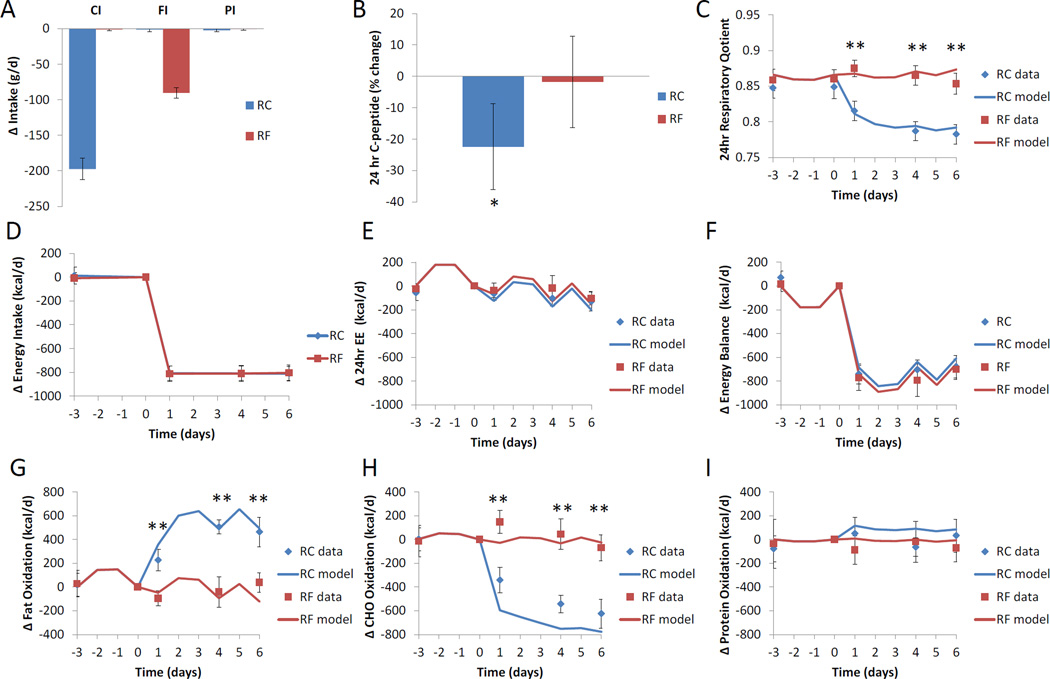
The experimental diets were designed such that they were 30% lower in calories than the baseline diet (Table 2) and the reduced carbohydrate (RC) and reduced fat (RF) diets led to selective reductions in carbohydrate intake, and fat intake, respectively, whereas protein intake was practically unchanged from baseline (Figure 2A). Note also that the RF diet did not have a decrease in sugar content compared to baseline (Table 2). This was important since a decrease in sugar content with the RF diet would be expected to decrease insulin secretion despite no change in total carbohydrate content compared to baseline. As a result, only the RC diet resulted in a 22.3±7.0% decrease in daily insulin secretion (p=0.001) as measured by 24hr urinary excretion of C-peptide and depicted in Figure 2B. Therefore, the experimental reduced-energy diets resulted in substantial differences in insulin secretion despite being isocaloric.
Table 2.
Nutrient content of the baseline and reduced carbohydrate (RC) and reduced fat (RF) diets.
| Baseline Diet |
RC Diet | RF Diet | |
|---|---|---|---|
| Energy (kcal) | 2740 | 1918 | 1918 |
| Energy Density (kcal/g) | 1.27 | 1.36 | 0.79 |
| Protein (g) | 101 | 101 | 105 |
| Fat (g) | 109 | 108 | 17 |
| Carbohydrate (g) | 350 | 140 | 352 |
| Total Fiber (g) | 24 | 16 | 21 |
| Sugars (g) | 152 | 37 | 170 |
| Saturated Fat (g) | 39 | 36 | 4 |
| Monounsaturated Fat (g) | 43 | 40 | 4 |
| Polyunsaturated Fat (g) | 21 | 24 | 4 |
| Cholesterol (mg) | 472 | 522 | 189 |
| Sodium (mg) | 4514 | 4514 | 4533 |
| Protein (% energy) | 14.5 | 20.9 | 21.1 |
| Fat (% energy) | 35.3 | 50.1 | 7.7 |
| Carbohydrate (% energy) | 50.2 | 29 | 71.2 |
| Saturated Fat (% energy) | 13.2 | 17.3 | 1.9 |
|
Monounsaturated Fat (% energy) |
14.6 | 19.7 | 2.1 |
|
Polyunsaturated Fat (% energy) |
7 | 11.9 | 1.9 |
| Omega-3 Fatty Acids (g) | 2.2 | 2.9 | 0.6 |
| Omega-6 Fatty Acids (g) | 18.1 | 21.3 | 3.5 |
| Omega-6:Omega-3 ratio | 8.3 | 7.4 | 6 |
Fig. 2.
Changes in daily diet, insulin secretion, and energy metabolism. (A) The reduced carbohydrate (RC) diet achieved 30% energy restriction via selective reduction in carbohydrate intake (CI) whereas the isocaloric reduced fat (RF) diet resulted from selective reduction of fat intake (FI). Protein intake (PI) was unchanged from baseline on both diets. (B) Insulin secretion throughout the day was assessed by 24hr urinary C-peptide excretion and was significantly reduced only following the RC diet. (C) The 24hr respiratory quotient was practically unchanged during the RF diet but fell during the RC diet indicating an increased reliance on fat oxidation. (D) Energy intake was reduced equivalently during the RC and RF diets. (E) Energy expenditure as measured in the metabolic chamber (24hr EE) decreased minimally with the RC and RF diets. (F) Energy balance was similar between RF and RC diets. (G) Net fat oxidation increased substantially during the RC diet and reached a plateau after several days, whereas the RF diet appeared to have little effect. (H) Net carbohydrate oxidation decreased during the RC diet and was relatively unchanged during the RF diet apart from a slight initial increase on the first day. (I) Net protein oxidation was not significantly altered by the RF or RC diets. Mean ± 95%CI. * indicates a significant difference from baseline at p=0.001; ** indicates a significant difference between RC and RF at p<0.0001.
The 24hr respiratory quotient (RQ) provides a measure of the overall metabolic fuel mixture being used by the body to produce energy, with RQ values approaching 1 indicating primarily carbohydrate oxidation and values near 0.7 indicating primarily fat oxidation. The 24hr RQ was the primary endpoint of this study and the mathematical model of human macronutrient metabolism predicted in advance that the RF diet would lead to no significant change in RQ whereas the RC diet would lead to a decrease in RQ (Hall, 2012). Figure 2C illustrates the 24hr RQ data and mathematical model simulations in response to the RC and RF diets. In agreement with the model simulations, only the RC diet resulted in RQ changes indicating a shift towards increased fat oxidation. In contrast, only the first day of the RF diet led to a significant increase in RQ from baseline (p<0.0001), but there was no significant change in RQ overall implying that changes in dietary fat have little effect on carbohydrate or fat oxidation (Table 3).
Table 3.
Body composition and energy metabolism changes following the isocaloric reduced carbohydrate (RC) and reduced fat (RF) diets. The data were analyzed using a repeated measures mixed model controlling for sex and order effects and are presented as least squares mean ± SE. The p-values refer to the diet effects and were not corrected for multiple comparisons.
| All Subjects | Δ RC diet N=19 |
p-value | Δ RF diet N=17 |
p-value | p-value RC vs. RF |
|---|---|---|---|---|---|
| BW (kg) | −1.85±0.15 | <.0001 | −1.3±0.16 | <.0001 | 0.022 |
| BMI (kg/m2) | −0.615±0.067 | <.0001 | −0.387±0.071 | <.0001 | 0.028 |
| % Body Fat* | 0.161±0.15 | 0.3073 | −0.072±0.16 | 0.66 | 0.24 |
| Fat Mass (kg)* | −0.529±0.13 | 0.0015 | −0.588±0.14 | 0.001 | 0.78 |
| SMR (kcal/d) | −86.2±25 | 0.0034 | 4.33±26 | 0.87 | 0.0024 |
| 24hr EE (kcal/d) | −97.7±23 | 0.0007 | −49.6±24 | 0.058 | 0.099 |
|
Energy Balance (kcal/d) |
−707±35.9 | <.0001 | −765±36.6 | <.0001 | 0.052 |
| 24hr RQ | −0.0552±0.003 | <.0001 | 0.00453±0.0031 | 0.16 | <.0001 |
|
24hr Fat Ox (kcal/d) |
403±30 | <.0001 | −31.2±31 | 0.33 | <.0001 |
|
24hr CHO Ox (kcal/d) |
−520±33 | <.0001 | 43.9±35 | 0.22 | <.0001 |
|
24hr Urinary N (g/d) |
0.754±1.1 | 0.48 | −2.43±1.1 | 0.037 | 0.095 |
|
Cumulative Fat Imbalance (g) |
−245±21 | <.0001 | −463±37 | <.0001 | <.0001 |
One female subject had changes in DXA % body fat data that were not physiological and were clear outliers, so these data were excluded from the analyses.
Figure 2D illustrates that the RC and RF diets resulted in a reduction of energy intake by 810±10 kcal/d from baseline. The diets resulted in minor changes in 24hr EE (Fig. 2E) and similar degrees of negative energy balance (Fig 2F). During the RC diet, the sleeping metabolic rate (SMR) and 24hr EE were significantly decreased by 86.2±25 kcal/d (p=0.0034) and 97.7±23 kcal/d (p=0.0007), respectively, but were not significantly changed during the RF diet (Table 3). There was a trend for a greater degree of negative energy balance during the RF diet compared to the RC diet, but this was not statistically significant (p=0.052). Note that the model simulations accounted for the observed differences in physical activity between chamber and non-chamber days (Fig. 2E).
Only the RC diet led to significant sustained adaptations of carbohydrate and fat metabolism. At the end of the RC diet period, net fat oxidation increased by 463±63 kcal/d (p<0.0001) (Fig. 2G) and net carbohydrate oxidation decreased by 595±57 kcal/d (p<0.0001) (Fig. 2H). In contrast, only the first day of the RF diet led to a significant reduction in net fat oxidation by 96±64 kcal/d (p=0.01) (Fig. 2G) and an increase in net carbohydrate oxidation of 147±49 kcal/d (p=0.01) (Fig 2H) compared to baseline. The mathematical model simulations agreed well with the observed changes in fat oxidation (Fig 2G), but slightly overestimated the decrease in carbohydrate oxidation during the RC diet (Fig 2H). The model also indicated that the RC diet would lead to increased net protein oxidation compared to the RF diet (Fig 2I), a trend that was apparent in the 24hr urinary nitrogen data (Table 3).
The mean changes in overall energy expenditure, energy balance, 24hr RQ, fat oxidation and carbohydrate oxidation during the RC and RF diets are quantified in Table 3 and mirror the day by day results above that are presented in Figure 2.
Macronutrient balance and body composition changes
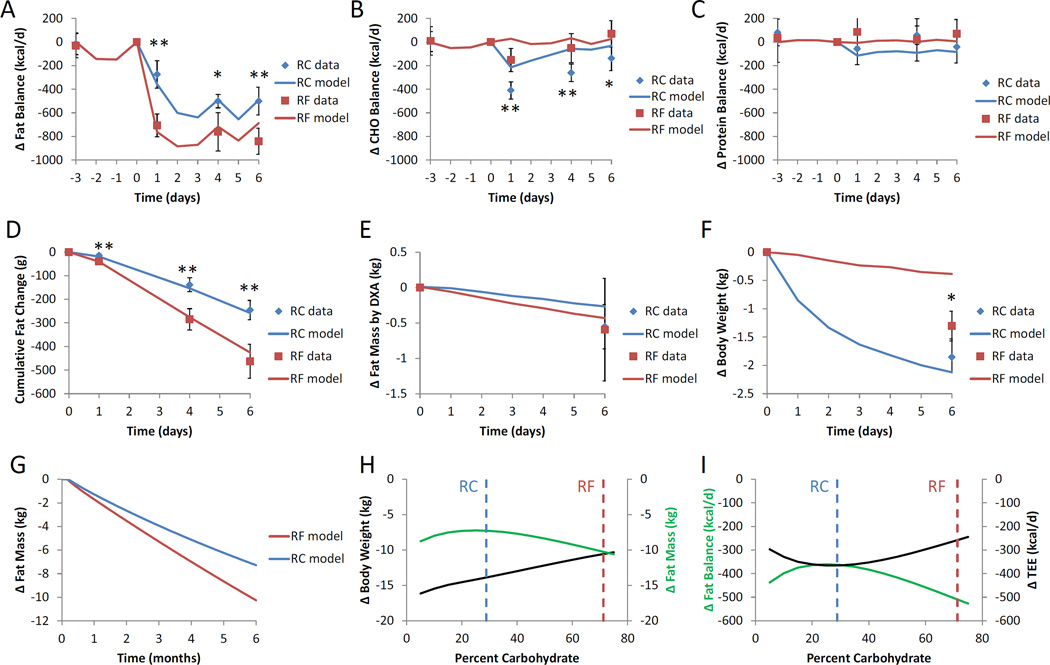
Several days of the RF diet led to a steady fat imbalance of 840±60 kcal/d, or equivalently 89±6 g/d of body fat loss (Fig. 3A) that was significantly greater that the than the steady rate of body fat loss of 500±60 kcal/d, or 53±6 g/d, achieved during the RC diet (p=0.0002) (Fig 3A). In contrast, the RC diet led to significantly greater transient carbohydrate imbalance (Fig 3B) with little difference in protein balance (Fig 3C) compared with the RF diet.
Fig. 3.
Macronutrient balance and body composition changes. (A) Daily fat balance was negative for both the RF and RC diets indicating loss of body fat. The RF diet led to consistently greater fat imbalance compared with the RC diet. (B) Net carbohydrate balance was more negative for the RC diet compared to the RF diet and returned towards balance at the end of the study with both diets. (C) Protein balance tended to be lower for the RC diet compared to the RF diet. (D) Cumulative fat balance indicated that both the RF and RC diets led to body fat loss, but the RF diet led to significantly more fat loss than the RC diet. (E) Fat mass change as measured by DXA revealed significant changes from baseline, but did not detect a significant difference between RF and RC diets. (F) The RC and RF diets both led to weight loss, but significantly more weight was lost following the RC diet. (G) Mathematical model simulations of 6 months of perfect adherence to the RC and RF diets predicted slightly greater fat mass loss with the RF diet compared with the RC diet. (H) Simulating 6 months of adherence to a 30% reduced-energy diets varying in carbohydrate and fat percentage, but with protein fixed at baseline, indicated that weight loss was linearly related to carbohydrate content, but fat mass was non-monotonic and relatively unaffected by carbohydrate content. (I) Model simulated changes in fat balance and average total energy expenditure (TEE) were reciprocally related and non-monotonic with respect to carbohydrate content. The experimental RC and RF diets are indicated by the vertical dashed lines. Mean ± 95%CI. ** indicates p<0.001 between RC and RF. * indicates p=0.004 between RC and RF.
Figure 3D shows that the greater net fat imbalance during the RF vs RC diet led to ∼80% greater cumulative body fat loss such that by the end of the 6 day period the RF diet resulted in 463±37 g of fat loss compared to 245±21 g of fat loss with the RC diet (p<0.0001) (Table 3). Dual-energy X-ray absorptiometry (DXA) is a widely used clinical method for estimating body fat percentage which was measured before and after the RC and RF diets (Fig. 1). While both diets led to significant decreases in DXA determined fat mass compared to baseline (p<0.002) (Table 3 and Fig. 3B), DXA was not sufficiently sensitive to detect a significant difference in fat mass change between the RC and RF diets. Figure 3C illustrates that both diets led to weight loss (p<0.0001) with the RC diet resulting in greater weight loss than the RF diet (p=0.02). The mathematical model simulations closely matched the cumulative fat loss measurements for both diets (Fig 3D). While the simulated weight loss with the RC diet was close to the observed value, the RF diet led to substantially more weight loss than was predicted by the model. This was likely due to body water losses that took place via mechanisms outside the scope of the current model (see the Supplementary Materials for a full description of the model).
Table 4 presents the baseline overnight-fasted plasma measurements along with the changes in response to the RC and RF diets. Both RC and RF diets appeared to significantly decrease plasma C-peptide, insulin, insulin resistance, leptin, adiponectin, total cholesterol and HDL. Plasma HDL and total cholesterol decreased to a greater extent with the RF diet and LDL decreased only with the RF diet. Plasma TG decreased only with the RC diet. Plasma β-hydroxybutyrate and ghrelin increased only with the RC diet. Plasma GIP increased with the RC diet and decreased with the RF diet. Sex specific data are presented in Supplementary Table S2.
Table 4.
Overnight fasted plasma hormone and metabolite levels during the last 3 days of the baseline diet and their changes over the last 3 days of the isocaloric reduced carbohydrate (RC) and reduced fat (RF) diets. The data were analyzed using a repeated measures mixed model controlling for sex and order effects and are presented as least squares mean ± SE. The p-values refer to the diet effects and were not corrected for multiple comparisons.
| All Subjects | Baseline | N | Δ RC diet | P-value | N | Δ RF diet | P-Value | N | P-value RC vs. RF |
|---|---|---|---|---|---|---|---|---|---|
| Glucose (mg/dl) | 87.5±1.2 | 19 | −2.69±1.7 | 0.13 | 19 | −7.1±1.7 | 0.0008 | 17 | 0.025 |
| Glycerol (mg/l) | 9.77±1.3 | 19 | 1.35±1.5 | 0.39 | 19 | −0.328±1.6 | 0.84 | 17 | 0.32 |
| BHB (mM) | 0.0682±0.009 | 19 | 0.0883±0.014 | <0.0001 | 19 | 0.00569±0.015 | 0.71 | 17 | <0.0001 |
|
Cholesterol (mg/dl) |
179±5.8 | 18 | −8.47±2.8 | 0.01 | 15 | −19.1±2.6 | <0.0001 | 16 | 0.024 |
| TG (mg/dl) | 101±11 | 18 | −17.5±5 | 0.0044 | 15 | −4.3±4.8 | 0.39 | 16 | 0.012 |
| LDL (mg/dl) | 114±4.2 | 18 | −1.77±2.6 | 0.52 | 15 | −11.4±2.5 | 0.0006 | 16 | 0.032 |
| HDL (mg/dl) | 44.8±2.4 | 18 | −2.67±0.66 | 0.0013 | 16 | −7.27±0.62 | <0.0001 | 16 | <0.0001 |
| Leptin (ng/ml) | 21.5±2.7 | 19 | −3.89±0.81 | 0.0002 | 19 | −2.89±0.86 | 0.0039 | 17 | 0.39 |
| Ghrelin (pg/ml) | 23.7±1.6 | 19 | 7.18±2.9 | 0.026 | 18 | −3.58±3.2 | 0.28 | 15 | 0.022 |
| MCP1 (pg/ml) | 150±11 | 19 | 1.96±4.9 | 0.69 | 19 | 2.41±5.1 | 0.64 | 17 | 0.95 |
| GIP (pg/ml) | 30.9±3.7 | 19 | 4.42±3.2 | 0.18 | 19 | −4.94±3.3 | 0.16 | 17 | 0.021 |
| GLP1 (pg/ml) | 37.8±4.4 | 19 | 0.543±0.75 | 0.48 | 19 | 0.628±0.79 | 0.44 | 17 | 0.95 |
| C-peptide (ng/ml) | 1.42±0.13 | 19 | −0.133±0.045 | 0.009 | 19 | −0.179±0.047 | 0.0017 | 17 | 0.52 |
| PYY (pg/ml) | 125±15 | 17 | 4.44±2.8 | 0.14 | 17 | −1.38±3.1 | 0.66 | 15 | 0.24 |
| Insulin (μU/ml) | 12.6±2 | 19 | −2.76±0.77 | 0.0024 | 18 | −2.04±0.8 | 0.021 | 17 | 0.48 |
| PP (ng/ml) | 54.6±24 | 19 | −1.02±5.6 | 0.86 | 18 | 0.511±6 | 0.93 | 16 | 0.88 |
| Adiponectin (mg/dl) | 0.978±0.13 | 17 | −0.118±0.035 | 0.0037 | 17 | −0.126±0.035 | 0.0024 | 17 | 0.73 |
| Resistin (ng/ml) | 56.2±13 | 17 | 8.99±3.7 | 0.028 | 17 | 4.2±3.8 | 0.28 | 17 | 0.26 |
| PAI1 (ng/ml) | 36±6.6 | 17 | −3.47±3.3 | 0.31 | 17 | −7.12±3.3 | 0.048 | 17 | 0.25 |
| Cortisol (pg/ml) | 4490±690 | 19 | 703±540 | 0.21 | 19 | −494±570 | 0.4 | 17 | 0.074 |
| CRP (mg/L) | 1.18±0.2 | 17 | −0.018±0.11 | 0.87 | 16 | −0.0887±0.12 | 0.46 | 14 | 0.55 |
| HOMA-IR | 2.72±0.43 | 19 | −0.489±0.15 | 0.0054 | 18 | −0.541±0.15 | 0.0028 | 17 | 0.8 |
Figure 3G illustrates the mathematical model simulations of 6 months of selective isocaloric restriction of dietary fat versus carbohydrate at the level implemented during the inpatient study. The model predicted that the RF diet would lead to approximately 3 kg more body fat loss after 6 months of perfect adherence to the isocaloric diets.
Since it might be possible that different ratios of carbohydrate and fat would lead to different results, we simulated body weight and fat mass changes after 6 months of eating a variety of 30% reduced-energy isocaloric diets varying in carbohydrate and fat, with protein fixed at baseline levels as illustrated in Figure 3H. The model predicted that weight loss increased with decreasing carbohydrate. However, body fat loss was relatively insensitive to isocaloric substitutions of dietary fat and carbohydrate suggesting that the body acts to minimize differences in fat loss when the diet calories and protein are held constant. In fact, the experimental RC and RF diets resulted in close to the maximum predicted differences in body fat loss. In other words, the modest differences in body fat loss achieved by the diets used in our experiment are probably greater than would be observed with other ratios of carbohydrate and fat.
Figure 3I shows the simulated average changes in fat balance and total energy expenditure during the 6 month simulations. There was a reciprocal relationship between fat balance and energy expenditure such that greater suppression of expenditure results in a lower rate of fat loss. Changes in whole-body metabolic fluxes, thermic effect of food, and body composition generated by isocaloric variations in carbohydrate and fat were responsible for the simulated differences in energy expenditure (not shown).
Discussion
This study demonstrated that, calorie for calorie, restriction of dietary fat led to greater body fat loss than restriction of dietary carbohydrate in adults with obesity. This occurred despite the fact that only the carbohydrate restricted diet led to decreased insulin secretion and a substantial sustained increase in net fat oxidation compared to the baseline energy-balanced diet.
In contrast to previous claims about a metabolic advantage of carbohydrate restriction for enhancing body fat loss (Ludwig and Friedman, 2014; Taubes, 2007, 2011; Westman et al., 2007), our data and model simulations support the opposite conclusion when comparing the RF and RC diets. Furthermore, we can definitively reject the claim that carbohydrate restriction is required for body fat loss (Taubes, 2011).
Dietary fat contributed only about 8% to the total energy content of the RF diet making it a very low-fat diet. The RF diet did not reduce refined carbohydrates from baseline and resulted in no significant changes in 24hr insulin secretion. In contrast, carbohydrates were about 29% of the energy content of the RC diet with a mean absolute carbohydrate intake of about 140 g/d, which induced a substantial drop in 24hr insulin secretion. Thus, while the RC diet qualifies as a low-carbohydrate diet, it was clearly not a very low-carbohydrate diet which typically requires carbohydrates to be less than 50 g/d (Westman et al., 2007). Given the composition of the baseline diet, it was not possible to design an isocaloric very low-carbohydrate diet without also adding fat or protein. We decided against such an approach due to the difficulty in attributing any observed effects of the diet to the reduction in carbohydrate as opposed to the addition of fat or protein.
Randomized controlled trials often involve hundreds or thousands of subjects prescribed to follow different diet regimens, with investigators providing instructions and support to participants on how to eat the prescribed diets. However, there is little evidence that people actually adhere to the diet prescriptions. Such studies actually test the effects of different diet prescriptions rather than the effects of different diets and cannot shed much light on the underlying physiology. As an alternative, controlled feeding studies can provide more useful physiological information, but diet adherence is often poor in outpatient studies even when participants are provided with all of their food (Das et al., 2007). Therefore, inpatient feeding studies are required to properly control the diets and measure physiological effects, but such studies are very expensive and labor intensive making them typically small in size.
Previous inpatient controlled-feeding studies have employed isocaloric reduced-energy diets with fixed protein and varying in carbohydrate and fat to investigate differences in weight loss (Anderson, 1944; Bell et al., 1969; Bogardus et al., 1981; Bortz et al., 1968; Bortz et al., 1967; Fletcher et al., 1961; Golay et al., 1996; Kekwick and Pawan, 1956; Kinsell et al., 1964; Lewis et al., 1977; Miyashita et al., 2004; Olesen and Quaade, 1960; Pilkington et al., 1960; Rabast et al., 1979; Rabast et al., 1981; Rumpler et al., 1991; Vazquez and Adibi, 1992; Vazquez et al., 1995; Werner, 1955; Yang and Van Itallie, 1976). Only two of these previous studies investigated more subjects per diet group than the present study (Golay et al., 1996; Rabast et al., 1979). Unlike the current study, all previous studies altered multiple macronutrients from their baseline values rather than selectively restricting individual macronutrients. Nevertheless, many studies agreed with our data showing greater weight loss with reduced carbohydrate diets (Anderson, 1944; Bell et al., 1969; Bogardus et al., 1981; Bortz et al., 1968; Bortz et al., 1967; Kekwick and Pawan, 1956; Lewis et al., 1977; Olesen and Quaade, 1960; Pilkington et al., 1960; Rabast et al., 1979; Rabast et al., 1981; Werner, 1955; Yang and Van Itallie, 1976). However, several studies did not detect significant differences in weight loss (Fletcher et al., 1961; Golay et al., 1996; Kinsell et al., 1964; Miyashita et al., 2004; Rumpler et al., 1991; Vazquez and Adibi, 1992; Vazquez et al., 1995). Often, the greater weight losses with the low carbohydrate diets were attributed to sodium and water imbalances (Anderson, 1944; Bell et al., 1969; Bortz et al., 1968; Bortz et al., 1967; Lewis et al., 1977; Olesen and Quaade, 1960; Pilkington et al., 1960; Werner, 1955; Yang and Van Itallie, 1976). Furthermore, nitrogen balance measurements in several previous studies have suggested greater lean tissue loss with low-carbohydrate diets (Bell et al., 1969; Bortz et al., 1968; Bortz et al., 1967; Vazquez and Adibi, 1992; Vazquez et al., 1995).
Fat loss is a more important goal than weight loss in the treatment of obesity. Five of the previous inpatient feeding studies attempted to measure differences in body fat resulting from varying carbohydrate and fat, but no significant differences were found (Bogardus et al., 1981; Golay et al., 1996; Miyashita et al., 2004; Rumpler et al., 1991; Yang and Van Itallie, 1976). Most of these studies used body composition assessment methodologies to measure body fat changes (Bogardus et al., 1981; Golay et al., 1996; Miyashita et al., 2004; Rumpler et al., 1991). But even high precision methods, such as DXA, may lack the sensitivity to detect small differences in body fat change (Hind et al., 2011; Lohman et al., 2009; Muller et al., 2012). Indeed, retrospective analysis of our data suggests that the minimal detectable difference between the diets for body fat mass using DXA was ∼0.4 kg. Thus, we suspect that the DXA measurements of fat mass change in the present study were insufficiently sensitive to detect differences between the diets. Furthermore, DXA may provide inaccurate results in situations of dynamic weight change and shifting body fluids (Lohman et al., 2000; Muller et al., 2012; Pourhassan et al., 2013; Valentine et al., 2008). This could be especially important with diets differing in their level of carbohydrate restriction since greater losses of body water are likely with lower levels of dietary carbohydrate.
The most sensitive method for detecting the rate of body fat change requires calculating daily fat balance as the difference between fat intake and net fat oxidation (i.e., fat oxidation minus de novo lipogenesis) measured by indirect calorimetry while residing in a metabolic chamber. At the end of the diet periods, our study had a minimum detectable difference in daily fat balance of 220 kcal/d (or 23 g/d) and the cumulative fat loss had a minimal detectable difference of 110 g. The observed differences in fat balance and cumulative body fat loss between RC and RF diets were substantially larger than these values and were statistically significant. While the fat balance method does not determine the anatomical location of lost fat, decreased adipose tissue triglyceride likely makes up the majority. Any additional loss of ectopic fat from liver or skeletal muscle would likely be even more beneficial.
Model simulations suggest that the differences in fat loss were due to transient differences in carbohydrate balance along with persistent differences in energy and fat balance. The model also implicated small persistent changes in protein balance resulting from the fact that dietary carbohydrates preserve nitrogen balance to a greater degree than fat (Bell et al., 1969; Vazquez and Adibi, 1992; Vazquez et al., 1995). The timing and magnitude of the observed change in net fat oxidation and fat balance with the RC diet were accurately simulated by the model and indicated that the adaptation to the experimental carbohydrate restriction achieved a plateau after several days. In contrast, the RF diet led to little adaptation with a relatively constant net fat oxidation rate thereby leading to a greater fat imbalance compared to the RC diet.
Our relatively short-term experimental study has obvious limitations in its ability to translate to fat mass changes over prolonged durations. It could be argued that perhaps the fat balance and body fat changes would converge with continuation of the diets over the subsequent weeks. However, this would require that the net fat oxidation rate somehow increase above the observed plateau with the RC diet and/or the RF diet would have to result in a swifter decrease in fat oxidation. Neither of these possibilities was apparent in the data and did not occur in the mathematical model simulations of prolonged diet periods. If such a convergence in body fat loss were to occur with prolonged RC and RF diets, the physiological mechanism is unclear.
The mathematical model simulations suggest that the diet with selective reduction in fat would continue to outpace the reduced carbohydrate diet over 6 months. However, further reducing dietary carbohydrate from the RC diet (with a corresponding addition of fat to maintain calories) was predicted to decrease body fat to a greater extent than the experimental RC diet. Very low carbohydrate diets were predicted to result in fat losses comparable to low fat diets. Indeed, the model simulations suggest that isocaloric reduced-energy diets over a wide range of carbohydrate and fat content would lead to only small differences in body fat and energy expenditure over extended durations. In other words, while the present study demonstrated the theoretical possibility that isocaloric diets differing in carbohydrate and fat can result in differing body fat losses, the body acts to minimize such differences. The endocrine and metabolic adaptations that allow for the relative insensitivity of body fat to dietary macronutrient composition may themselves have effects on health over the long term, but this was not investigated in the present study.
Translation of our results to real-world weight loss diets for treatment of obesity is limited since the experimental design and model simulations relied on strict control of food intake which is unrealistic in free-living individuals. While our results suggest that the experimental reduced fat diet was more effective at inducing body fat loss than the reduced carbohydrate diet, diet adherence was strictly enforced. We did not address whether it would be easier to adhere to a reduced fat or a reduced carbohydrate diet under free-living conditions. Since diet adherence is likely the most important determinant of body fat loss, we suspect that previously observed differences in weight loss and body fat change during outpatient diet interventions (Foster et al., 2010; Gardner et al., 2007; Shai et al., 2008) were primarily due to differences in overall calorie intake rather than any metabolic advantage of a low carbohydrate diet.
In summary, we found that selective reduction of dietary carbohydrate resulted in decreased insulin secretion, increased fat oxidation, and increased body fat loss compared to a eucaloric baseline diet. In contrast, selective isocaloric reduction of dietary fat led to no significant changes in insulin secretion or fat oxidation compared to the eucaloric baseline diet, but significantly more body fat was lost than during the carbohydrate restricted diet.
Experimental Procedures
Study protocol
The study protocol was approved by the Institutional Review Board of the National Institute of Diabetes & Digestive & Kidney Diseases (NCT00846040). Nine women and ten men with body mass indices (BMI) > 30 kg/m2 provided informed consent and were admitted to the NIH Metabolic Clinical Research Unit (MCRU). Participants were excluded if they were not weight stable (> ±5kg in the past 6 months), menopausal or pregnant or breastfeeding (women), impaired physical mobility, evidence of diseases or taking medications interfering with study outcomes, allergies to food or local anesthetics, regular use of caffeinated drinks and alcohol, eating disorders and psychiatric disorders, or strict dietary concerns (vegetarian or kosher diet).
Each inpatient visit included a 5-day baseline and a 6-day calorie restricted dietary intervention. Subjects were fed an energy balanced diet (50% carbohydrate, 35% fat, 15% protein) for 5 days followed by random assignment to isocaloric removal of 30% of total energy, either by a 60% reduction of dietary carbohydrate (RC) or 85% reduction of dietary fat (RF) for 6 days. Diets were designed using ProNutra software (version 3.4, Viocare, Inc., Princeton, NJ).
All subjects were confined to the metabolic ward throughout the study with no access to outside food. Subjects knew that it was imperative that they eat all of the food provided and nothing else. If they were not able to eat a study food, they were instructed to notify the study dietitian immediately so that other arrangements could be considered. Dietitians and health technicians met with the subjects regularly to discuss the diet and assess compliance. Visitors were allowed to meet with study subjects in a common area under observation of the nursing and/or research staff to avoid the exchange of food or beverages. Meals were consumed in a common area or in patient rooms with open doors. All meal trays were checked after consumption and any food that was not consumed at a given meal was weighed back by the dietitians and subsequent meals were modified to adjust for previously uneaten food, if necessary.
Every day, subjects completed 60 minutes of treadmill walking at a fixed self-selected pace and incline determined during the screening visit. Physical activity was quantified with activity monitors using high sampling frequencies (32 samples per second in the chamber – minute-to-minute sampling other times) during all waking periods using small, portable pager-type accelerometers (Mini Mitter / Respironics Co, Bend OR) worn on the hip.
Volunteers were readmitted after a 2–4 week washout period to repeat the 5-day balanced diet followed by the alternate 6-day RF or RC diet. Both in-patient visits were carried out during the follicular phase of the menstruation cycle in the female subjects. One male subject erroneously received the RF diet on the first day of the RC study period and one female subject erroneously received the RF diet on the final day of the RC study period. These data were retained in the analyses and their removal did not affect the statistical significance of any comparisons. Two male subjects dropped out of the study after completing the first inpatient stay on the RC diet and therefore did not contribute data to the RF diet phase.
Respiratory quotient, energy expenditure and net macronutrient oxidation rates
The primary aim of the study was to measure differences in the twenty-four hour respiratory quotient (RQ) during the RC and RF diets as measured in a metabolic chamber for at least 23 continuous hours on days 2 and 5 of the baseline diet and days 1, 4, and 6 of the reduced energy diets. We extrapolated the chamber measurements to represent 24hr periods by assuming that the mean of the measured periods was representative of the 24hr period. The mechanistic mathematical model described below was used to design and power the study as well as predict this primary outcome.
Energy expenditure (24hr EE) and net macronutrient oxidation rates were calculated using the coefficients derived by Livesey and Elia (Livesey and Elia, 1988) such that the indirect calorimetry equations for net fat, carbohydrate, and protein oxidation were:
where VO2 and VCO2 were the volumes of oxygen consumed and carbon dioxide produced, respectively, and N was the 24hr urinary nitrogen excretion measured by chemiluminescence (Antek MultiTek Analyzer, PAC, Houston, TX).
The net macronutrient oxidation rates determined by the indirect calorimetry equations above include the influence of gluconeogenesis (GNG) from amino acids and de novo lipogenesis (DNL) from carbohydrates (Frayn, 1983). In other words, the net fat oxidation rate determined by the equation above is actually the difference between fat oxidation and DNL. Similarly, the net carbohydrate oxidation rate is the sum of carbohydrate oxidation and DNL minus GNG, and the net protein oxidation rate is the sum of protein oxidation and GNG. Therefore, the macronutrient balances calculated by subtracting the net oxidation rates from macronutrient intake rates are given by:
where FI, CI, and PI are the metabolizable fat, carbohydrate and protein intake rates, respectively. Summing the above equations gives the energy balance equation:
Therefore, energy expenditure (EE) was determined by summing the net macronutrient oxidation rates and, using the indirect calorimetry equations above along with the energy densities of fat, carbohydrate and protein, results in the following equation for EE:
One male subject was not compliant during the 24hr urine collection procedure, so we assumed nitrogen balance for this subject when calculating macronutrient oxidation rates and energy expenditure. Sleeping metabolic rate (SMR) was determined during chamber periods of zero physical activity between 2am-5am.
On the morning of the first day of both in-patient periods, subjects drank from a stock solution of 1.5 g per kg body weight of 10% 18O enriched H2O and 0.08 g of 99% enriched 2H2O per kg of body weight followed by 100–200 mL tap water to rinse the dose container. Spot urine samples were collected daily. Isotopic enrichments of urine samples were measured by dual inlet chromium reduction and continuous flow CO2 equilibration isotope ratio mass spectrometry. The CO2 production rate was estimated from the differential disappearance of the 2 isotopes (kO and kD) over the baseline period according the equation by Speakman (Speakman, 1997):
where and Rdilspace was calculated as the mean of the ND/NO values.
The average total energy expenditure (TEE) during each baseline period was calculated as:
where the respiratory quotient, RQ, was calculated as the average RQ measured during the metabolic chamber days.
Anthropometry and body composition
Body weight (Scale-Tronix 5702, Carol Stream, IL, USA) and height (Seca 242, Hanover, MD, USA) were measured to the nearest 0.1 kg and 0.1 cm, respectively, with subjects wearing light clothes and following an overnight fast. Since body composition assessment methods are insufficiently sensitive to measure short term body fat change during active energy imbalance (Lohman et al, 2000; Muller et al, 2012; Pourhassan et al., 2013; Valentine et al, 2008), body fat change was determined using cumulative net fat balance as determined by indirect calorimetry. Nevertheless, body fat percentage was also measured using dual-energy X-ray absorptiometry scanner (Lunar iDXA, GE Healthcare, Madison, WI, USA). One female subject had fat mass changes measured via DXA that were not physiological and were clear outliers. These data were excluded from the analyses.
Analytical measurements
Blood was drawn into EDTA coated tubes containing DPPIV (EMD-Millipore, Billerica, MA) and protease inhibitors (S-cocktail, Sigma-Aldrich, St. Louis, MO). Samples were processed immediately after blood collection and stored at −80 °C for the subsequent measurement of biomarkers.
Plasma ghrelin (active), GLP-1 (active), pancreatic polypeptide, PYY, leptin, MCP-1, c-peptide, insulin and GIP were measured using the Milliplex magnetic bead human metabolic hormone multiplex panel (HMHMAG-34K; EMD-Millipore, Billerica MA) and plasma adiponectin, resistin and PAI-1 were measured using the Milliplex magnetic bead human serum adipokine multiplex panel A (HADK1-61K-A; EMD-Millipore, Billerica MA). Both assays are based on the Luminex xMAP technology (Luminex Corp, Austin, TX). The intra- and inter- assay CV were 5.8% and 4.9% for ghrelin (active), 6.8% and 3.4% for GLP-1 (active), 4.9% and 4.6% for pancreatic polypeptide (PP), 4.4% and 3.8% for PYY, 7.3% and 5.9% for leptin, 4.0% and 3.5% for MCP-1, 3.0% and 4.6% for c-peptide, 4.4% and 5.2% for insulin and 3.2% and 5.0% for GIP, respectively.
Beta hydroxybutyrate (βHB), glucose and glycerol and were measured using colorimetric kits from Cayman Chemical Co (Ann Arbor MI). The intra- and inter- assay CV were 5.0% and 3.6% for βHB, 3.6% and 3.9% for glucose and 4.0% and 2.1% for glycerol respectively. Cortisol was measured using an ELISA from Cayman Chemical Company (Ann Arbor, MI). The intra- and inter- assay CV were 3.6% and 3.9%, respectively.
24hr urinary C-peptide excretion was measured during chamber days using an ELISA from Mercodia (Uppsala, Sweden). The intra- and inter- assay CV were 5.1% and 6.7%, respectively.
Mathematical modeling
A detailed description of the mathematical model is presented in the Supplementary Materials. The model quantitatively tracks the metabolism of all three dietary macronutrients and simulates how diet changes result in adaptations of whole-body energy expenditure, metabolic fuel selection, and alterations in the major whole-body fluxes contributing to macronutrient balance (Hall, 2010). Other than the initial conditions for body composition and energy expenditure and the physical activity differences between chamber and non-chamber days, no model parameters were adjusted to fit the data from this study. Model simulations were used to design the study and the successful predictions of the observed 24hr RQ changes were included in the clinical protocol (NCT00846040).
Statistical analyses
Statistical analyses were performed using SAS (version 9.3; SAS Institute Inc, Cary, NC). The baseline data are presented as mean ± SE and were analyzed by analysis of variance (PROC GLM, SAS). The data tables present least squares mean ± SE and were analyzed using a repeated measures mixed model with a covariance structure of compound symmetry (PROC MIXED, SAS). We controlled for sex and order effects by including these parameters in the statistical model. The figures depict mean ± 95% CI at each time point and two-sided t-tests were used to compare the diet groups. Outliers were identified by Cook’s distance with a cutoff of 4/n, where n is the number of observations. Significance was declared at p < 0.05. Retrospective calculations of minimal detectable effect sizes were performed using the measured variances with a type I error probability of 0.05 and 80% power for pairwise comparison of 17 subjects (PROC POWER, SAS).
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes & Digestive & Kidney Diseases. We thank the nursing and nutrition staff at the NIH MCRU for their invaluable assistance with this study. We thank Terri Wakefield, Dwayne Staton, and Erica Vass for their support in managing the study. We are most thankful to the study subjects who volunteered to participate in this demanding protocol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AB. Loss of weight in obese patients on sub-maintenance diets and the effect of variation in the ratio of carbohydrate to fat in the diet. Quarterly Journal of Medicine. 1944;13:27–36. [Google Scholar]
- Bell JD, Margen S, Calloway DH. Ketosis, weight loss, uric acid, and nitrogen balance in obese women fed single nutrients at low caloric levels. Metabolism. 1969;18:193–208. doi: 10.1016/0026-0495(69)90039-0. [DOI] [PubMed] [Google Scholar]
- Bogardus C, LaGrange BM, Horton ES, Sims EA. Comparison of carbohydrate-containing and carbohydrate-restricted hypocaloric diets in the treatment of obesity. Endurance and metabolic fuel homeostasis during strenuous exercise. J Clin Invest. 1981;68:399–404. doi: 10.1172/JCI110268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz WM, Howat P, Holmes WL. Fat, carbohydrate, salt, and weight loss. Further studies. Am J Clin Nutr. 1968;21:1291–1301. doi: 10.1093/ajcn/21.11.1291. [DOI] [PubMed] [Google Scholar]
- Bortz WM, Wroldson A, Morris P, Issekutz B., Jr Fat, carbohydrate, salt, and weight loss. Am J Clin Nutr. 1967;20:1104–1112. doi: 10.1093/ajcn/20.10.1104. [DOI] [PubMed] [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- Fletcher RF, Mc CM, Crooke AC. Reducing diets. Weight loss of obese patients on diets of different composition. Br J Nutr. 1961;15:53–58. doi: 10.1079/bjn19610007. [DOI] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, Stein RI, Mohammed BS, Miller B, Rader DJ, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. Jama. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- Golay A, Allaz AF, Morel Y, de Tonnac N, Tankova S, Reaven G. Similar weight loss with low- or high-carbohydrate diets. Am J Clin Nutr. 1996;63:174–178. doi: 10.1093/ajcn/63.2.174. [DOI] [PubMed] [Google Scholar]
- Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab. 2010;298:E449–E466. doi: 10.1152/ajpendo.00559.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD. Metabolism of mice and men: mathematical modeling of body weight dynamics. Curr Opin Clin Nutr Metab Care. 2012;15:418–423. doi: 10.1097/MCO.0b013e3283561150. [DOI] [PubMed] [Google Scholar]
- Hind K, Oldroyd B, Truscott JG. In vivo precision of the GE Lunar iDXA densitometer for the measurement of total body composition and fat distribution in adults. Eur J Clin Nutr. 2011;65:140–142. doi: 10.1038/ejcn.2010.190. [DOI] [PubMed] [Google Scholar]
- Kekwick A, Pawan GL. Calorie intake in relation to body-weight changes in the obese. Lancet. 1956;271:155–161. doi: 10.1016/s0140-6736(56)91691-9. [DOI] [PubMed] [Google Scholar]
- Kinsell LW, Gunning B, Michaels GD, Richardson J, Cox SE, Lemon C. Calories Do Count. Metabolism. 1964;13:195–204. doi: 10.1016/0026-0495(64)90098-8. [DOI] [PubMed] [Google Scholar]
- Lewis SB, Wallin JD, Kane JP, Gerich JE. Effect of diet composition on metabolic adaptations to hypocaloric nutrition: comparison of high carbohydrate and high fat isocaloric diets. Am J Clin Nutr. 1977;30:160–170. doi: 10.1093/ajcn/30.2.160. [DOI] [PubMed] [Google Scholar]
- Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr. 1988;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- Lohman M, Tallroth K, Kettunen JA, Marttinen MT. Reproducibility of dual-energy x-ray absorptiometry total and regional body composition measurements using different scanning positions and definitions of regions. Metabolism. 2009;58:1663–1668. doi: 10.1016/j.metabol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Harris M, Teixeira PJ, Weiss L. Assessing body composition and changes in body composition. Another look at dual-energy X-ray absorptiometry. Annals of the New York Academy of Sciences. 2000;904:45–54. doi: 10.1111/j.1749-6632.2000.tb06420.x. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311:2167–2168. doi: 10.1001/jama.2014.4133. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Koide N, Ohtsuka M, Ozaki H, Itoh Y, Oyama T, Uetake T, Ariga K, Shirai K. Beneficial effect of low carbohydrate in low calorie diets on visceral fat reduction in type 2 diabetic patients with obesity. Diabetes research and clinical practice. 2004;65:235–241. doi: 10.1016/j.diabres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Bosy-Westphal A, Lagerpusch M, Heymsfield SB. Obesity. Vol. 20. Silver Spring; 2012. Use of Balance Methods for Assessment of Short-Term Changes in Body Composition; pp. 701–707. [DOI] [PubMed] [Google Scholar]
- Olesen ES, Quaade F. Fatty foods and obesity. Lancet. 1960;1:1048–1051. doi: 10.1016/s0140-6736(60)90933-8. [DOI] [PubMed] [Google Scholar]
- Pilkington TR, Rosenoer VM, Gainsborough H, Carey M. Diet and weight-reduction in the obese. Lancet. 1960;1:856–858. doi: 10.1016/s0140-6736(60)90736-4. [DOI] [PubMed] [Google Scholar]
- Pourhassan M, Schautz B, Braun W, Gluer CC, Bosy-Westphal A, Muller MJ. Impact of body-composition methodology on the composition of weight loss and weight gain. Eur J Clin Nutr. 2013;67:446–454. doi: 10.1038/ejcn.2013.35. [DOI] [PubMed] [Google Scholar]
- Rabast U, Schonborn J, Kasper H. Dietetic treatment of obesity with low and high-carbohydrate diets: comparative studies and clinical results. Int J Obes. 1979;3:201–211. [PubMed] [Google Scholar]
- Rabast U, Vornberger KH, Ehl M. Loss of weight, sodium and water in obese persons consuming a high- or low-carbohydrate diet. Ann Nutr Metab. 1981;25:341–349. doi: 10.1159/000176515. [DOI] [PubMed] [Google Scholar]
- Rumpler WV, Seale JL, Miles CW, Bodwell CE. Energy-intake restriction and diet-composition effects on energy expenditure in men. Am J Clin Nutr. 1991;53:430–436. doi: 10.1093/ajcn/53.2.430. [DOI] [PubMed] [Google Scholar]
- Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Doubly labelled water: Theory and practice. London: Chapman & Hall; 1997. [Google Scholar]
- Taubes G. Good calories, bad calories: challenging the conventional wisdom on diet, weight control, and disease. New York: Alfred A. Knopf; 2007. [Google Scholar]
- Taubes G. Why we get fat and what to do about it. New York: Alfred A. Knopf; 2011. [Google Scholar]
- Valentine RJ, Misic MM, Kessinger RB, Mojtahedi MC, Evans EM. Location of body fat and body size impacts DXA soft tissue measures: a simulation study. Eur J Clin Nutr. 2008;62:553–559. doi: 10.1038/sj.ejcn.1602770. [DOI] [PubMed] [Google Scholar]
- Vazquez JA, Adibi SA. Protein sparing during treatment of obesity: ketogenic versus nonketogenic very low calorie diet. Metabolism. 1992;41:406–414. doi: 10.1016/0026-0495(92)90076-m. [DOI] [PubMed] [Google Scholar]
- Vazquez JA, Kazi U, Madani N. Protein metabolism during weight reduction with very-low-energy diets: evaluation of the independent effects of protein and carbohydrate on protein sparing. Am J Clin Nutr. 1995;62:93–103. doi: 10.1093/ajcn/62.1.93. [DOI] [PubMed] [Google Scholar]
- Werner SC. Comparison between weight reduction on a high-calorie, highfat diet and on an isocaloric regimen high in carbohydrate. N Engl J Med. 1955;252:661–665. doi: 10.1056/NEJM195504212521604. [DOI] [PubMed] [Google Scholar]
- Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, Yancy WS, Phinney SD. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86:276–284. doi: 10.1093/ajcn/86.2.276. [DOI] [PubMed] [Google Scholar]
- Winkler JT. The fundamental flaw in obesity research. Obes Rev. 2005;6:199–202. doi: 10.1111/j.1467-789X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Yang MU, Van Itallie TB. Composition of weight lost during short-term weight reduction. Metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. J Clin Invest. 1976;58:722–730. doi: 10.1172/JCI108519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.