SUMMARY
Regulatory T (Treg) cells suppress immune responses to a broad range of non-microbial and microbial antigens and indirectly limit immune inflammation-in-flicted tissue damage by employing multiple mechanisms of suppression. Here, we demonstrate that selective Treg cell deficiency in amphiregulin leads to severe acute lung damage and decreased blood oxygen concentration during influenza virus infection without any measureable alterations in Treg cell suppressor function, antiviral immune responses, or viral load. This tissue repair modality is mobilized in Treg cells in response to inflammatory mediator IL-18 or alarmin IL-33, but not by TCR signaling that is required for suppressor function. These results suggest that, during infectious lung injury, Treg cells have a major direct and non-redundant role in tissue repair and maintenance—distinct from their role in suppression of immune responses and inflammation—and that these two essential Treg cell functions are invoked by separable cues.
Graphical Abstract

INTRODUCTION
Regulatory T (Treg) cells expressing X-chromosome-encoded transcription factor Foxp3 represent a specialized lineage of T lymphocytes whose key function is suppression of T cell responses to self, the commensal microbiota, and dietary and environmental antigens (Josefowicz et al., 2012; Sakaguchi et al., 2008). Congenital deficiency in Treg cells in mice and humans—or their acute elimination—results in fatal autoimmunity, associated with splenomegaly and lymphadenopathy and destructive inflammatory damage to numerous non-lymphoid organs, including the lung, stomach, small and large intestine, pancreas and other endocrine glands, liver, and skin (Fontenot et al., 2003; Khattri et al., 2003). In addition to the maintenance of immunological tolerance to “self” and non-”self” antigens that the organism is chronically exposed to, Treg cells have been implicated in limiting immune responses to acute and chronic microbial infections and also limiting corresponding tissue damage (see for review Josefowicz et al. [2012] and Veiga-Parga et al. [2013]). Treg cells employ multiple mechanisms of suppression (Josefowicz et al., 2012), and genetic ablation of the T cell receptor (TCR) in differentiated Treg cells recently revealed that TCR signaling is prerequisite for their suppressor function (Levine et al., 2014).
Aside from limiting tissue damage through suppression of inflammatory responses following infection, Treg cells may promote tissue repair. One way in which Treg-cell-mediated tissue repair is thought to occur is by suppressing pro-inflammatory chemokine production, endothelial cell activation, and pro-inflammatory responses of cells of the innate and adaptive immune system (Burzyn et al., 2013a).
In addition to secondary lymphoid organs, Treg cells reside within a number of non-lymphoid organs, where circulatory Treg cells are rapidly recruited, and the resident Treg cells expand upon tissue damage or injury (Burzyn et al., 2013a; D’Alessio et al., 2009). Therefore, we reasoned that, in addition to their aforementioned indirect role in response to tissue injury and stress, Treg cells likely play a direct role in tissue repair and function by elaborating mediators acting on parenchymal cells. In support of this idea, analysis of published datasets and unpublished data from our laboratory indicates that tissue-resident populations of Treg cells exhibit features evoking tissue-remodeling capability (data not shown) (Burzyn et al., 2013b). Specifically, the epidermal growth factor receptor (EGF-R) ligand amphiregulin is expressed in Treg cells isolated from visceral adipose tissue (VAT), muscle, and the intestinal lamina propria (LP) during inflammation (Burzyn et al., 2013b; Cipolletta et al., 2012; Feuerer et al., 2009; Schiering et al., 2014). Amphiregulin plays an important role in development and maintenance of numerous organs, including mammary glands and ovaries. It also promotes repair under inflammatory conditions and organ injury by acting locally in its membrane-bound form and upon its cleavage, primarily by TACE (ADAM17) protease (Berasain and Avila, 2014).
As indirect evidence of a biological role for amphiregulin production by Treg cells, acute ablation of Treg cells during muscle injury has been shown to impede tissue repair and could be ameliorated by administration of recombinant amphiregulin protein (Burzyn et al., 2013b). However, amphiregulin production by multiple cell types, including group 2 innate lymphoid cells (ILC2), and basophils has been implicated in tissue repair (Meulenbroeks et al., 2015; Monticelli et al., 2011). Furthermore, it is not clear to what extent therapeutic dosing of recombinant amphiregulin corresponds to its physiological systemic and local concentrations (Berasain and Avila, 2014). Therefore, it is possible that systemic delivery of amphiregulin can override compromised tissue repair resulting from Treg cell depletion. Moreover, several recent studies suggested that AREG produced by mast cells and basophils has a non-redundant immunosuppressive function and that amphiregulin may act in an autocrine manner on Treg cells to facilitate their suppressor capacity (Meulenbroeks et al., 2015; Zaiss et al., 2013, 2015). Thus, it remains unknown whether its production by Treg cells has a distinct non-redundant role in, or is dispensable for, tissue repair or whether amphiregulin expression by Treg cells has immunosuppressive function along with other mediators.
We sought to unequivocally address these questions and test the hypothesis that amphiregulin production by Treg cells defines their distinct tissue repair modality. The absence of Treg-cell-derived amphiregulin conferred a marked increase in acute lung damage upon influenza virus infection, whereas viral load and T cell responses were unaffected. Our results suggest that Treg cells can adopt distinct—albeit non-mutually exclusive—effector states depending on the type of activating stimuli they receive. Through coordinate utilization of these effector mechanisms, tissue-resident Treg cells are capable of acting as universal sentinels of mucosal barriers—rapidly responding to tissue damage and immune-mediated inflammation to promote tolerance, tissue repair, and restore function.
RESULTS
Treg- and T-Cell-Specific Ablation of Amphiregulin
Knowing that both Treg cells and conventional T cells have been reported to produce amphiregulin during an immune response (Burzyn et al., 2013b; Jamieson et al., 2013; Qi et al., 2012; Zaiss et al., 2006), we assessed its production by purified T cell populations following in vitro stimulation. T cells isolated from the spleen and axillary and inguinal (pLN) and mesenteric (MLN) lymph nodes, as well as from the lung and large intestine lamina propria (LILP), produced amphiregulin upon stimulation with phorbol-12-myristate-13-acetate (PMA) and ionomycin, albeit to different extents (Figure 1A). Further analysis of the cell-surface phenotype of amphiregulin-producing Foxp3− cells (Figure S1) showed that the majority of amphiregulin+ cells exhibited very low expression of the homing receptor L-selectin (CD62L) and high expression of CD44, whereas naive T cells failed to produce amphiregulin (data not shown). Similarly, amphiregulin-producing Treg cells were also mostly CD44hiCD62Llo and exhibited elevated expression of CD103, PD-1, GITR, CTLA-4, and KLRG1 in comparison to their amphiregulin-negative counterparts, in agreement with two recent studies (Burzyn et al., 2013b; Qi et al., 2012) (Figure 1B). Considering that a large number of Treg cells expressing amphiregulin also exhibit higher levels of effector molecules that are important for their suppressor function, we sought to unequivocally define the role and function of amphiregulin expression by Treg cells using conditional genetic deletion.
Figure 1. In Vitro Amphiregulin Production by Treg and Conventional CD4+ T Cells.
(A and B) Amphiregulin expression by Treg cells and conventional CD4+ T cells with an effector/memory cell phenotype.
(A) Flow cytometric analysis for amphiregulin (Areg) and Foxp3 expression in CD4+TCRβ+ cells isolated from spleens, axillary and inguinal (pLN) and mesenteric (MLN) lymph nodes, lungs, and LILP of C57BL/6 mice following in vitro stimulation for 3 hr with PMA and ionomycin in the presence of brefeldin A. Amphiregulin+ Treg (green) and conventional CD4+ T cells (orange) and amphiregulin− Treg cells (blue). The frequencies of cells within each gate are shown.
(B) Flow cytometric analysis of surface and intracellular Treg cell activation markers. Histograms depict expression in amphiregulin−CD4+Foxp3+ (blue) or CD4+Foxp3+amphiregulin+ (green) populations as gated in (A). Data represent one of three independent experiments, each with n ≥ 3 mice.
See also Figure S1.
Mice expressing an amphiregulin (Areg) conditional allele on a B6 genetic background (AregFl/Fl) were crossed to Foxp3YFP-cre or CD4-cre mice to ablate amphiregulin in Foxp3+ Treg or all T cells, respectively. Intracellular staining for amphiregulin following stimulation with PMA and ionomycin confirmed its Treg or pan-T-cell-specific loss in spleen, pLN, MLN, lung, and LILP (Figure 2A; data not shown). Animals were born in normal Mendelian ratios with no gender bias, suggesting that T-cell-restricted amphiregulin deficiency—contrary to its germline deficiency—did not impair normal reproductive organ development (data not shown) (Berasain and Avila, 2014). Furthermore, animals with amphiregulin deficiency in either Treg (Foxp3YFP-cre) or all T cells (CD4-cre) showed similar frequencies and numbers of B or T cells and activated T cells, assessed by CD44 expression, to those found in littermate controls up to ~4 months of age (Figures 2B and S2; data not shown). Germline Areg deficiency had previously been shown to result in skin and intestinal abnormalities exacerbated upon exposure to ionizing irradiation (Shao and Sheng, 2010; Zaiss et al., 2013). In contrast, in unchallenged mice, pan-T or Treg-cell-specific Areg deletion resulted in no gross phenotypic or histological abnormalities (data not shown). Further assessment of CD4+ T helper (Th) cell subsets following in vitro stimulation of lymphocytes isolated from various secondary and non-lymphoid tissues indicated that amphiregulin deficiency did not influence the frequency or total number of Th1, Th2, and Th17 cells (Figure 2C). Taken together, these data suggest that, in a physiologic setting, amphiregulin expression by T cells is dispensable for either organ development or the differentiation of lymphocyte populations or their effector Th cell polarization.
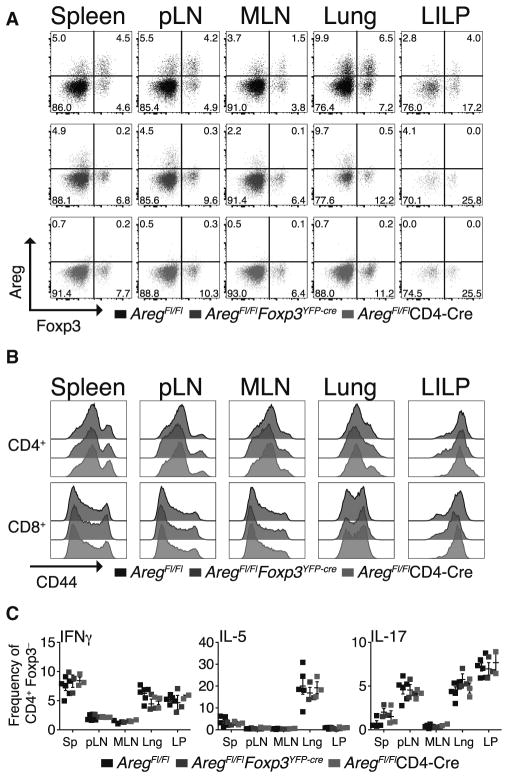
Figure 2. Treg- and T-Cell-Specific Ablation of Amphiregulin Does Not Result in Immune Activation.
(A–C) Flow cytometric analysis of cells isolated from spleens, axillary and inguinal (pLN), and mesenteric (MLN) lymph nodes, lungs, and LILP of ~3-month-old AregFl/Fl (black), AregFl/FlFoxp3YFP-cre (blue), and AregFl/FlCD4-cre (red) mice (see Experimental Procedures).
(A) Intracellular staining for amphiregulin (Areg) and Foxp3 within CD4+ T cells following in vitro stimulation performed as described in Figure 1A. Specific loss of amphiregulin expression in Foxp3+ Treg cells in AregFl/FlFoxp3YFP-cre mice (middle) or all CD4+TCRβ+ T cells in AregFl/FlCD4-cre mice (bottom) as compared to AregFl/Fl control animals (top). The frequencies of cells within each quadrant are shown.
(B) Ex vivo surface staining of CD4+Foxp3− (top) and CD8+ (bottom) conventional T cells isolated from the indicated organs. Overlaid shaded histograms depict expression of CD44 on T cells isolated from AregFl/Fl (black), AregFl/Fl Foxp3YFP-cre (blue), and AregFl/FlCD4-cre (red) mice.
(C) Cytokine production by CD4+Foxp3− T cells stimulated in vitro as described in Figure 1 legend. The data are shown as the frequency of CD4+Foxp3− T cells producing each cytokine indicated in the top left corner of the plot. Shown as mean ± SEM; comparisons to AregFl/Fl not statistically significant. Lng, lung; LP, large intestine lamina propria (LILP). Data shown are representative of more than 3 independent experiments (n ≥ 3 mice per each group).
See also Figure S2.
Amphiregulin Deficiency Does Not Affect Treg Cell Suppressor Function
Recently, it was proposed that amphiregulin produced by Treg cells may act in an auto- or paracrine manner to activate EGF-R expressed on Treg cells and enhance their suppressive capacity (Zaiss et al., 2013, 2015). However, we failed to identify heightened inflammatory responses in unchallenged mice harboring amphiregulin-deficient Treg cells (or all T cells) as would be expected if Treg cell immunosuppressive function were noticeably impaired (Figures 2B and 2C). To directly test whether amphiregulin production by Treg or effector T cells can affect Treg-cell-mediated suppression in vitro, we assessed the ability of amphiregulin-sufficient or -deficient CD4+CD25hi Treg cells to suppress CD3-antibody-induced proliferation of naive CD44loCD62LhiCD25−CD4+ T cells (Figure 3A). Irrespective of whether responder or Treg cells were proficient or deficient for amphiregulin, a similar degree of suppression was observed (Figure 3B). Furthermore, antigen-presenting cell (APC)-stimulated amphiregulin-deficient effector (“effector only”) or suppressor (“Treg only”) cells responded identically to their amphiregulin-sufficient counterparts (Figures 3A and 3B). Thus, amphiregulin production by T cells is dispensable for suppressing immune activation in vitro.
Figure 3. Amphiregulin Does Not Influence Effector Differentiation or Mediate Suppression.
(A and B) In vitro suppressor capacity of Treg cells is independent of amphiregulin expression. FACS-sorted naive CD4+CD44loCD62Lhi amphiregulin-sufficient or -deficient T cells from (A) AregFl/Fl (solid lines/symbols) or (B) AregFl/FlCD4-Cre (dashed lines/open symbols) mice, respectively, were cultured with graded numbers of CD4+CD25hi Treg cells FACS sorted from AregFl/Fl (black) or AregFl/FlCD4-Cre (red) animals in the presence of irradiated T-cell-depleted splenocytes and 1 μg/ml CD3 antibody for 80 hr. T cell proliferation was assessed by incorporation of [3H]-thymidine added during the final 8 hr of culture. Controls show proliferation under the same conditions for naive CD4+ T cells in the absence of Treg cells (“EFFECTOR ONLY”) and Treg cells incubated without effector cells (“Treg only”). Data are shown as mean ± SEM and represent one of two independent experiments.
(C) Amphiregulin does not influence T helper cell polarization in vitro. Flow cytometric analysis of cytokine production by FACS-sorted naive CD4+CD44loCD62LhiCD25−Foxp3− T cells from Foxp3GFP reporter mice cultured for 5 days with plate-bound CD3 and soluble CD28 antibodies in the presence of IL-2 alone (Th0; left); in combination with IL-12, IFNγ, and anti-mouse IL-4 antibody (Th1; middle); or IL-4 in combination with IFNγ and IL-12 neutralizing antibodies (Th2; right) with or without increasing amounts of recombinant amphiregulin (as indicated by symbol size). Intracellular staining for each indicated cytokine was performed following restimulation with PMA and ionomycin in the presence of brefeldin A. Data are representative of three independent experiments.
(D–G) Equivalent in vivo suppressor capacity of amphiregulin-deficient and -sufficient Treg cells. Magnetic-bead-purified CD4+ T cells from Foxp3−(CD45.1+) mice were transferred into Tcrb−/−Tcrd−/− recipients alone (white symbols; “Foxp3− effector only”) or in combination with CD4+YFP+CD25hi Treg cells from Aregwt/wtFoxp3YFP-cre (black) or AregFl/FlFoxp3YFP-cre (blue) mice. (D) Changes in body weight were monitored and plotted as percent from weight at the date of transfer. Mice receiving only effector cells (“Foxp3− effector only”) succumbed to autoimmunity and were euthanized (the dashed line). The data are shown as mean ± SEM; n = 5 (Aregwt/wtFoxp3YFP-cre), n = 5 (AregFl/FlFox-p3YFP-cre), and n = 2 (Foxp3− Effector only). (E and F) Flow cytometric analysis of (E) the frequency of proliferating (Ki67+; left) and IFNγ-producing (right) transferred CD45.1+CD4+ and (F) CD45.1+CD8+ Foxp3− effector cells and (G) the frequency and expression levels of (H) Foxp3 (left) and CD25 (right) of transferred CD45.2+ Treg cells from each indicated group, as labeled in (D). The data are shown as mean ± SEM (n = 5 per group). No significant differences were observed in recipients receiving Aregwt/wtFoxp3YFP-cre and AregFl/FlFoxp3YFP-cre.
Nevertheless, it was possible that we may have missed a potential modulatory role of amphiregulin in T cell responses. In this regard, amphiregulin elaborated by bone-marrow-derived cells was suggested to promote Th2 responses during worm infection (Zaiss et al., 2006). However, addition of increasing concentrations of recombinant mouse amphiregulin (rmAREG) to fluorescence-activated cell-sorted (FACS) naive CD4+CD44loCD62Lhi CD25− Foxp3− T cells from Foxp3GFP mice stimulated with plate-bound CD3 and soluble CD28 antibodies under Th0, Th1, and Th2 polarizing conditions did not significantly suppress effector T cell activation or cytokine production. In the presence of amphiregulin, we observed comparable frequencies of cells producing IFNγ, IL-5, IL-13, and TNF-α within each set of polarization conditions when compared to controls (Figure 3C). Since conventional Foxp3−CD4+ T cells were also proficient for amphiregulin production, it was possible that amphiregulin produced by these cells could increase the immunosuppressive capacity of nearby Treg cells. However, consistent with some previous reports, we failed to observe EGF-R expression or signaling in Treg cells or an effect of endogenous amphiregulin expressed in T cells or rmAREG in enhancing the induction or proliferation of Treg cells in vitro (Figure 3B; J.A.G. and A.Y.R., unpublished data) (Burzyn et al., 2013b).
To assess a role of amphiregulin production by Treg cells for suppression in vivo, we adoptively transferred CD45.2+ amphiregulin-sufficient or -deficient YFP+ Treg cells FACS purified from Aregwt/wtFoxp3YFP-cre and AregFl/FlFoxp3YFP-cre mice, respectively, together with Foxp3-deficient effector CD45.1+CD4+ T cells isolated from Foxp3− mice into T-cell-deficient recipients and monitored for signs of immune activation. Whereas animals that received effector CD4+ T cells alone succumbed to autoimmune disease ~5 weeks after transfer, those that received amphiregulin-sufficient and -deficient Treg cells were equally protected, showed no histopathological sequelae, and exhibited comparable numbers, activation status, and pro-inflammatory cytokine production by effector T cells (Figures 3D–3F; data not shown). Furthermore, transferred Foxp3+ Treg cells were present in similar numbers and expressed comparable amounts of Foxp3 and CD25 on a per-cell basis (Figures 3G and 3H). Thus, Treg-cell-derived amphiregulin was dispensable for elaboration or potentiation of suppressor function or maintenance.
Amphiregulin Production by Lung Treg Cells Prevents Tissue Damage but Is Dispensable for Regulation of Virus-Specific Immune Responses
Next, we asked whether Treg-cell-derived amphiregulin has a non-redundant role in tissue repair. It remained possible that, in settings of tissue injury, amphiregulin produced by Treg cells could suppress immune responses. To address this question, we used an intranasal influenza virus infection model, where a general role for amphiregulin in repairing lung injury and Treg-cell-mediated suppression of pathogen-specific immune responses has been well documented (Brincks et al., 2013; Jamieson et al., 2013; Monticelli et al., 2011). In addition, flu infection enables tracking of virus-specific immune responses, as well as pathogen and antigen load in the affected tissue (Bedoya et al., 2013; Brincks et al., 2013; Moser et al., 2014). Intranasal infection of mice with influenza A virus strain A/Puerto Rico/8/34 (PR8) encoding H-2Kb ovalbumin peptide epitope, OVA257-264 (PR8-OTI), induces robust alveolar epithelial damage early after infection, and the virus-specific T cell response is required for viral clearance (Epstein et al., 1998).
Since multiple cell types are capable of amphiregulin production, we first sought to assess dynamics of amphiregulin expression during the course of infection. We observed a rapid increase in both transcript and protein levels of amphiregulin in whole-lung tissue homogenate beginning at day 3 post-infection, followed by a decrease after day 5 (Figure S3A). The observed changes in total amphiregulin amounts in the infected lungs tightly corresponded with an increase in amphiregulin production by lung Treg cells assessed by flow cytometry, with amphiregulin staining performed directly following ex vivo isolation of cells (Figure 4A). The observed high level of amphiregulin expression by lung Treg cells was not a result of its buildup due to an impaired proteolytic cleavage of cell-surface-bound amphiregulin, as evidenced by a marked increase in amphiregulin staining following in vitro incubation of lung lymphocytes with the matrix metalloproteinase (MMP) inhibitor, marimastat, when compared to control (Figure S3B). The marked early increase in amphiregulin-expressing cells on days 3 and 5 post-infection was limited to the lung Treg cell population. In contrast, an increase in the number of other lung lymphocytes producing amphiregulin, namely Foxp3−CD4+amphiregulin+ T cells and amphiregulin+ ILC2, was not observed until days 8 and 10 post-infection. This exclusive increase in amphiregulin+ Treg cells did not seem to be a result of preferential expansion of preexisting amphiregulin-expressing lung Treg cells at early time points. In fact, overall numbers of lung effector CD4+ and CD8+ T cells were already increased at day 3, whereas lung ILC2 displayed a delayed increase in numbers between days 5 and 8 post-infection when compared to numbers of corresponding cell subsets in lungs from control uninfected mice (Figures 4B, and S3C, and S3D).
Figure 4. Production of Amphiregulin by Treg Cells Does Not Affect Anti-Viral Immune Responses.
(A and B) Flow cytometric analyses for amphiregulin (Areg) expression by cells isolated from lungs of C57BL/6 mice that were intranasally instilled with PBS (dpi 0) or infected with 0.5 LD50 PR8-OTI influenza (flu) virus at the indicated time points (dpi: days post-infection).
(A) Representative flow cytometry plots of amphiregulin and Foxp3 expression by CD4+ T cells directly following ex vivo isolation.
(B) Numbers of amphiregulin-expressing CD4+Foxp3− conventional T cells (squares; “AREG+ Th”), CD4+Foxp3+ Treg cells (circles; “AREG+ Treg”), and Lin−CD90+CD127+KLRG1+ST2+ ILC2 (triangles; “AREG+ILC2”). Mean ± SEM. Data are representative of 2 independent experiments (n ≥ 3 mice per time point in each group, except n ≥ 2 per group in dpi 10 group in one of the two experiments).
(C) AregFl/Fl (black), AregFl/FlFoxp3YFP-cre (blue), and AregFl/FlCD4-cre (red) mice were intranasally instilled with PBS (solid symbols; “mock”) or infected with 0.5 median lethal dose (LD50) PR8-OTI (open symbols; “PR8-OTI”). Changes in body weight were monitored daily in infected and control groups (dpi). The data are shown as mean percent weight ± SEM of weight at dpi 0. No significant differences were found between infected AregFl/FlFoxp3YFP-cre or AregFl/FlCD4-cre groups as compared to infected AregFl/Fl group (n = 4 per group except for n = 3 per group for mock-treated AregFl/FlFoxp3YFP-cre mice). Data are representative of ≥3 independent experiments.
(D–G) Flow cytometric analysis of the frequency of virus-specific (D) CD8+CD44hi and (E) CD4+Foxp3−CD44hi, (F) IFNγ (left), and IL-17-producing (right) CD4+Foxp3−, and (G) IFNγ-producing CD8+ T cells in spleen, lung, and lung-draining lymph nodes (LDLN) of AregFl/Fl (black), AregFl/FlFoxp3YFP-cre (blue), and AregFl/FlCD4-cre (red) mice infected with 0.5 LD50 PR8-OTI at dpi 10. Mean ± SEM. Data are representative of ≥3 independent experiments (n ≥ 3 mice per group).
(H) Influenza virus load on dpi 8 in lung tissue of AregFl/Fl (black), AregFl/Fl Foxp3YFP-cre (blue), and AregFl/FlCD4-cre (red) mice infected as in (C), as determined by TCID50. Data are shown as TCID50/mg of lung tissue analyzed. Mean ± SEM. Data are representative of three independent experiments.
See also Figure S3.
Next, we tested whether the observed early amphiregulin production by Treg cells has a role in promoting tissue repair during infection or in controlling antiviral immune responses by infecting AregFl/FlFoxp3YFP-cre and littermate control mice. To account for the potential contribution of amphiregulin production by effector T cells, we also examined flu-infected AregFl/FlCD4-Cre mice. Following intranasal infection, all groups of mice displayed an equivalent reduction in body weight (Figure 4C). Analysis of lung T cells at day 8 or 10 post-infection revealed comparable frequencies of CD44hiCD62Llo and Ki67+ cells, suggesting that overall T cell activation is unaffected by Treg or T-cell-restricted amphiregulin deficiency (Figure S3E). Furthermore, virus-specific CD8+ and CD4+ T cell responses in the lung, lung-draining lymph nodes, (LDLN) and spleen, assessed by H2-KbOVA257-264 and H2-I-Ab NP311-325 tetramer staining, respectively, were comparable in all groups of mice at the peak of the response on days 8–10 post-infection (Figures 4D and 4E). In addition, cytokine production by lung T cells was comparable in all groups of mice (Figures 4F and 4G). Importantly, similar antigen-specific T cell expansion and activation was accompanied with a comparable viral load in control mice and those harboring amphiregulin-deficient Treg cells or all T cells (Figure 4H). Thus, amphiregulin production by Treg cells was fully dispensable for Treg-cell-mediated suppression of anti-viral immunity.
To account for a potential role of Treg-cell-derived amphiregulin in tissue repair, we assessed physiological and histological parameters of lung damage following infection. In the absence of amphiregulin production by Treg cells, we observed a rapid decline in lung function, as measured by pulse oximetry, with average blood oxygen saturation levels falling below 80% as early as day 4 and below ~60% at later time points post-infection of AregFl/FlFoxp3YFP-cre mice (Figure 5A). In contrast, control animals did not exhibit blood oxygen saturation levels measuring less than 80% throughout the course of the infection. The comparably severe impairment in lung function observed in AregFl/FlCD4-Cre (Figure S4A) mice suggested that Treg cells—not effector T cells—serve as a key source of amphiregulin and was consistent with the dynamics of amphiregulin produced by lung adaptive and innate lymphocyte subsets (Figures 4A, 4B, and S3D). Furthermore, histopathological analysis revealed a markedly increased severity and extent (E1) of overall tissue damage, as quantified by bronchial epithelial loss (BEL), lymphoid aggregation (LA), and increased accumulations of cell debris and edema (AE) in the alveolar space of mice lacking amphiregulin in Treg cells or in all T cells (Figures 5B–5D and S4B). It must be noted that both AregFl/FlFoxp3YFP-cre and AregFl/FlCD4-cre mice eventually clear the infection and repair lung damage, whereas low-dose infection does not result in substantial lung damage or impaired blood oxygenation due to rapid viral clearance (data not shown).
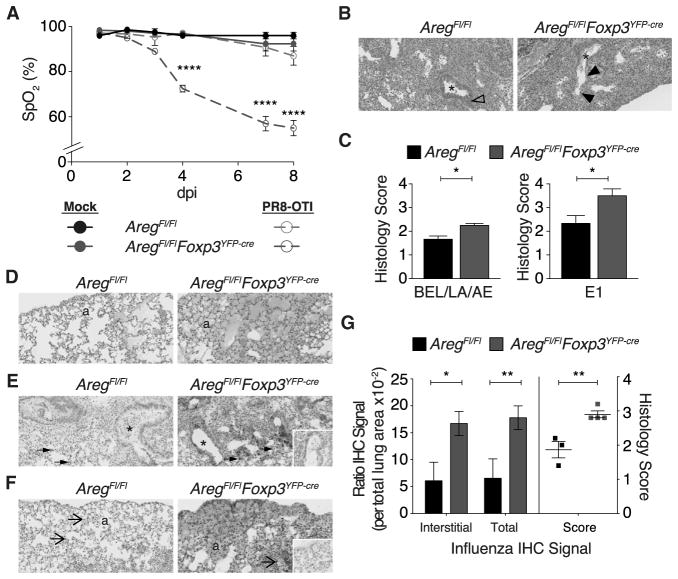
Figure 5. Lung Treg Cells Prevent Tissue Damage through the Production of Amphiregulin.
(A) Blood oxygen saturation (SpO2) in AregFl/Fl (solid black and dashed green) and AregFl/FlFoxp3YFP-cre (blue) mice following control treatment with PBS (solid symbols; “mock”) or infection with 0.5 LD50 PR8-OTI flu virus (open symbols; “PR8-OTI”) measured at the indicated dpi. The data are shown as mean ± SEM (n = 4 per group except for n = 3 per group for mock-treated AregFl/FlFoxp3YFP-cre mice). Depicted p value corresponds to comparison of infected AregFl/Fl with infected AregFl/FlFoxp3YFP-cre; the difference between control AregFl/Fl to control AregFl/FlFoxp3YFP-cre was not statistically significant. (B–F) Histopathological analysis of lungs from AregFl/Fl and AregFl/FlFoxp3YFP-cre mice following infection with 0.5 LD50 PR8-OTI virus as in (A), at dpi 8.
(B) Representative H&E stained histologic sections depicting prominent BEL in AregFl/FlFoxp3YFP-cre lung (between filled arrowheads; right) in contrast to hyperplastic bronchial epithelium in AregFl/Fl (open arrowhead; left). Asterisk (*) marks bronchiolar lumen.
(C) Quantification of increased severity (left) and extent (E1; right) of tissue damage, as measured by BEL, LA, increased cell debris, and AE. Mean ± SEM. (D) AE is marked in the subpleural region of histological sections from lungs of AregFl/FlFoxp3YFP-cre (right); alveoli (“a”).
(E and F) Immunohistochemical staining for influenza virus protein antigens is (E) intensely positive in AregFl/FlFoxp3YFP-cre (right) bronchial epithelium, alveolar macrophages (solid arrows), and sloughed cellular debris. Similar but less intense staining is noted in infected AregFl/Fl (left) lung. (F) Alveolar edema stains intensely positive for influenza antigen by immunohistochemistry; in contrast, in AregFl/Fl lung sections (left), only cell-associated immunohistochemical signal (open arrows) is noted. Insets are negative control of same section; alveoli (“a”), bronchiolar lumen (*).
(G) Quantification of interstitial and total signal intensity (see Experimental Procedures) and disease severity using image analysis software (left) and histological scoring (right) of sections from all mice stained as in (F). Original magnification all panels is 20×. Mean ± SEM. Data are representative of one of 2 independent experiments (n ≥ 3 mice per group).
See also Figure S4.
Consistent with a role of Treg cells as an early source of amphiregulin, both AregFl/FlFoxp3YFP-cre and AregFl/FlCD4-cre mice exhibited decreased total amounts of amphiregulin in bronchoalveolar lavage (BAL) fluid and lung tissue on day 5 of infection in comparison to control mice (Figure S4C). Immunohistochemical staining for viral protein provided additional evidence that barrier integrity and tissue architecture were compromised (Figure 5E). Whereas control animals exhibited punctate staining localized within alveolar and bronchiolar epithelial cells or sloughed cells confined to the luminal space, lung tissue of infected AregFl/FlFoxp3YFP-cre and AregFl/FlCD4-cre mice exhibited increased staining throughout sections. Thus, despite comparable control of viral replication, viral proteins were diffusing—most likely with necrotic debris and edema fluid—into the parenchyma, potentially impeding the transfer of oxygen into circulating blood (Figures 5F, 5G, and S4B). The observed decrease in blood oxygenation was unlikely a result of increased microbial translocation due to increased gut permeability because C-reactive protein (CRP) and serum LPS levels were unaffected (Figure S4D; data not shown). Likewise, it was not a consequence of anemia because AregFl/FlFoxp3YFP-cre and AregFl/FlCD4-cre mice had unperturbed hematocrit, red blood cell counts, and hemoglobin concentrations (Figure S4E). Consistent with the decreased capillary oxygenation, we observed decreased surface temperature in AregFl/FlFoxp3YFP-cre and AregFl/FlCD4-cre mice, whereas core body temperature was comparable to that in respective control groups (Figure S4F). These results demonstrate a key role for Treg-cell-derived amphiregulin in mediating tissue protection and maintaining barrier integrity in response to infectious stimuli-induced tissue damage.
IL-18 and IL-33 but Not TCR Signaling Elicit Amphiregulin Production in Treg Cells
We next questioned whether amphiregulin production by Treg cells—a distinct functional modality for promoting tissue protection—is mobilized in response to the same or separable cues from those eliciting their suppressor function, the latter being elicited by, and dependent upon, TCR signaling (Levine et al., 2014; Wu et al., 2006). Thus, it was possible that amphiregulin production is also induced in response to TCR signaling to simultaneously invoke Treg cell suppressor and tissue repair function. Alternatively, inflammatory mediators or alarmins released upon tissue damage might serve as distinct and biologically relevant cues for amphiregulin production in Treg cells. Indeed, previous studies showed that the alarmin IL-33 induces amphiregulin production in lung ILC2 in vitro, and high levels of amphiregulin expression were reported in ST2-expressing Treg cells in the gut and VAT (Burzyn et al., 2013b; Cipolletta et al., 2012; Feuerer et al., 2009; Monticelli et al., 2011; Schiering et al., 2014). Thus, alarmins IL-33 and IL-1α and inflammatory cytokines IL-18 and IL-1β, prominently expressed at mucosal epithelial surfaces, were likely candidates to mobilize an amphiregulin-dependent tissue repair functionality of Treg cells.
To test this hypothesis, FACS-purified GFP+ Treg cells from Foxp3GFP mice were incubated in vitro with IL-33, IL-18, IL-1α, IL-1β, or related cytokines IL-36α, β, or γ in the presence of IL-2 and IL-7, with or without stimulation with CD3 and CD28 antibody-coated beads. Quantification of cell-associated and secreted amphiregulin by flow cytometry and ELISA, respectively, showed that both IL-18 or IL-33 stimulation led to a marked increase in the level of secreted amphiregulin and was independent of TCR engagement, whereas IL-1α, IL-1β, and IL-36 family members failed to increase amphiregulin over 4 days in culture (Figures 6A and S5A). Amphiregulin production was limited to Treg cells expressing receptors for IL-18 (IL18R1; CD218α) and IL-33 (ST2), and amphiregulin secretion was dependent on receptor expression (Figures S5B and S5C; data not shown). Although IL-18 and IL-33 aided Treg cell expansion, amphiregulin protein levels produced on a per cell basis were still markedly increased (Figure 6B). In contrast, TCR stimulation in the presence of IL-2 induced proliferation and production of IL-10, whose non-redundant role in Treg cell suppressor function has been well documented (Figure 6C) (Asseman et al., 1999; Rubtsov et al., 2008). Notably, TCR stimulation not only failed to potentiate amphiregulin production on a per cell basis but also led to a reduction in cell-associated amphiregulin levels, likely due to expansion of amphiregulin-negative cells (Figures 6B and 6D). These results support the idea that amphiregulin is produced in response to signals distinct from those eliciting suppressor function.
Figure 6. IL-18 and IL-33 Signaling in Treg Cells Induces Production of Amphiregulin.
(A–D) FACS-sorted CD4+Foxp3+ Treg cells from Foxp3GFP reporter mice were cultured in vitro with each of the indicated alarmins, or IL-1β, in the presence of IL-2 and IL-7, with (“TCRstim”; red) or without (“Unstim”; black) stimulation with CD3 and CD28 antibody-coated beads for 4 days followed by quantification of (A) total and normalized per cell (B) amphiregulin amounts and (C) IL-10 protein levels by ELISA of cell-free culture supernatants and flow cytometric analysis of cell pellets for determining total viable cell numbers (used for normalization in B and C) and (D) remaining protein levels of cell-associated amphiregulin. In the absence of TCR stimulation, IL-10 levels are below the limit of detection. The data are shown as mean ± SEM. Data are representative of ≥3 independent experiments. (E and F) The (E) frequency of amphiregulin+ST2+ cells, (F) representative flow cytometry plots depicting amphiregulin and ST2 (top) or IL-18R (bottom) expression, and the (G) frequency of amphiregulin expressing IL-18R+ST2−, IL-18R−ST2+, and IL-18R+ST2+ cells determined by flow cytometric analysis of CD4+Foxp3+ Treg cells isolated from lungs of C57BL/6 mice following intranasal instillation with (F) PBS (dpi 0) or (E−G) infection with 0.5 LD50 PR8-OTI virus on the indicated dpi. Data are representative of 2 independent experiments (n ≥3 mice per group except n ≥2 in dpi 10 group in one of the two experiments). Data in (E) and (G) are presented as mean ± SEM.
(H) Treg cell production of amphiregulin in response to influenza infection-induced tissue damage occurs in a TCR-independent manner. Tcrawt/FlFoxp3eGFP-Cre-ERT2 mice were gavaged twice with tamoxifen to induce deletion of a conditional Tcra allele and TCR ablation. Two weeks later, treated mice were infected with 0.5 LD50 PR8-OTI, and lung leukocytes were analyzed directly ex vivo by flow cytometry for amphiregulin production on dpi 5 using intracellular staining. Representative histograms (left) and MFI plots (right) depict the level of amphiregulin expression in TCR+ and TCR− CD4+Foxp3+ Treg cells. TCR expression was detected using TCRβ-specific monoclonal antibody staining. MFI data are presented as mean ± SEM. Data are representative of n ≥10 mice.
See also Figure S5.
Next, we assessed lung tissue levels of IL-18 and IL-33 and expression of their receptors, IL-18R and ST2, respectively, and amphiregulin production by lung Treg cells after infection. Throughout the course of the infection, ST2 was present on no greater than ~50% of cells staining positively for amphiregulin, and at day 3 post-infection, when Treg cells first began expressing detectable levels of amphiregulin, only ~30% of cells were positive for both amphiregulin and ST2 (Figure 6E). We observed, however, significant expansion of IL-18R+ Treg cells. This subset of Treg cells exhibited the highest proliferative activity very early following infection (Figures S5D and S5E). Furthermore, whereas few amphiregulin+ Treg cells were ST2+, ~80% of amphiregulin-producing Treg cells were IL-18R+ (amphiregulin+IL-18R+) and expressed the highest amount of amphiregulin on a per-cell basis (Figures 6F and S5F). Accordingly, the majority of amphiregulin+ST2+ Treg cells co-expressed IL-18R (amphiregulin+IL-18R+ST2+; Figure 6G). Consistent with these observations, IL-18 was present at approximately two log higher concentration than IL-33 in BAL fluid 5 days post-infection (data not shown).
To investigate whether Treg cells are capable of producing amphiregulin in a TCR-independent manner in vivo, previously characterized Tcrawt/FlFoxp3eGFP-Cre-ERT2 mice were treated with tamoxifen 14 days prior to infection with PR8 influenza virus to ablate TCR in a portion of Treg cells due to Tcra allelic exclusion. Efficient production of amphiregulin by ex-vivo-isolated TCR-deficient Treg cells on day 5 post-infection was indicative of independence from TCR signaling (Figure 6H). Thus, these studies suggested that the amphiregulin-mediated tissue protective function of Treg cells is induced in response to distinct cues, namely, inflammatory mediator IL-18 and alarmin IL-33, but not TCR signaling, which promotes suppressor function.
Our finding that the majority of amphiregulin-producing Treg cells expressed IL-18R presented an opportunity to explore the transcriptional features associated with amphiregulin expression in Treg cells and their tissue repair function. For comparison, we sought to examine RNA expression in IL-10-expressing cells as representatives of “effector” Treg cells with a TCR signaling-dependent immunosuppressive function. To assess the transcriptional profiles of cells receiving these different contextual inputs, the following activated Thy1.1+ Treg cell populations were FACS-purified from infected Il10GFP Foxp3Thy1.1 mice based on the expression of either IL-10 (GFP+) or IL-18R (CD218α+): (1) IL-10−IL-18R−, (2) IL-10+IL-18R−, and (3) IL-10−IL-18R+. We isolated cells on day 5 post-infection, when significant numbers of Treg cells produce amphiregulin and when amphiregulin-producing conventional T cells have yet to undergo marked expansion (Figure 4A). Genome-wide transcriptional analysis by RNA sequencing (RNA-seq) confirmed that lung IL-10+IL-18R− and IL-10−IL-18R+ Treg cells exhibited a similar activation phenotype relative to double-negative IL-10−IL-18R− cells, with > 90% of shared differentially expressed genes (p < 0.01; > 2-fold change) (Figures 7A, 7B, and S6A). Lung IL-10+IL-18R− Treg cells exhibited a heightened, previously defined TCR-dependent activation signature of CD44hiCD62Llo Treg cells (Figure S6B) (Arvey et al., 2014; Levine et al., 2014). Enrichment for a Treg cell activation signature specific to IL-10+IL-18R− cells suggested that these cells experienced potent TCR-dependent stimulation unlike IL-10−IL-18R+ cells. Despite being markedly different in amphiregulin protein expression, both populations of cells featured increased amounts amphiregulin transcript in comparison to IL-10−IL-18R−Treg cells. This was likely because exposure of some of these cells to IL-33, or a yet-to-be-identified factor, induced amphiregulin mRNA in IL-10+IL-18R− Treg cells. In addition, the IL-10+IL-18R− Treg cell subset showed statistically increased amounts of IL-18R (Il18r1) mRNA in comparison to the IL-10−IL-18R− Treg cell subset, suggesting that some of the former may have been stimulated by IL-18, but downregulated IL-18R on the cell surface. Finally, it must be noted that the increased amphiregulin mRNA amounts does not necessarily mean that membrane-bound or secreted amphiregulin protein is also expressed.
Figure 7. Treg Cells Poised for Amphiregulin Production Display a Distinct Gene Expression Profile.
(A–C) RNA-seq analysis of Treg cell subsets isolated from lungs of Il10GFPFoxp3Thy1·1 mice. Thy1.1+ Treg cells were FACS sorted into IL-10−IL-18R−, IL-10+IL-18R−, and IL10−IL-18R+ populations on day 5 following intranasal infection with 0.5 LD50 PR8-OTI. Differential gene expression between IL-10+IL-18R− and IL10−IL-18R+ populations is shown as (A) fold-change-fold-change plot of IL-10+IL-18R− versus IL-10−IL-18R− and IL10−IL-18R+ versus IL-10−IL-18R− highlighting genes exhibiting >2-fold (p < 0.01) increase in IL-10+IL-18R− versus IL10−IL-18R+ (green dots) and IL10−IL-18R+ versus IL-10+IL-18R− (blue dots) or (B) >2-fold increase (p < 0.01; shown in [A] as IL-10+IL-18R− versus IL-10−IL-18R−, green dashed line and IL10−IL-18R+ versus IL-10−IL-18R−, blue dashed line) in IL-10+IL-18R− versus IL-10−IL-18R− and IL10−IL-18R+ versus IL-10−IL-18R− (beige), IL-10+IL-18R− versus IL-10−IL-18R− relative to IL10−IL-18R+ versus IL-10−IL-18R− (green), or IL-10−IL-18R+ versus IL-10−IL-18R− relative to IL10+IL-18R− versus IL-10−IL-18R− (blue), as depicted by Venn diagram and (C) heatmap of genes with significant gene expression (n = 5 mice per population sequenced).
See also Figure S6.
A number of genes unique to IL-10+IL-18R− Treg cells have previously been shown to correlate with high immunosuppressive potential (Figure 7C). Interestingly, this gene set was highly reminiscent of those previously reported in Treg cells isolated from VAT during high-fat-diet-induced obesity and from injured skeletal muscle (Burzyn et al., 2013b; Cipolletta et al., 2012; Feuerer et al., 2009). Of these genes, the most pronounced overlap was observed for genes related to Treg cell suppressor function, e.g., Lag3, Gzmb, Tnfrsf8 (CD30), and Entpd1 (CD39) (Josefowicz et al., 2012). This pattern highlighted the notion that lung IL-10-producing Treg cells from infected mice exhibited high immunosuppressive potential. Analysis of transcripts selectively upregulated in IL-10−IL-18R+ versus IL-10+IL-18R− Treg cells (Figure 7C) suggested that the former population is proficient in extracellular matrix (ECM) remodeling and tissue repair. Genes expressed by this population encompass core matrisome and matrisome-associated components (http://matrisomeproject.mit.edu). These results suggest that Treg cells capable of tissue repair exhibit a discrete gene expression pattern consistent with the idea that distinct contextual inputs facilitate Treg cell tissue repair and immunosuppressive effector modalities.
DISCUSSION
It has been proposed that Treg cells serve multiple functions beyond their initially characterized role in suppressing autoimmunity (Burzyn et al., 2013a; Josefowicz et al., 2012). Depending on tissue localization, transcription factor expression, and activation status, Treg cells may adopt specialized capabilities for affecting function of hematopoietic and non-hematopoietic cells. Here, using influenza lung infection as a model of pathogen-induced immunity, inflammation, and tissue damage, we demonstrate an essential role for Treg cells in tissue protection—separable from their well-established function in suppression of the immune response. The ability of Treg cells to preserve lung structural and functional integrity early during influenza infection was conditional upon their production of amphiregulin.
Recently, Treg cells have been implicated in response to tissue damage based on an impairment in muscle regeneration observed upon systemic Treg cell ablation following muscle injury, with systemic administration of recombinant amphiregulin capable of rescuing the defect (Burzyn et al., 2013b). However, acute Treg cell deficiency results in autoimmunity, with massive expansion and recruitment of pro-inflammatory monocytes and activation of myeloid cells, which fuel severe inflammation and tissue damage (Kim et al., 2007). Furthermore, generalized delivery of amphiregulin is capable of rescuing tissue function in a variety of settings, including lung failure during bacterial and viral co-infection, and many cell types are capable of producing amphiregulin, including basophils, ILC2, mast cells, Treg, effector CD4 and CD8 T cells, and smooth-muscle cells (Deacon and Knox, 2015; Jamieson et al., 2013; Zaiss et al., 2015). Treg cells muffle pro-inflammatory gene activation; the blunted recruitment and responses of inflammatory cells curtail positive-feedback loops mediated by activated T cells and promote a switch from an inflammatory to a tissue repair program. Thus, it is possible that the impairment in tissue repair observed in the absence of Treg cells was an indirect consequence of increased systemic immune activation and inflammation.
Our study suggests that Treg cells have the capacity to directly exert tissue repair function, at least in part, through production of amphiregulin. Although seemingly unexpected in light of the aforementioned multiple cellular sources, the non-redundant role for amphiregulin produced by Treg cells was consistent with our finding that they represented the most numerous cellular source of amphiregulin mobilized early in the response to tissue damage. It must be noted that AregFl/FlFoxp3YFP-cre mice cleared the infection, and lungs were eventually repaired likely due to contribution of amphireglin, produced by other cell types, and of epiregulin and EGF, which, together with amphiregulin, act upon an overlapping set of receptors. The importance of amphiregulin production by T cells was dependent on the extent of infection-induced damage because we observed comparable lung function in mutant and wild-type mice upon a low flu infection (data not shown). It is also likely that the outcome of Treg-restricted amphiregulin deficiency could be much more dramatic upon bacterial co-infection.
Recently, ILC2-derived amphiregulin was shown to be similarly required for limiting lung tissue damage in influenza-virus-infected Rag1−/ − mice lacking T and B cells (Monticelli et al., 2011). Although we cannot formally demonstrate or dismiss a role for amphiregulin+ ILC2 in response to influenza infection in lymphoreplete animals, we find that amphiregulin+ Treg cells far outnumber amphiregulin+ ILC2 as early as 3 days post-infection, when differences in lung function begin to be observed. It is also important to consider that ILC2 are present in higher numbers in RAG-deficient mice than in wild-type mice and that, upon challenge, ILC2 may expand more significantly in the former animals.
Treg cells are capable of suppressing immune responses against influenza virus and assisting in animal recovery after viral infection is cleared (Bedoya et al., 2013; Brincks et al., 2013; Moser et al., 2014). In light of these results, it is important to emphasize that no detectable impairment in Treg cell suppressor capacity or their numbers or activation status was observed in unchallenged animals, in settings of T cell-mediated systemic autoimmunity, or during infection. These findings were contrary to previous conjecture of an autocrine role for amphiregulin in immunosuppression (Meulenbroeks et al., 2015; Zaiss et al., 2013, 2015).
In contrast to TCR and IL-2-signaling-induced suppressor function, Treg cell tissue repair capacity manifested by production of amphiregulin was not facilitated in an “adaptive manner” by TCR engagement in vitro or in vivo. In contrast, it can be induced in an “innate” manner by IL-18 and IL-33, whose release is associated with inflammation and tissue injury. Although the extent of their relative contribution to the induction of a Treg cell tissue repair modality in vivo remains to be experimentally tested, the majority of IL-18R-expressing Treg cells produced amphiregulin in the lungs of infected animals. Particular transcriptional features of these cells suggested that they were proficient in tissue protection or repair. It is noteworthy that Treg cells isolated from other tissues, including injured skeletal muscle, inflamed colon, and adipose tissue, express ST2 and IL-18R (Burzyn et al., 2013b; Harrison et al., 2015; Schiering et al., 2014; Vasanthakumar et al., 2015). Our analysis of influenza infection in mixed bone marrow chimeras generated upon transfer of IL-18R-deficient and Foxp3-deficient bone marrow cells into irradiated lymphopenic recipients also supported a prominent role for IL-18R signaling in Treg cells in their tissue protective function in vivo (S.H., N.A., and A.Y.R., unpublished data). Our studies suggest that IL-18 likely has a dual role in promoting inflammatory responses when acting upon immune effector cells and alleviating or preventing tissue damage and loss of tissue function when acting upon Treg cells. Such a dual role for IL-18 is reminiscent of IL-2, which promotes both effector T cell responses and Treg cell suppressor function (Liao et al., 2013).
In conclusion, our study provides evidence that Treg cells have a distinct function in protective responses to tissue injury, repair, and maintenance and that this function of Treg cells is invoked in response to cues that are separable from those invoking suppressor capacity.
EXPERIMENTAL PROCEDURES
Mice
AregFl/Fl mice were generated using mouse B6 ES cells obtained through the EUCOMM (European Conditional Mouse Mutagenesis Program) consortium. AregFl/Fl allele was bred to Foxp3YFP-cre and CD4-cre mice, and mice were screened for maintenance of the C57BL/6N Nnt allele. C57BL/6NCr mice were purchased from Charles River. For details of AregFl/Fl and other mice used in this study, see the Supplemental Experimental Procedures. Generation and treatments of mice were performed under protocol 08-10-023 approved by the Sloan Kettering Institute (SKI) Institutional Animal Care and Use Committee. All mouse strains were maintained in the SKI animal facility in accordance with institutional guidelines.
Cell Isolation
Spleens and lymph node cell suspensions were prepared and red blood cell (RBC) were lysed using ammonium chloride (ACK; Sigma) buffer followed by repeated washing in RPMI1640 medium, 5% fetal bovine serum (FBS; Corning). For isolation of lymphocytes from lung and LILP, tissues were digested with collagenase A (1 mg/ml, Roche) and DNase I (0.5 μg/ml, Roche) in RPMI1640 for 30 min at 37°C. For some experiments, CD4+ T cells were enriched using the Dynabeads FlowComp mouse CD4 kit (Life Technologies) and sorted using an Aria II cell sorter (BD Biosciences).
In Vitro Assays
For the analysis of amphiregulin production, ex vivo isolated cells were stimulated, where noted, with PMA (50 ng/ml, Sigma) and ionomycin (1 nM, Calbiochem) for 3 hr in the presence of marimastat (10 μM, Sigma) and GolgiPlug (brefeldin A) and GolgiStop (monensin; BD Biosciences) or stained directly ex vivo following isolation from infected lungs. Secreted amphiregulin and IL-10 were quantified using mouse Amphiregulin DuoSet (R&D) and IL-10 Ready-SET-Go! (eBioscience) ELISA kits. In vitro suppression assays were performed as previously described (Arpaia et al., 2013). For a complete list of recombinant cytokine concentrations and in vitro polarization conditions, see the Supplemental Experimental Procedures.
Influenza Virus Infection
Recombinant influenza A strain PR8-OTI (H1N1) was grown for 40 hr in the allantoic cavity of 10-day-old embryonated chicken eggs (Charles River) and titer was determined by plaque immunostaining. For details, see the Supplemental Experimental Procedures.
RNA Sequencing
Treg cells isolated from lungs of flu-infected Il10GFPFoxp3Thy1.1 mice were FACS sorted directly into TRIzol LS reagent (Life Technologies) based on expression of GFP and IL-18R (CD218α) staining at 5 days post-infection. Extracted RNA was amplified by SMART amplification (Clontech) prior to generation of cDNA libraries (see the Supplemental Experimental Procedures).
Statistical Analyses
Unless otherwise noted, data are presented as mean ± SEM, with significance calculations determined by Student’s t test or ANOVA using Prism software (GraphPad). A value of p > 0.05 was deemed not statistically significant (ns); *p≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001.
Supplementary Material
Highlights.
Treg cells serve as a major early source of amphiregulin during influenza infection
Amphiregulin expression in Treg cells is dispensable for their suppressor function
Amphiregulin deficiency in Treg cells results in severe damage of infected lungs
Treg cell production of amphiregulin is IL-18/IL-33 dependent but TCR independent
Acknowledgments
We thank B.W. Johnson for assistance with histopathological analysis, S. Dikiy, C. Campbell, A.G. Levine, B. Hoyos, S.Y. Lee, and A.H. Bravo for experimental support. This work was supported by NIH/NCI Cancer Center Support Grant (CCSG) P30CA008748, NIH grant R37AI034206 (A.Y.R.), the Ludwig Center at Memorial Sloan Kettering Cancer Center, the Hilton-Ludwig Cancer Prevention Initiative (Conrad N. Hilton Foundation and Ludwig Cancer Research) (A.Y.R.), the Robert Black Fellowship of the Damon Runyon Cancer Research Foundation DRG-2143-13 (N.A.), and an Irvington Fellowship of the Cancer Research Institute (J.A.G.). A.Y.R. is an investigator with the Howard Hughes Medical Institute.
Footnotes
ACCESSION NUMBERS
High-throughput RNA-seq data from this project have been deposited within the NCBI Gene Expression Omnibus (GEO) database under accession number GEO: GSE71588.
Supplemental Information includes Supplemental Experimental Procedures and six figures and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2015.08.021.
AUTHOR CONTRIBUTIONS
This study was conceived and designed by N.A. and A.Y.R.; N.A. performed experiments and analyzed data jointly with A.Y.R.; A.A. performed RNA-seq data analysis; B.M. provided assistance with flu infection experiments; P.M.T. performed histological analyses; J.A.G., S.H., and S.Y. assisted with the analysis of amphiregulin production; N.A. and A.Y.R. wrote the manuscript.
References
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15:580–587. doi: 10.1038/ni.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya F, Cheng GS, Leibow A, Zakhary N, Weissler K, Garcia V, Aitken M, Kropf E, Garlick DS, Wherry EJ, et al. Viral antigen induces differentiation of Foxp3+ natural regulatory T cells in influenza virus-infected mice. J Immunol. 2013;190:6115–6125. doi: 10.4049/jimmunol.1203302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol. 2014;28:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Brincks EL, Roberts AD, Cookenham T, Sell S, Kohlmeier JE, Blackman MA, Woodland DL. Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J Immunol. 2013;190:3438–3446. doi: 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013a;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013b;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon K, Knox AJ. Human airway smooth muscle cells secrete amphiregulin via bradykinin/COX-2/PGE2, inducing COX-2, CXCL8 and VEGF expression in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2015 doi: 10.1152/ajplung.00390.2014. Published online June 5, 2015 http://dx.doi.org/10.1152/ajplung.00390.2014. [DOI] [PMC free article] [PubMed]
- Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J Immunol. 1998;160:322–327. [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Srinivasan N, Pott J, Schiering C, Krausgruber T, Ilott NE, Maloy KJ. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3 Treg cell function in the intestine. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.13. Published online March 4, 2015 http://dx.doi.org/10.1038/mi.2015.13. [DOI] [PMC free article] [PubMed]
- Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, Medzhitov R. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340:1230–1234. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbroeks C, van Weelden H, Schwartz C, Voehringer D, Redegeld FA, Rutten VP, Willemse T, Sijts AJ, Zaiss DM. Basophil-derived amphiregulin is essential for UVB irradiation-induced immune suppression. J Invest Dermatol. 2015;135:222–228. doi: 10.1038/jid.2014.329. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EK, Hufford MM, Braciale TJ. Late engagement of CD86 after influenza virus clearance promotes recovery in a FoxP3+ regulatory T cell dependent manner. PLoS Pathog. 2014;10:e1004315. doi: 10.1371/journal.ppat.1004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Operario DJ, Georas SN, Mosmann TR. The acute environment, rather than T cell subset pre-commitment, regulates expression of the human T cell cytokine amphiregulin. PLoS ONE. 2012;7:e39072. doi: 10.1371/journal.pone.0039072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Sheng H. Amphiregulin promotes intestinal epithelial regeneration: roles of intestinal subepithelial myofibroblasts. Endocrinology. 2010;151:3728–3737. doi: 10.1210/en.2010-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol Rev. 2013;255:182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Zaiss DM, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Gröne A, Sibilia M, van Bergen en Henegouwen PM, Roovers RC, Coffer PJ, Sijts AJ. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314:1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.