Abstract
De novo fear conditioning paradigms have served as a model for how clinical anxiety may be acquired and maintained. To further examine variable findings in the acquisition and extinction of fear responses between clinical and non-clinical samples, we assessed de novo fear conditioning outcomes in outpatients with either anxiety disorders or depression and healthy subjects recruited from the community. Overall, we found evidence for attenuated fear conditioning, as measured by skin conductance, among the patient sample, with significantly lower fear acquisition among patients with depression and posttraumatic stress disorder. These acquisition deficits were evident in both the simple (considering the CS+ only) and differential (evaluating the CS+ in relation to the CS−) paradigms. Examination of extinction outcomes were hampered by the low numbers of patients who achieved adequate conditioning, but the available data indicated slower extinction among the patient, primarily panic disorder, sample. Results are interpreted in the context of the cognitive deficits that are common to the anxiety and mood disorders, with attention to a range of potential factors, including mood comorbidity, higher-and lower-order cognitive processes and deficits, and medication use, that may modulate outcomes in fear conditioning studies, and, potentially, in exposure-based cognitive behavioral therapy.
Keywords: fear conditioning, classical conditioning, extinction learning, anxiety disorders, depression
Investigations of de novo fear conditioning provide an important strategy for studying how clinical anxiety may be acquired and maintained. In such conditioning studies, a neutral stimulus is paired repeatedly with an aversive unconditioned stimulus (UCS) such that the neutral stimulus becomes a conditioned stimulus (CS+) capable of eliciting a fear response. In simple conditioning paradigms, the strength of fear conditioning is assessed by the magnitude of responding to the CS+. In differential conditioning, one CS is paired with the UCS (designated as the CS+) during acquisition, and a second CS is not (designated as the CS−). Fear conditioning is measured as the difference between responses to the CS+ and CS−, providing an index that reflects the extent to which subjects successfully discriminate a threat cue (CS+) from a safety cue (CS−). It is important to recognize that differential conditioning paradigms control for individual differences in reactivity (by subtracting reactivity to the CS− from reactivity to the CS+), whereas simple conditioning paradigms typically do not.
Both the acquisition and extinction of de novo fear responses are important, because the development and persistence of anxiety disorders may be influenced by differences in: (1) the ease by which new fears are learned, (2) the difficulty by which relative safety is (re)learned during fear extinction, and/or (3) the degree to which an individual can discriminate safe from unsafe conditions, thereby influencing the predictability of perceived danger (Orr et al., 2000). Studies of fear conditioning have focused on the examination of each of these processes, with surprisingly complex outcomes, particularly concerning the acquisition of fears in de novo fear-conditioning paradigms. Lissek et al. (2005) conducted a meta-analysis of the effect sizes for conditioning among anxiety patients relative to controls, with positive scores representing greater acquisition of conditioned fear responses among anxiety patients. Of the 46 effect sizes computed from 20 studies in the literature, 25 (55%) were positive (reflecting better acquisition among anxious samples), 8 (17%) were negative, and 13 (28%) were near zero.
The disparity in effect sizes reported by Lissek et al. (2005) is attenuated somewhat by examining the differences in results between simple and differential conditioning procedures. Although more consistently positive effects are evident for simple conditioning results (mean d = .42 ± .28), compared to differential conditioning (mean d = .08 ± .23), there continues to be an abundance of heterogeneity within these two paradigms. For example, anxiety patients have been shown to learn differential fear conditioning both dramatically better and worse than healthy control participants (with effect sizes ranging from +1.03 to −.91) in the Lissek et al. (2005) meta-analysis.
Some of the variability in these results may be a function of the intensity and/or relevance of the stimuli used in de novo fear conditioning paradigms. Grillon (2009) has emphasized a dual-model theory of fear conditioning in humans: with fear conditioning potentially engaging a lower-order defensive process that is outside conscious awareness as well as a higher order cognitive system linked to conscious awareness of anticipation and danger. Whereas rodent fear conditioning is dependent on the lower-order process, Grillon argues that human fear conditioning may preferentially rely on higher-order cognitive processes that are more dependent on hippocampal rather than amygdala function, unless the stimuli are particularly fear relevant or an intense UCS is used. The less relevant the conditioning paradigm is to actual clinical fear, the more de novo fear conditioning will engage higher-order processes that are hippocampally-mediated and potentially different from those processes activated by clinical fears (Grillon, 2009).
One implication of this distinction between higher- and lower-order processes is that hippocampally-mediated functions may be more disrupted in anxiety disorders due to associated cognitive deficits. Although findings vary somewhat across studies (Gladsjo et al., 1998; Twamley, Hami, & Stein, 2004), memory and executive function deficits are frequently observed among patients with anxiety disorders (panic disorder: (Airaksinen, Larsson, & Forsell, 2005; Asmundson, Stein, Larsen, & Walker, 1994; Deckersbach, Moshier, Tuschen-Caffier, & Otto, 2011; Samuelson et al., 2006), PTSD: (Koso & Hansen, 2006; Peri, Ben-Shakhar, Orr, & Shalev, 2000; Polak, Witteveen, Reitsma, & Olff, 2012), social phobia: (Hermann, Ziegler, Birbaumer, & Flor, 2002), as are hippocampal and memory and executive function deficits among patients with depression (Bora, Fornito, Pantelis, & Yucel, 2012; Lee, Hermens, Porter, & Redoblado-Hodge, 2012; McKinnon, Yucel, Nazarov, & MacQueen, 2009). These cognitive deficits may help to account for the negative effect sizes observed in some de novo fear acquisition paradigms, where anxiety patients show weaker, rather than stronger, acquisition of a conditioned fear response (Grillon & Morgan, 1999; Veit et al., 2002). Cognitive deficits may also contribute to the difficulties extinguishing learned fears observed in anxiety patients relative to healthy samples (Blechert, Michael, Vriends, Margraf, & Wilhelm, 2007; Grillon, 2002b; Grillon, Lissek, McDowell, Levenson, & Pine, 2007; Peri et al., 2000; Pitman & Orr, 1986), as well as the difficulty discriminating “safe” from “unsafe” environments observed in studies of context conditioning in anxiety patients (Hermann et al., 2002).
Another potential contributor to the variability observed in conditioning results is the presence of depression. Major depression is frequently comorbid with anxiety disorders (Brown, Campbell, Lehman, Grisham, & Mancill, 2001), yet the role of depression in de novo fear conditioning has been relatively unstudied. Deficits in associative conditioning (eyeblink) have been found for depressed patients (Greer, Trivedi, & Thompson, 2005), but relative to fear conditioning, the available research has been specific to fear potentiated startle paradigms. Specifically, Jovanovic et al. (2010) used an aversive conditioning procedure (airblasts to the throat) to examine a range of conditioning indices relative to healthy controls in patients with comorbid PTSD and depression, PTSD alone, and depression alone. Patients with depression alone had responses most similar to controls, although comorbid depression did appear to enhance fear potentiated startle to the safety cue among patients with PTSD, suggesting that depression may further impair the safety signal processing documented for PTSD patients or may reflect more severe PTSD.
Although much has been learned about the nature of fear learning and extinction through de novo conditioning studies, the variability of results and the unknown role of depression have been significant limitations of the literature. The current study aims to address these limitations. To further study differences in the acquisition of fear responses between clinical and non-clinical samples, we assessed simple and differential de novo fear conditioning outcomes in outpatients with either anxiety disorders or depression and healthy subjects recruited from the community. We used a mild electric shock to the fingers as the UCS and skin conductance as the measure of conditioned fear.
Because differential conditioning requires the learning and subsequent discrimination of the meaning of both a CS+ and CS−, we hypothesized that, due to the attentional and memory deficits common to anxiety and mood disorders, the patient sample would show poorer acquisition of a differential conditioned fear response than the healthy community sample, with the poorest differential conditioning in the depressed participants. However, because simple conditioning is assessed by measuring reactivity only to the fear-relevant cue (CS+), we hypothesized that anxiety patients, but not depressed patients, would demonstrate stronger simple conditioning than the healthy control participants. These hypotheses are in accord with the more consistently positive effect sizes observed for simple, compared to differential, conditioning in anxiety samples (Lissek et al., 2005). The present study expands upon these findings by examining conditioned fear in depressed patients relative to both clinically anxious and healthy control participants.
Method
Participants
A total of 168 participants (88 females), ages 18 to 64 years, were enrolled in the study. The sample included 102 healthy controls (HC) with no history of mood disorder, and 66 treatment-seeking participants with anxiety and mood disorders. In the clinical sample, participants met DSM-IV criteria as determined by the Structured Clinical Interview for DSM-IV (SCID-IV; First, Spitzer, Gibbon, & Williams, 1995) for a current primary diagnosis of either 1) posttraumatic stress disorder (PTSD, n=27), 2) panic disorder (PD, n=24) or 3) unipolar major depressive disorder (MDD, n=15) without current PTSD or PD comorbidity. Exclusionary criteria for all participants included serious medical conditions, current use of beta-blockers, past or present psychosis, current suicidality or homicidality, substance use disorder, and current pregnancy. Participants with a primary diagnosis of MDD were excluded if they also met criteria for PTSD or PD. Additionally, healthy control participants were excluded for current or past DSM-IV Axis I disorders, as determined by the SCID-IV.
Participants failing to show an average unconditioned skin conductance response (UCR) greater than 0.1 μ Siemens to the five presentations of the UCS were considered to be electrodermal non-responders and were not included in the analyses (n = 21; HC = 5, PTSD = 11, PD = 3, MDD = 2). One healthy control participant was excluded from the analysis due to incomplete data. The final sample consisted of 146 participants (HC = 96, PTSD = 16, PD = 21, MDD = 13). Of the final sample, rates of MDD comorbidity among the PD and PTSD groups were 24% and 25%, respectively. Sixty-six percent of PD patients had any psychiatric comorbidity, compared with 44% of PTSD patients and 31% of MDD patients (see Table 1). Of the PD patients, 91% met criteria for panic disorder with agoraphobia.
Table 1.
Demographic Characteristics for the Samples
| Characteristic | Healthy Controls | PTSDa | Panic Disorder | MDDb |
|---|---|---|---|---|
| N | 96 | 17 | 21 | 13 |
| Sex (%, n female)c | 49.0 (47) | 81.3 (13) | 47.6 (11) | 30.8 (4) |
| Age (mean, sd)d | 29.6 (10.3) | 39.5 (11.4) | 32.5 (7.9) | 41.2 (10.6) |
| Race | ||||
| Caucasian, % (n) | 86.5 (83) | 87.5 (15) | 90.5 (19) | 84.6 (11) |
| African-American, % (n) | 7.3 (7) | 12.5 (2) | 4.8 (1) | 15.4 (2) |
| Asian, % (n) | 4.2 (4) | 0 (0) | 4.8 (1) | 0 (0) |
| Other, % (n) | 1.0 (1) | 0 (0) | 0 (0) | 0 (0) |
| Hispanic Ethnicity, % (n) | 3.2 (3) | 6.3 (1) | 4.8 (1) | 0 (0) |
| Comorbid Diagnoses, % (n) | ||||
| MDD | - | 21.0 (4) | 19.0 (4) | - |
| Dysthymia | - | 0 (0) | 4.8 (1) | 0 (0) |
| GADe | - | 6.3 (1) | 33.3 (7) | 15.4 (2) |
| OCDf | - | 0 (0) | 4.8 (1) | 7.7 (1) |
| Social phobia | - | 6.3 (1) | 14.3 (3) | 7.7 (1) |
| Panic Disorder | - | 12.5 (2) | - | 0 (0) |
| PTSD | - | - | 0 (0) | 0 (0) |
| Specific phobia | - | 0 (0) | 14.3 (3) | 7.7 (1) |
| Agoraphobia without panic disorder | - | 0 (0) | 0 (0) | 7.7 (1) |
PTSD: Posttraumatic Stress Disorder
MDD: Major Depressive Disorder
p < .05 for the difference between the HC sample vs. PTSD and MDD
p < .05 for the chi-square test of sex distribution
GAD: Generalized Anxiety Disorder
OCD: Obsessive Compulsive Disorder
Stimuli
The conditioned stimuli (CS+ and CS−) were represented by a yellow circle and a white square, respectively. A computer monitor positioned 4 ft in front of the subject displayed the colored CSs. The unconditioned stimulus (UCS) was a 500 ms electrical shock delivered through electrodes attached to the second and third fingers of the subject’s dominant hand. The shock was generated by a Coulbourn Transcutaneous Aversive Finger Stimulator (E13-22), which uses a 9-V dry cell battery attached to an adjustable step-up transformer. At the beginning of testing, each participant established their own shock level, as described below.
Psychophysiological Measures
Skin conductance (SC)
SC was chosen as the physiological outcome measure due to its utility in the investigators’ previous work (see Orr et al., 2000; Otto et al., 2007) as well as its frequent use across the conditioning literature (Lissek et al., 2005). A Coulbourn Lablinc V, Human Measurement System was used to record SC and control the presentation of experimental stimuli. Skin conductance was measured by a Coulbourn Isolated Skin Conductance coupler (SV71-23) using a constant 0.5 V through 9 mm (sensor diameter) Invivo Metric Ag/AgCl electrodes placed on the hypothenar surface of the non-dominant hand (see Fowles et al., 1981). The SC electrodes were separated by 14 mm, as determined by the width of the adhesive collar.
Procedure
Study day one
After participants provided written informed consent, trained raters administered the SCID-IV to assess for the presence or absence of anxiety and mood disorders. Participants were asked to refrain from alcohol consumption in the 24 hours, and caffeine and nicotine intake in the 2 hours, prior to their visit. Female subjects were given a urine pregnancy screen, with no participant screening positive on this assessment.
Following completion of the self-report measures, participants were led to a sound-attenuated, humidity- and temperature-controlled room, connected by wires to an adjoining laboratory in which the experimental apparatus was located. Participants were seated in a comfortable chair and were monitored through an unobtrusive video camera. The technician attached electrodes to the second and third fingers of the participants’ hand and instructed participants to select a level of electric stimulation considered to be “highly annoying but not painful” (UCS; ranging between 0.2 and 4.0 mA). Once established, the UCS intensity remained constant throughout the experiment.
The technician then left the room and the computer took over the administration of the experiment. During a 5-min baseline recording period, SC level was sampled at 1,000 Hertz. Habituation consisted of 5 presentations of each stimulus type (later serving as CS+ and CS−). The duration of the CS was 8 s, and the inter-trial interval was 20 +/− 5 s, determined at random by the computer. Acquisition involved 5 presentations of each stimulus type; a 500 ms shock pulse occurred immediately after each CS+ offset.
Study day two
The technician informed participants that they “may or may not” receive the annoying shock stimuli delivered at the previously established UCS intensity. Participants were again connected to the shock electrodes but no shocks were administered. Extinction consisted of 10 presentations each of the CS+ and CS− in pseudo-random order in the absence of the shock stimulus. After completion of this phase, participants were unhooked from all equipment and queried regarding their awareness of the contingency between the CS+ and shock administration. Participants were compensated $80 for completion of all study procedures.
Data Reduction
Data reduction procedures were chosen to be consistent with the methods of previous studies (Orr et al., 2000; Otto et al., 2007; Pineles, Vogt, & Orr, 2009). Physiological responses to the stimuli were calculated by subtracting the mean SC level for the 2-s interval preceding CS onset from the highest SC level value among those recorded during the CS interval (i.e., 0–8 s following CS onset). SC responses to the UCS were calculated by subtracting the average SC level within 6–8 s after CS onset from the maximum increase in SC level during the 6-s interval following UCS offset. A SC orienting response was calculated by averaging the response to the first presentation of the to-be CS+ with the response to the first presentation of the to-be CS− during habituation. A differential SC response score for the acquisition phase was calculated by subtracting the average SC response for the five CS− trials from the average SC response for the five CS+ trials. Larger differential scores reflect greater fear conditioning; in other words, subjects have learned the contingency between the CS+ and shock and the CS− and absence of shock. Simple conditioning was calculated by averaging only the five CS+ trials independent of the response to CS− trials. A differential extinction SC response score was calculated separately for the first and second halves of the extinction phase. These scores consisted of the difference between the average SC responses to 5 CS+ presentations minus the 5 CS− presentations during the first or second half of the extinction phase.
Data Analysis
Broad comparisons between healthy controls and the patient group as a whole were completed with independent t-tests. Comparisons between diagnostic groups were completed with one-way ANOVA and ANCOVA, with follow-up planned pairwise comparisons, with control of inflation to alpha with Fisher’s PLSD. As noted below, ANCOVA was used to adjust for variables that differed between groups at baseline.
Results
Preliminary Analyses
Demographic information is presented in Table 1. We examined potential differences among groups for relevant demographic characteristics, including age, sex, ethnicity, and race. A significant difference emerged for age (F (3, 140) = 8.23, p = .00), with post-hoc Tukey’s tests showing that the depression and PTSD groups had a significantly higher mean age than controls (p < .01). In addition, the groups differed significantly in sex distribution (X2 (3, n = 146) = 8.36, p = .04), with a higher proportion of women in the PTSD group (81%). In a preliminary analysis, the relationship between age and SC simple and differential conditioning scores for habituation and acquisition were examined using Pearson correlations and were found to be non-significant for each individual diagnostic group (all ps > .05). However, age was significantly correlated with SC differential conditioning scores during phase 1 of the extinction procedure (r= −.67, p =.001) for individuals with panic disorder . Given recent evidence that sex may influence fear conditioning (Inslicht et al., 2012), we conducted preliminary analysis of sex effects on conditioning variables. No significant differences existed between men and women on SC simple or differential conditioning scores within any diagnostic group (all p > .14).
Mean (± standard deviation) scores for stimulus levels chosen by participants in each diagnostic group were as follows HC: 2.25±1.02, PTSD: 1.63±0.79, PD: 2.17 ±1.13, and MDD: 1.76±0.75. One-way ANOVA was used to examine the differences in the UCS level set by participants in each diagnostic group. Overall, a non-significant but trending effect was obtained (F (3,141) = 2.32, p =.078, partial eta squared = .047. reflecting a medium effect). Examination of all pair-wise differences with post-hoc Tukey tests revealed no significant differences between any two diagnostic groups (all p > .1). According to Pearson correlation, shock level was not associated with SC simple (r = .03) or differential (r = −.05) conditioning scores.
Seventeen percent of participants were taking psychiatric medications at the time of the study (HC = 1, PTSD = 7, PD = 9, and MDD = 8). The types of medications by diagnostic group are presented in Table 2. The one healthy control participant taking a psychiatric medication was taking trazodone for sleep difficulties. In a preliminary analysis, independent t-tests were conducted to examine potential differences between those patients taking psychiatric medication and those who were not. There was no significant difference between these groups in the magnitude of the SC unconditioned response (t (48) = −.103, p =.92, d = .22), SC simple conditioning score for acquisition (t (48) =−.62, p = .53, d = .17), or SC differential conditioning score for acquisition (t (48) = −.98, p = .33, d = .28). Additionally, there was no difference in SC differential scores for extinction (phases 1 and 2) between medicated and non-medicated participants when covarying the level of acquisition (all p > .6). Repeating these tests specific to antidepressant or benzodiazepine medications led to the same conclusions.
Table 2.
Psychiatric medication use by diagnosis
| Diagnostic Group | Benzodiazepine | SSRI/SNRI | Other Antidepressant | Othera |
|---|---|---|---|---|
| Healthy Controls (n=96) | 0 | 0 | 1 | 0 |
| PTSDb (n=16) | 3 | 3 | 2 | 0 |
| Panic Disorder (n=21) | 5 | 6 | 2 | 1 |
| MDDc (n =13) | 0 | 5 | 3 | 1 |
Topiramate, Buspar
PTSD: Posttraumatic Stress Disorder
MDD: Major Depressive Disorder
Habituation
An independent t-test revealed no significant differences between healthy controls and patients in their SC orienting response (t (144) = −.41, p = .68, d = .07). ANOVA was used to examine differences between healthy controls and each diagnostic group. The overall model showed no significant effect of diagnosis on SC orienting response (F (3, 142) = 1.83, p = .14, partial eta squared = .04, reflecting a small-to-moderate effect). Post-hoc LSD tests revealed a significant difference between the PD and PTSD groups (MPTSD = .23, MPD = .56, p = .029, d= .80) and a trend toward a significant difference between PD and control group (MHC = .36, MPD = .56, p = .07, d = .41). These results suggest a stronger orienting response to the stimuli prior to conditioning within the panic disorder group.
Response Acquisition
Results for fear acquisition by diagnostic group and paradigm are depicted in Figures 1 and 2. The ANOVA results for both simple and discriminant conditioning are described in more detail below.
Figure 1.
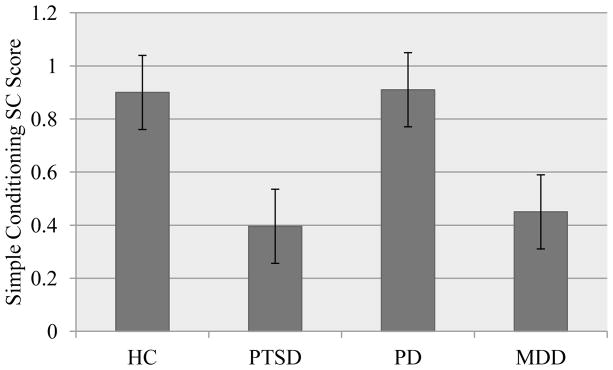
Acquisition of simple conditioned response by diagnostic group.
Note. HC: healthy control; PTSD: posttraumatic stress disorder; PD: panic disorder; MDD: major depressive disorder
Figure 2.
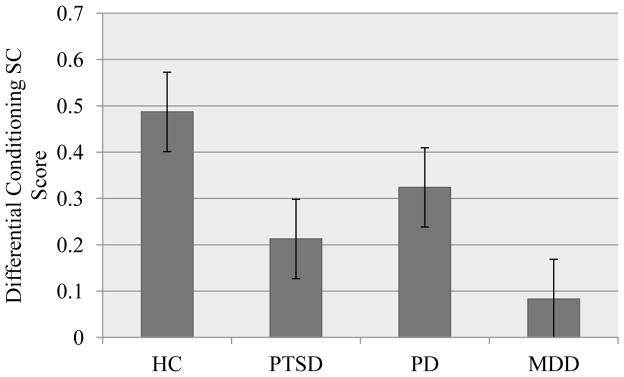
Acquisition of differential conditioned response by diagnostic group.
Note. HC: healthy control; PTSD: posttraumatic stress disorder; PD: panic disorder; MDD: major depressive disorder
Simple conditioning scores
We first examined differences in simple conditioning broadly using a t-test comparing healthy controls and patients. A trend toward a significant difference existed such that controls demonstrated greater SC simple conditioning scores than the patient group (MHC = .90, MPatient =.63, p = .057, d = 35). This difference remained at a trend level when covarying for the use of psychiatric medication (F (1, 143) = 3.03, p = .084 partial eta squared = .026).
We then examined group differences between healthy controls and each diagnostic group. A main effect of diagnostic condition for SC simple conditioning scores was observed (F (3, 142) = 2.75, p = .045, partial eta squared = .055). Follow up tests using Fisher’s PLSD revealed a significant difference between the HC group and the PTSD patient group (MHC = .90, MPTSD = .40, p = .021, d = −.72). In addition, there was a trend toward a significant difference between the HC group and the MDD patient group (MHC = .90, MMDD = .45, p = .062, d = −.57). Finally, a trend toward a significant difference existed between the PD and PTSD groups, representing a large effect (MPD = .91, MPTSD = .40, p = .057, d = −.89). When adding medication use to the model, the effect of diagnostic group remained at a trend level (F (3, 141) = 2.56, p = .057, partial eta squared = .052).
To further address the confounding between age and diagnostic group, we examined a subsample of healthy control subjects (n = 50) matched for mean age with the diagnostic groups, all effect sizes for simple SC scores were maintained for this analysis, indicating that age could not explain the differences between diagnostic groups.
Differential conditioning scores
We first examined differences in SC differential conditioning scores broadly using a t-test comparing healthy controls and patients. A significant difference existed such that controls demonstrated greater differential conditioning scores than the patient group group (MHC = .49, MPatient =.23, p = .02, d = .45). This difference remained significant when covarying psychiatric medication use (F (1, 143) = 5.102 p = .025, d = .38).
We then examined group differences between healthy controls and each diagnostic group. A trend toward a main effect of diagnostic condition was observed for differential conditioning scores (F (3, 142) = 2.29, p = .081, partial eta squared =.05). Follow-up tests using Fisher’s PLSD revealed a significant difference between the HC group and the MDD patient group (MHC = .49, MMDD = .08, p = .031, d = −.72), and trends for the difference between the HC group and the PTSD group (MHC = .49, MPTSD = .22, p = .11, d = −.50). The effect of diagnostic group remained at a similar trend level when covarying for the use of psychiatric medication (F (3, 141) = 2.16, p = .095, partial eta squared = .044).
Again, to address the confounding between age and diagnostic group, we examined a subsample of healthy control subjects (n = 50) matched for mean age with the diagnostic groups. All effect sizes for differential SC scores were maintained for this analysis, indicating that age could not explain the differences between diagnostic groups.
Extinction
Analysis of the extinction phase included only those individuals who acquired a conditioned response during the acquisition phase. This was defined as an average difference of at least 0.1 μ Siemens between SCR during the habituation and acquisition phase trials. Of the original 146 participants included in the acquisition phase data, 95 acquired a SC simple conditioned response to the CS+ (HC = 69, PTSD = 8, PD = 14, and MDD = 4). A total of 83 participants acquired a differential conditioned response (HC = 59, PTSD = 7, PD = 15, MDD = 2). Due to the small sample size in both the MDD and PTSD groups, subsequent analyses combined all three diagnostic groups to examine differences in extinction data between healthy controls and those with a psychiatric diagnosis. ANCOVA was used to evaluate the main effect of diagnostic group (control vs. psychiatric diagnosis) on SC differential conditioning scores for phase 1 and phase 2 of extinction when controlling for the level of differential acquisition as a covariate. The model for the first half of the extinction procedure indicated a trend toward better extinction among the healthy sample (F (1, 79) = 2.72, p = .10, d = .37.) that was not evident by the second half of extinction (F (1, 80) = .31, p = .58, d = .12. The effect of diagnostic status decreased when adding psychiatric medication use to the model (first half: F (1, 78) = .88, p = .35, d = .21; second half: F (1, 79) = .1, p = .75, d = .07).
Again, to address the confounding between age and diagnostic group, we examined a subsample of healthy control subjects (n = 50) matched for mean age with the diagnostic groups. Of these 50 individuals, 29 acquired a differential conditioned response and were included in the comparison analysis. We found a significant effect of diagnostic status on extinction for phase one, such that patients were characterized by reduced extinction (MHC = .109, MPatient = .279, F(1, 50) = 7.98, p = .007, d = .80). There was no significant difference between controls and patients on extinction in phase two (F (1, 50) = 1.45, p = .23, d = .34)
Contingency Awareness
Participants were also asked to identify the contingency between the CS and the shock administration. Of 135 participants for whom data was available, 106 identified the contingency, and 29 did not. The proportion of individuals who identified the contingency in each group was as follows: HC: 84%, PTSD: 71%, PD: 67%, and MDD: 62%. The relationship between groups and contingency awareness did not reach significance (χ2 (3, n=135) = 5.12, p = .16), although a trend was evident for the difference in proportion between the healthy control group and patients with major depression (Fisher’s Exact p = .066, phi = .193).
Discussion
The current study offers two particular contributions to the de novo fear conditioning literature. First, this was a particularly large study, with statistical analysis of 96 healthy controls and 55 treatment-seeking patients with anxiety and mood disorders. This compares well to the average total sample size of 47 in the fear acquisition studies reviewed by Lissek et al. (2005). Second, in the current study, we provide the first published data, to our knowledge, on simple and differential fear-conditioning outcomes for depressed patients without panic disorder or PTSD.
Overall, our results indicated that our patient samples conditioned less well than the healthy control participants. The difference between the combined-patient and non-patient samples reached significance and reflected a small-to-medium effect size, which was not substantively changed when controlling statistically for medication status. Follow-up tests indicated significant differences between the patients with major depression and the healthy participants for SC differential conditioning scores (approaching a large effect size), with a similar strong trend for the SC simple conditioning score (reflecting in excess of a medium effect size). We also found a significant difference between patients with PTSD and the healthy participants for the SC simple conditioning score, but non-significant effects for the SC differential conditioning score (with these results spanning moderate to large effect sizes). As evident in Figures 1 and 2, we obtained similar patterns of results for simple and differential conditioning scores, with similar effect sizes that differentially reached the point of significance. As such, we did not find support for our hypothesis that differences between the diagnostic groups and healthy control participants would be stronger for the simple conditioning score, rather, both scores indicated poorer fear learning among the patient samples.
Given the huge variability evident between studies in the literature (Lissek et al., 2005), our fear conditioning results are well within the range of findings for differences between healthy control participants and anxiety patients. Our results argue against the assumption that anxiety patients are particularly susceptible to acquiring conditioned fear responses, at least when a mild UCS is used. Yet, in part due to the limited number of participants who conditioned sufficiently, we provide only a limited perspective on whether associative learning deficits might translate into (1) difficulties by which relative safety is relearned during fear extinction, and/or (2) the degree to which an individual can discriminate safe from unsafe conditions, such that fear reactivity is generalized due to the belief that aversive outcomes are unpredictable. Specifically, those patients showing the poorest acquisition learning (predominantly those with major depression and PTSD) were unavailable for analysis of extinction effects, restricting the sample size for analysis of extinction effects. Nonetheless, among those that did acquire a conditioned response, we found significantly slower extinction (evident only in the first phase of extinction) for the diagnostic groups, these participants extinguished more slowly than the health control sample, reflecting a large effect size. Yet, during continued extinction, differences between groups were no longer significant. Slower extinction may have clinical relevance, if those with anxiety and mood disorders do not persist in exposure long enough to achieve the same extinction of their non-anxious and non-depressed counterparts. As such, we provide partial support for the hypothesis that anxiety and mood disorders may be influenced by relative conditioning deficits that translate to somewhat slower extinction of learned fears.
Even so, the sheer variability in conditioning results between studies leads us to be circumspect with regard to the meaning of our conditioning findings, and encourages attention to those factors that may moderate the degree of fear/extinction learning observed variably across anxious samples. One factor may be the presence of depression. In the current study we show that depressed patients have associative-learning deficits for fear stimuli. Thus, there is a potential for depression to modulate associative learning abilities in anxiety patients.
A second factor may be the degree to which the feared stimulus engages higher order vs. lower order processes (Grillon, 2002a, 2009; Grillon et al., 2007), and the degree to which cognitive deficits differentially affect these processes. One potential index of higher order learning is the degree to which individuals abstract the contingencies among stimuli. In the current study, we observed a trend toward poorer abstraction of the contingency among the patient sample. For example, only 62% of depressed participants accurately described which stimulus predicted shock, as compared to 84% of healthy participants. As such, we have evidence in the current study of some impairment in higher-order abstraction abilities among the patient cohort that parallels the significant results for skin conductance. Yet, it is not clear whether cognitive impairments, including those commonly documented for anxiety and mood disorders (e.g., Airaksinen et al., 2005; Asmundson et al., 1994; Lee et al., 2012), influence both higher and lower associative learning processes. A limitation of the current study is that we did not assess neuropsychological functioning, nor the link between neuropsychological functioning and conditioning outcomes. This issue has received initial examination in a substance-dependent cohort. In a pilot study, Basden (2010) found deficits in the acquisition of conditioned fear in a poly-substance abusing sample and deficits in neuropsychological functioning among these patients. Most importantly, the relative degree of impairment in attentional functioning predicted the ability to correctly identify the CS+/US contingency in this sample. There is also evidence of impaired extinction of eyeblink conditioning linked to intellectual level (Lobb & Hardwick, 1976), and a recent study indicates a greater likelihood of enduring post-trauma stress symptoms among those with lower intellectual functioning (Orr et al., 2012). These findings underscore the potential importance of cognitive impairment to conditioning processes, and raises questions about whether similar effects are evident in learning-based treatments.
The examination of the impact of cognitive impairment on CBT outcomes more generally has been relatively absent from the literature. We are aware of two positive studies in specialty populations, and neither includes exposure-based treatment. Specifically, there is evidence for poorer CBT outcome linked to neuropsychological performance for both depressed Parkinson’s patients (Dobkin et al., 2012) and elderly adults with generalized anxiety disorder (Caudle et al., 2007). These findings encourage further investigation of the link between cognitive abilities and outcomes from CBT, with particular attention to exposure-based treatments.
In the current study we included individuals taking medications, introducing a study limitation while allowing for a particularly real-world perspective on the effects of medications for subsequent fear acquisition or extinction. We detected no significant effects of medication (primarily antidepressant) use on fear acquisition. Likewise, no effects on fear acquisition were found in a study of a two-week antidepressant trial on de-novo fear conditioning in humans (Bui et al., 2013). Nonetheless, antidepressant medications appear to have complex effects on fear extinction, with some evidence of facilitation in a human trial (Bui et al., 2013) and reports of both impairment (Burghardt, Sigurdsson, Gorman, McEwen, & LeDoux, 2013) and facilitation (Melo et al., 2012; Yang et al., 2012) of extinction following chronic antidepressant administration in animals, effects that may be modified by hormonal variations (Lebron-Milad et al., 2013).
Overall, laboratory testing of fear acquisition and extinction in humans has, over time, resulted in a literature of highly variable results. The current larger-scale study of fear acquisition provided additional evidence for impaired rather than the enhanced fear acquisition we had hypothesized. There is a potential for this impaired learning to be explained by the cognitive impairments associated with anxiety disorders, and, indeed, clinical findings encourage attention to the role of cognitive abilities in attenuating CBT outcomes. Also, our findings draw attention to the role of depression in impairing associative fear learning. In continuing to try to provide a more nuanced model of fear acquisition or maintenance for the anxiety disorders, the fear conditioning literature must be able to place findings within the broader context of the commonly seen presentations of mood comorbidity, higher- and lower-order cognitive processes and deficits, and medication use.
Highlights.
We assess fear conditioning in controls and patients with depression, PTSD, or panic
Fear conditioning was attenuated in those with PTSD or depression
Effect sizes suggest slower extinction among patients, particularly those with panic
We discuss results in context of cognitive deficits common to anxiety and mood disorders
Acknowledgments
Funding
This research was supported by a NIMH translational research grant (MH072165) to the first author. The funding source had no other role other than financial support.
Footnotes
Disclosure Statement
The authors are aware of no conflicts with the content of this manuscript, nonetheless in the past two years, Dr. Otto has received consulting income from MicroTransponder Inc., ProPhase, and Concert Pharmaceuticals, as well as royalties for the use of the SIGH-A from ProPhase. Dr. Simon reports past 2 years research support from American Foundation for Suicide Prevention, Forest Laboratories, NIMH, DOD, honoraria from the MGH Psychiatry Academy and spousal equity in Elan, Dandreon, G Zero and Gatekeeper. Dr. Pollack reports the following disclosures over the 12 months: Advisory Boards and Consultation: Concept Pharma, Edgemont Pharmaceuticals, Eli Lilly, Ironwood Pharmaceuticals, Medavante, Merck: Research Grants: NIH; Equity: Doyen Medical, Medavante, Mensante Corporation, Mindsite, Targia Pharmaceuticals; Royalty/patent: SIGH-A, SAFER interviews. Ms. Moshier and Drs. Kinner and Orr have no disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39(2):207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Asmundson GJ, Stein MB, Larsen DK, Walker JR. Neurocognitive function in panic disorder and social phobia patients. Anxiety. 1994;1(5):201–207. [PubMed] [Google Scholar]
- Basden SL. Acquisition and Extinction in a Substance Dependent Sample: A De Novo Fear Conditioning Study. Boston University; Ann Arbor: 2010. ProQuest (Order No. 3411710 ) [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour Research & Therapy. 2007;45(9):2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. 0. Journal of Affective Disorders. 2012;138(1–2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110(4):585–599. doi: 10.1037/0021-843X.110.4.585. [DOI] [PubMed] [Google Scholar]
- Bui E, Orr SP, Jacoby RJ, Keshaviah A, Leblanc NJ, Milad MR, Simon NM. Two weeks of pretreatment with escitalopram facilitates extinction learning in healthy individuals. Human Psychopharmacology. 2013;28(5):447–456. doi: 10.1002/hup.2330. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, LeDoux JE. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biological Psychiatry. 2013;73(11):1078–1086. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle DD, Senior AC, Wetherell JL, Rhoades HM, Beck JG, Kunik ME, Stanley MA. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. American Journal of Geriatric Psychiatry. 2007;15(8):680–689. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Moshier SJ, Tuschen-Caffier B, Otto MW. Memory dysfunction in panic disorder: an investigation of the role of chronic benzodiazepine use. Depression and Anxiety. 2011;28(11):999–1007. doi: 10.1002/da.20891. [DOI] [PubMed] [Google Scholar]
- Dobkin RD, Rubino JT, Allen LA, Friedman J, Gara MA, Mark MH, Menza M. Predictors of treatment response to cognitive-behavioral therapy for depression in Parkinson’s disease. Journal of Consulting and Clinical Psychology. 2012;80(4):694–699. doi: 10.1037/a0027695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition. New York: New York Psychiatric Institute; 1995. [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Committee report. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18(3):232–239. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Rapaport MH, McKinney R, Lucas JA, Rabin A, Oliver T, Judd LL. A neuropsychological study of panic disorder: negative findings. Journal of Affective Disorders. 1998;49(2):123–131. doi: 10.1016/s0165-0327(98)00006-8. http://dx.doi.org/10.1016/S0165-0327(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Greer TL, Trivedi MH, Thompson LT. Impaired delay and trace eyeblink conditioning performance in major depressive disorder. Journal of Affective Disorders. 2005;86(2–3):235–245. doi: 10.1016/j.jad.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry. 2002a;51(11):851–858. doi: 10.1016/S0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002b;52(10):958–975. doi: 10.1016/S0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C. D-cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biological Psychiatry. 2009;66(7):636–641. doi: 10.1016/j.biopsych.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Lissek S, McDowell D, Levenson J, Pine DS. Reduction of trace but not delay eyeblink conditioning in panic disorder. American Journal of Psychiatry. 2007;164(2):283–289. doi: 10.1176/appi.ajp.164.2.283. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108(1):134–142. doi: 10.1037/0021-843X.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hermann C, Ziegler S, Birbaumer N, Flor H. Psychophysiological and subjective indicators of aversive pavlovian conditioning in generalized social phobia. Biological Psychiatry. 2002;52(4):328–337. doi: 10.1016/S0006-3223(02)01385-9. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety. 2010;27(3):244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim B, Wilhelm FH, Temp L, Margraf J, Wiederhold BK, Rasch B. Sleep enhances exposure therapy. Psychological Medicine. 2013:1–9. doi: 10.1017/S0033291713001748. [DOI] [PubMed] [Google Scholar]
- Koso M, Hansen S. Executive function and memory in posttraumatic stress disorder: a study of Bosnian war veterans. European Psychiatry. 2006;21(3):167–173. doi: 10.1016/j.eurpsy.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Tsareva A, Ahmed N, Milad MR. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behavioural Brain Research. 2013;253:217–222. doi: 10.1016/j.bbr.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. Journal of Affective Disorders. 2012;140(2):113–124. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behavour Research and Therapy. 2005;43(11):1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lobb H, Hardwick C. Eyelid conditioning and intellectual level: effects of repeated acquisition and extinction. American Journal Mental Deficiency. 1976;80(4):423–430. [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. Journal of Psychiatry and Neuroscience. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Melo TG, Izidio GS, Ferreira LS, Sousa DS, Macedo PT, Cabral A, Silva RH. Antidepressants differentially modify the extinction of an aversive memory task in female rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;37(1):33–40. doi: 10.1016/j.pnpbp.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Macklin ML, Pineles SL, Chang Y, Pitman RK. Predicting post-trauma stress symptoms from pre-trauma psychophysiologic reactivity, personality traits and measures of psychopathology. Biology of Mood & Anxiety Disorders. 2012;2(1):8. doi: 10.1186/2045-5380-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109(2):290–298. http://dx.doi.org/10.1037FF0021-843X.109.2.290. [PubMed] [Google Scholar]
- Otto MW, McHugh RK, Kantak KM. Combined pharmacotherapy and cognitive-behavioral therapy for anxiety disorders: Medication effects, glucocorticoids, and attenuated outcomes. Clinical Psychology: Science and Practice. 2010;17:91–103. doi: 10.1111/j.1468-2850.2010.01198.x. http://dx.doi.org/10.1111j.1468-2850.2010.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry. 2000;47(6):512–519. doi: 10.1016/s0006-3223(99)00144-4. http://dx.doi.org/10.1016/S0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP. Test of the conditioning model of neurosis: differential aversive conditioning of angry and neutral facial expressions in anxiety disorder patients. Journal of Abnormal Psychology. 1986;95(3):208–213. doi: 10.1037//0021-843X.95.3.208. [DOI] [PubMed] [Google Scholar]
- Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. Journal of Affective Disorders. 2012;141(1):11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Samuelson KW, Neylan TC, Metzler TJ, Lenoci M, Rothlind J, Henn-Haase C, Marmar CR. Neuropsychological functioning in posttraumatic stress disorder and alcohol abuse. Neuropsychology. 2006;20(6):716–726. doi: 10.1037/0894-4105.20.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Hami S, Stein MB. Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatry Research. 2004;126(3):265–274. doi: 10.1016/j.psychres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328(3):233–236. doi: 10.1016/s0304-3940(02)00519-0. http://dx.doi.org/10.1016/S0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Yang CH, Shi HS, Zhu WL, Wu P, Sun LL, Si JJ, Yang JL. Venlafaxine facilitates between-session extinction and prevents reinstatement of auditory-cue conditioned fear. Behavioral Brain Research. 2012;230(1):268–273. doi: 10.1016/j.bbr.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Zalta AK, Dowd S, Rosenfield D, Smits JA, Otto MW, Simon NM, Pollack MH. Sleep quality predicts treatment outcome in CBT for social anxiety disorder. Depression and Anxiety. doi: 10.1002/da.22170. in press. http://dx.doi.org/10.1002/da.22170. [DOI] [PMC free article] [PubMed]


