Abstract
Objectives
Neurogranin (NRGN) is a small neuronal protein that plays an important role in synaptic signaling by regulating calmodulin (CaM) availability. In this study, we developed an ELISA to measure NRGN quantitatively in serum samples from a cohort of acute traumatic brain injury (TBI) patients and a non-TBI control cohort, and explored the potential value of NRGN as a circulating biomarker for TBI.
Design and methods
Recombinant His-NRGN protein was used to develop mouse monoclonal capture and rabbit polyclonal detection antibodies, and they were used to develop a sandwich ELISA. After validation, we used this ELISA to measure serum samples from a cohort of typical adult acute TBI patients (N = 76 TBI cases) and non-TBI control patients (N = 150 controls).
Results
The NRGN ELISA lower limit of detection was 0.055 ng/mL, lower limit of quantification was 0.2 ng/mL, and interassay CVs were ≤ 10.7%. The average recovery was 99.9% (range from 97.2–102%). Serum NRGN concentrations in TBI cases were significantly higher than in controls (median values were 0.18 ng/mL vs. 0.02 ng/mL, p < 0.0001), but did not discriminate TBI cases with intracranial hemorrhage (p = 0.09).
Conclusions
We have developed a highly sensitive and reproducible ELISA for measuring circulating NRGN in blood samples. Serum NRGN concentrations in acute TBI patients were significantly higher than in controls, indicating that NRGN could have utility as a circulating biomarker for acute TBI. This report provides evidence to support larger and controlled TBI clinical studies for NRGN validation and prediction of outcomes.
Keywords: ELISA, Neurogranin, Biomarker, Traumatic brain injury
Introduction
Neurogranin (NRGN or Ng) is a highly conserved (+96% protein sequence homology among mammals) small neuronal protein (78 AAs, ~7.6 kDa) originally identified in rat brain [1–3]. It binds to calmodulin (CaM) and serves as a substrate for protein kinase C (PKC) [2,4–7], from where its another name BICKS (B-50 immunoreactive C kinase substrate) originated [8]. NRGN protein is found in the cell bodies of cerebral cortex neurons in layers II–VI, and in apical and basal dendrites of pyramidal neurons [9]. It is concentrated in dendritic spines where it participates in the synaptic signaling events by regulating the availability of calmodulin (CaM) [2,10]. NRGN plays an important role in synaptic plasticity and cognitive function where it serves as a key second messenger in mediating the effects of thyroid hormone on the brain [11]. Although NRGN knockout mice display a structurally normal phenotype, they have a severe functional impairment of spatial learning and a decrease in long term potentiation (LTP) induction, most likely due to the defective activation of calcium/CaM kinase II (CaMKII) auto-phosphorylation [12]. Cognitive, learning and memory deficits have been reported after traumatic brain injury (TBI), however, the mechanisms underlining these deficits are not well understood [13]. As NRGN is also a small protein, it may be able to cross an intact and or damaged blood brain barrier with relative ease, therefore, we hypothesize that NRGN may serve as a circulating biomarker of acute TBI. In this report we describe the development of a NRGN ELISA and the diagnostic accuracy of circulating NRGN in distinguishing between acute TBI cases and non-TBI controls in the Emergency Department.
Materials and methods
Recombinant NRGN protein production
The construction of the expression vector for His tagged NRGN and the recombinant NRGN protein production has been previously described [14]. Briefly, we purified cDNA fragment of full-length human NRGN gene by restriction enzyme digestion (Sgf I and Mlu I) and aga-rose gel purification from human cDNA ORF clone (RC201209, Origene, Rockville, MD), and then ligated into bacterial expression vector pEX-N-His (PS100030, Origene, Rockville, MD) with the same restriction enzymes sites. The DNA sequence of the expression construct (pEX-N-His-NRGN) was verified by DNA sequencing to ensure the His tag was in frame with the NRGN cDNA. pEX-N-His-NRGN was then transformed into Rosetta 2 (DE3) E. coli strain (71405, EMD Millipore, Billerica, MA) for recombinant protein expression. The resulting strain was grown in Overnight Express Instant TB medium (71757, EMD Millipore, Billerica, MA) supplemented with ampicillin (100 μg/mL) and chloramphenicol (50 μg/mL) at 37 °C for 16–18 h to produce the recombinant protein. The overexpressed His-NRGN protein was extracted from a 1 L culture of bacteria cells by lysis in TEN buffer (50 mM Tris, pH 8.0, 0.5 mM EDTA and 0.15 M NaCl) supplemented with 1% NP-40, and then affinity chromatography on Ni-NTA agarose as recommended by the supplier (30210, Qiagen, Valencia, CA). The eluted His-NRGN protein was dialyzed against 3 L 1× PBS overnight, and the protein concentration was determined by Coomassie Plus Protein Assay (23236, Thermo Fisher Scientific, Grand Island, NY). Purified His-NRGN was visualized by SDS-PAGE followed by Coomassie staining.
Mass spectrometry and data analysis
Approximately 100 μg of the proteins was reduced with DTT and alkylated with iodoacetimide, then digested with trypsin for 16 h at 37 °C. LC-MS/MS of the desalted peptides was performed on an Agilent 1200 nanoflow LC system coupled on-line to a LTQ OrbiTrap mass spectrometer (Thermo Scientific). BioBasic C18 reverse-phase PicoFrit column (300 A, 5 μm, 75 μm × 10 cm, 15 μm tip, New Objective) was used to separate the peptides. Peptides were eluted with a 142-min linear gradient from 5 to 45% B (mobile phase A: 2% v/v ACN containing 0.1% v/v formic acid; mobile phase B: 90% v/v ACN containing 0.1% v/v formic acid) at 200 nL/min flow rate. The OrbiTrap was operated with an applied electrospray potential of 1.71 kV and capillary transfer tube temperature of 185 °C in a data-dependent mode where each full MS scan was followed by ten MS/MS scans in which the ten most abundant peptide molecular ions detected from the MS scan were dynamically selected for MS/MS analysis using a normalized CID energy of 35%. A dynamic exclusion of 60-s was applied to reduce redundant selection of peptides. SEQUEST (Thermo Electron) and Mascot (Matrix Science) search engines were used to analyze the MS/MS spectra.
Production of anti-NRGN antibodies
A mouse anti-human NRGN monoclonal antibody was produced at The Monoclonal Antibody Core Facility (MACF) at Johns Hopkins University, Department of Neuroscience. Briefly, five 6 week old BALB/c female mice (Charles River, Wilmington, MA) were immunized with 100 μg of His-NRGN by intraperitoneal injection and then boosted twice with 50 μg of His-NRGN. A direct ELISA was used to screen sera collected from immunized mice. The mouse with the best titer by direct ELISA was selected for a final intravenous boost. The mouse spleen was harvested on day 89, and spleen cell and myeloma cell fusion and seeding were performed following standard protocols. After 10 days of undisturbed culture in selection medium (DMEM [D5796, Sigma, Brooklyn, NY] containing 20% HyClone FCS [SH30073, GE Healthcare Life Science, Logan, UT], supplemented with 1× OPI [O5003, Sigma, Brooklyn, NY], 100 μM hypoxanthine [H9636, Sigma, Brooklyn, NY], 0.4 μM aminopterin [A3411, Sigma, Brooklyn, NY], and 160 μM thymidine [T1895, Sigma, Brooklyn, NY]), supernatants were tested by direct ELISA. Positive colonies identified by direct ELISA were cloned twice by limiting dilution on splenocytes from normal BALB/c mice as feeder cells. Clone 30.5.2 was found to bind NRGN at high dilution in direct ELISA.
The cloned hybridoma cell line was grown in DMEM containing 10% defined FCS supplemented with 1× OPI for 4 to 6 days. Then, the hybridoma cells were adapted to grow in serum free media. When the cells reached log growth phase, 2 × 107 cells were inoculated into a CELLine Bioreactor (Integra, Hudson, NH). The culture supernatant (antibody) was collected every 5 days. The antibody concentration was measured by Coomassie Plus Protein Assay (23236, Thermo Fisher Scientific, Grand Island, NY).
We used the same recombinant His-NRGN protein to commercially generate a rabbit polyclonal antibody (Covance, Conshohocken, PA).
Development of a sandwich ELISA for neurogranin
We developed an electrochemiluminescent sandwich immunoassay for measuring NRGN using the Meso Scale Discovery platform (Meso Scale Discovery [MSD], Gaithersburg, MD). First we coated standard 96-well sector plates (MSD) with 30 μL/well (100 ng) monoclonal anti-NRGN antibody diluted with 1× phosphate-buffered saline (PBS) and incubated overnight at room temperature. In preparation for the NRGN ELISA the coated plates were blocked with 5% BSA/PBS and incubated with shaking (600 rpm) at room temperature for 1 h. The His-NRGN recombinant protein described above was used as calibrator, with a concentration range from 40 ng/mL to 0.055 ng/mL, by 1:3 series dilution with 1% BSA/PBS. Serum samples were diluted 1:1 using 1% BSA/PBS, then 25 μL of calibrators and diluted samples were added in duplicate to the plate. After 2 h of incubation with shaking at room temperature, the plates were washed three times (ELx405, BioTek, Winooski, VT) with 150 μL/well of 1× PBS supplemented with 0.05% Tween-20 (wash buffer). The mixture of the rabbit anti-NRGN polyclonal antibody and MSD SULFO-TAG labeled anti-rabbit antibody (R32AB, MSD) was used as detection reagents (dilute in 1% BSA/PBS to a final concentration of 1 μg/mL of each antibody). Twenty-five microliters of detection reagents was added to each well, and the plates were incubated with shaking for another 1 h, then washed three times to remove non-bound detection antibodies. Then 100 μL of 1× read buffer (MSD) was dispersed into each well and the plate was read in a Sector Imager 2400 (MSD) immediately.
Serum samples
We measured NRGN values in serum samples from acute TBI cases and non-TBI controls. The institutional review board approved the study protocol. Written informed consent was waived for TBI cases (we utilized excess clinical serum samples) but was obtained for non-TBI controls. Cases were patients presenting to the Johns Hopkins Emergency Department (ED) after sustaining acute blunt traumatic head injury. They were eligible for inclusion if they: presented within 24 h of injury; met the American College of Emergency Physicians (ACEP) criteria for obtaining head CT scans in TBI; received a non-contrast head CT scan as part of their clinical evaluation; and had excess serum samples available in the clinical chemistry lab. Eligible cases were excluded if they had one of the following prior medical conditions: demyelinating disease; neurodegenerative disease; dementia; stroke; brain tumor; intracranial surgery; or active cancer. For cases included in the present study, excess serum samples stored in a 4 °C refrigerator were retrieved from the clinical chemistry laboratory and stored in a −80 °C freezer. These samples were in the 4 °C refrigerator for variable amounts of time (median of 5 days). We sought to compare NRGN levels in TBI subjects to NRGN levels in control subjects who were ED patients evaluated for non-trauma related complaints. Therefore we selected subjects from an ongoing prospective cohort of ED patients evaluated for suspected acute coronary syndrome [15]. Selected subjects had no blunt head trauma in the preceding 7 days; and were deemed to have a non-cardiac condition (for example gastroesophageal reflux disease or musculoskeletal chest pain) and discharged home from the ED. All control subjects had a GCS of 15. Subjects were excluded from being controls if they met any of the exclusion criteria for eligible cases (see above). Serum samples were obtained prospectively from control subjects and processed and stored in a −80 °C freezer within 2 h of collection.
Statistical analysis
Descriptive statistics were used to summarize clinical and demographic data. NRGN data were not normally distributed and therefore were summarized using medians and interquartile ranges. Groupwise comparisons were made with the Kruskal–Wallis test. Proportions were compared using the chi-squared test. We quantified the discriminative ability of NRGN for distinguishing between TBI cases and controls using area under the receiver operator curve (AUC). We also adjusted for potential confounders (age, gender and race). A two-tailed p-value of <0.05 was considered statistically significant. Statistical analyses were performed using STATA/MP statistical software version 11.2 (StataCorp, College Station, Texas), and RStudio statistical software version 0.97.312.
Results
Expression construct and recombinant protein production
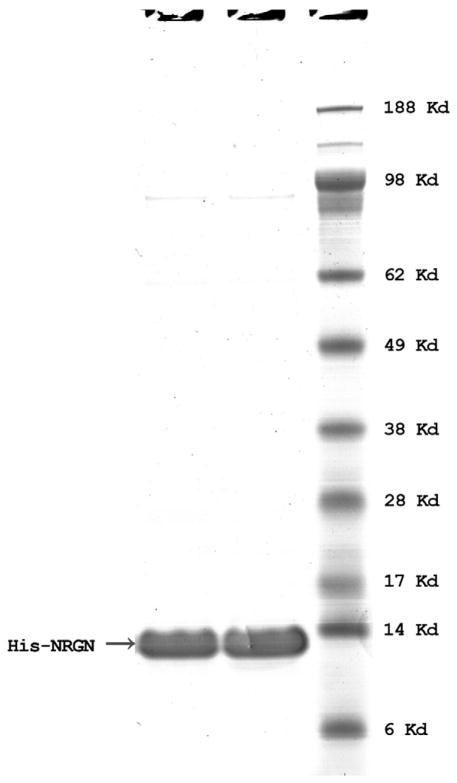
To develop antibodies for NRGN, we cloned the entire human NRGN coding region into pEX-N-His expression vector using Sgf I and Mlu I restriction enzyme sites, giving an N-terminal 6XHis tagged NRGN protein. His-NRGN recombinant protein was extracted from E. coli lysate in native condition and affinity purified on a Ni-NTA beads and dialyzed against 1× PBS. The purified His-NRGN recombinant protein migrated in SDS-PAGE at approximately 13 kDa (Fig. 1) as a result of the addition of His tag. The expressed recombinant protein sequence was verified by MS/MS analysis (OrbiTrap Elite, Thermo Fisher Scientific, Grand Island, NY, data not shown).
Fig. 1.
SDS-PAGE of His-NRGN in reduced condition. Recombinant His-NRGN (1 μg) was run on 4–12% gradient SDS-PAGE in MES buffer, and the gel was then stained with Coomassie blue. Two samples of His-NRGN were loaded; molecular weight standard is indicated in kDa.
NRGN ELISA validation
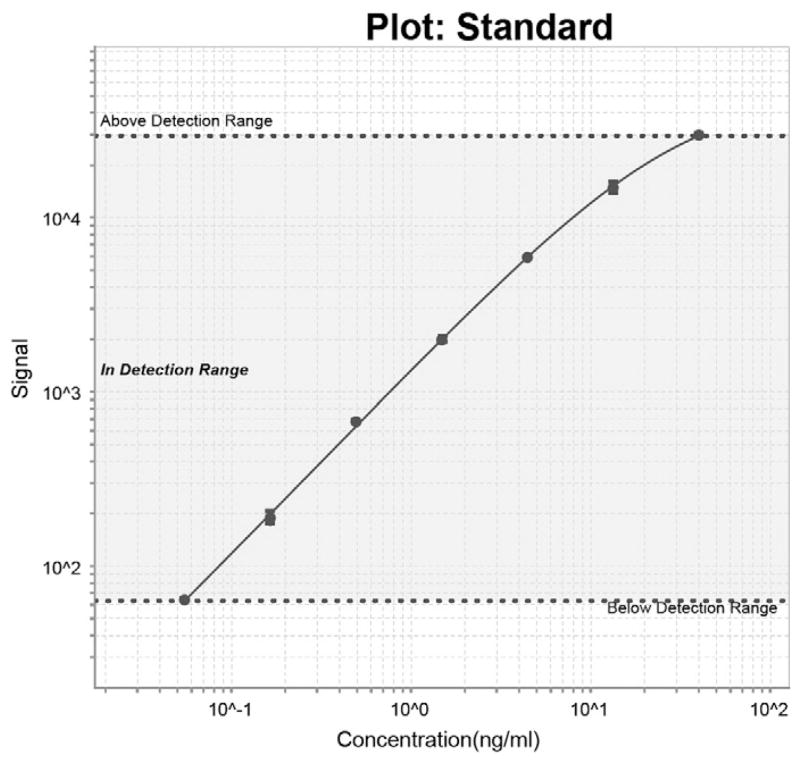
We assessed the purity of the recombinant His-NRGN by running the proteins on SDS-PAGE under reduced condition, as shown in Fig. 1. We then used His-NRGN recombinant protein as calibrator to develop a sandwich ELISA to measure circulating NRGN concentrations. During the development of the NRGN ELISA, we found the highest and most consistent signal was obtained using the monoclonal mouse anti-human NRGN antibody developed in the JHU Monoclonal Antibody Core Laboratory as capture antibody and our polyclonal rabbit anti-human NRGN antibody as detection antibody. We also verified the specificity of the monoclonal capture antibody to detect NRGN in serum by performing a spike in of NRGN recombinant protein followed by immunoprecipitation with the monoclonal capture antibody with subsequent MS/MS analysis (data not shown). This approach readily detected NRGN. The optimal reagent concentrations and sample dilutions were described in Materials and methods. The recombinant His-NRGN was used as a calibrator to generate the concentration curve of the NRGN ELISA. A typical calibration curve is shown in Fig. 2. The lower limit of detection was 0.055 ng/mL (CV ≤ 1.7%), which was estimated as the minimum analyte concentration to produce a significant signal greater than the zero calibrator and a lower limit of quantification of 0.2 ng/mL. The interassay CVs were calculated by averaging the CVs of high and low controls in a total of 11 runs, over a period of 18 months, the value was ≤ 10.7%. The average recovery was 99.9% (range 97.2–102%), which was calculated by dividing calculated concentrations by the input concentrations of the calibrators (n = 8).
Fig. 2.
Typical NRGN ELISA standard curve. A typical calibration curve is shown. His-NRGN was used as calibrator, and 7 points of duplicated calibrators were assayed by NRGN ELISA (40.0–0.055 ng/mL).
NRGN values among TBI cases and controls
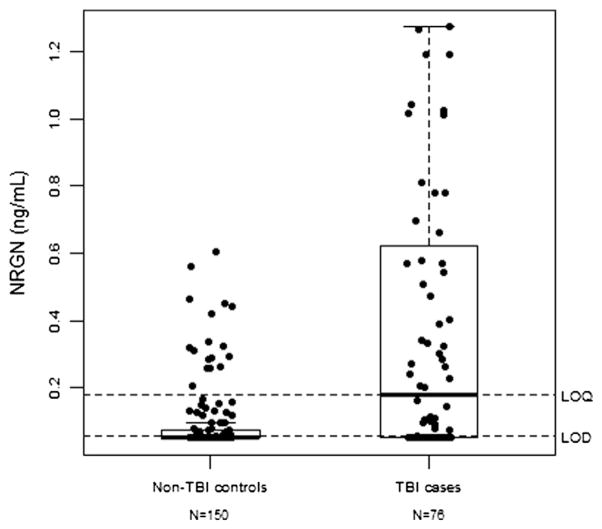
We studied 76 TBI cases and 150 non-TBI controls with the distribution of clinical and demographic data for cases and controls presented in Table 1. Control subjects were more likely to be older, female and African-American, compared to cases. Median NRGN values were significantly higher among the 76 TBI cases (0.18 ng/mL, interquartile range [IQR]: 0.05–0.64 ng/mL) than 150 non-TBI controls (0.02 ng/mL, IQR: 0.05–0.07 ng/mL), p < 0.0001 (Fig. 3). NRGN discriminated between TBI cases and non-TBI controls with an area under the receiver operator curve (AUC) of 0.72 (95% CI: 0.65–0.79). The AUC remained 0.72 after adjusting for age, gender and race. However, median NRGN values were not statistically different between TBI cases with intracranial abnormalities on head CT scan (0.08 ng/mL (IQR: 0.05–0.32 ng/mL)) than those without (0.27 ng/mL (IQR: 0.05–0.78 ng/mL)), p = 0.09.
Table 1.
Demographic and clinical characteristics of study population.
| JHH no-TBI controls n = 150 | JHH TBI cases n = 76 | p | |
|---|---|---|---|
| Median age in years (IQR) | 54 (47–62) | 47 (30–56) | <0.001 |
| Female (%) | 79 (52.7) | 29 (38.2) | 0.04 |
| Race (%) | <0.001 | ||
| • African-American | 116 (77.3) | 41 (54.0) | |
| • White | 30 (20.0) | 25 (32.9) | |
| • Other | 4 (2.7) | 10 (13.2) | |
| Mchanism of injury (%) | |||
| • Assault | 19 (25.0) | ||
| • Fall | 26 (34.2) | ||
| • MVC | 21 (27.6) | ||
| • Pedestrian struck | 4 (5.3) | ||
| • Struck by/against | 3 (4.0) | ||
| • Other trauma | 3 (4.0) | ||
| Glasgow outcome scale (%) | |||
| • 3–8 | 5 (6.6) | ||
| • 9–12 | 3 (4.0) | ||
| • 13 | 2 (2.6) | ||
| • 14 | 11 (14.5) | ||
| • 15 | 55 (72.4) | ||
| Traumatic intracranial abnormality on head CT | 21 (27.6) |
Fig. 3.
NRGN values among acute TBI cases and controls. This figure is a graphical representation of the distribution of neurogranin among TBI cases and non-TBI control subjects. Neurogranin values are higher in TBI cases than in non-TBI controls. The rectangle of the box plot represents the interquartile range (25th–75th percentiles) and the thick line within the box represents the median value of neurogranin. LOQ = limit of quantitation for neurogranin assay. LOD = limit of detection for neurogranin assay. Statistically significant difference between the control cohort and TBI cohort was observed, p < 0.0001.
Discussion
In this study, we developed a sensitive NRGN sandwich ELISA with initial evidence that NRGN deserves further investigation as a candidate biomarker of acute TBI. The human NRGN gene is mapped to chromosome 11q24, spans ~12.5 kb and contains 4 exons and 3 introns, which encodes a 78-amino acid protein product [16]. NRGN gene expression in brain starts at embryonic day 18 (E18) in the rat, following another 2 peaks of protein expression at postnatal day 14 and 20 respectively. These developmental periods are regarded as critical stages for dendritic and cortical synapse development, thus NRGN is implicated as playing an important role in the synaptogenesis [17–21] and functionally in learning, likely as a mediator of thyroid hormone signaling.
The precise local concentration of CaM distributes Ca2+ signal through various effectors, such as protein kinase, adenylate cyclases or nitric oxide synthase to carry out different biological events. An IQ motif (IQXXXRGXXXR) in NRGN is responsible for its CaM binding property, which is also found in many other CaM binding proteins [22]. The NRGN affinity to CaM is Ca2+ dependent, when local Ca2+ concentration reaches micromolar or higher, NRGN quickly releases CaM and vice versa [23]. The posttranslational status of NRGN also dramatically regulates its affinity to CaM. There is a PKC phosphorylation site located inside of NRGN IQ motif (Ser36); knockout mouse model demonstrated that PKCγ is the only PKC isoform to phosphorylate NRGN. Phosphorylated NRGN lost its binding capacity to CaM [3,4,23,24]. NRGN can also be oxidized by nitric oxide (NO) at 4 cysteine sites [25–27]. The oxidation also attenuates the CaM binding affinity of NRGN [25,28,29]. We have also found NRGN undergoes a deimidation reaction converting arginine (R68) to citrulline by peptidylarginine deiminase (PAD) [14]. As PAD is a calcium activated enzyme, and increased intracellular calcium is part of the cellular injury process in TBI, citrullinated NRGN could serve as an additional measure of acute injury. Previously, NRGN has been shown to have potential efficacy in Alzheimer's disease as levels were found to be elevated in CSF of Alzheimer's patients, using mass spectrometry [30].
TBI is an important cause of death and disability across all age groups and especially among athletes and military personnel. The Center for Disease Control and Prevention (CDC) estimates that there were approximately 2.5 million visits to emergency departments (ED) across the US in 2010 for TBI (National Center for Injury Prevention and Control. Traumatic Brain Injury in the United States: Fact Sheet. Atlanta, GA: Centers for Disease Control and Prevention; 2014. Available from: http://www.cdc.gov/traumaticbraininjury/get_the_facts.html). TBI accounts for approximately 30% of all injury-related deaths (Faul M, Xu L, Wald M, Coronado V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf) and leaves approximately 5.3 million Americans with permanent TBI-related disability (National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003 Sep: 1–47). Objective diagnosis of TBI remains challenging. Currently, there are no Food and Drug Administration (FDA) approved biomarkers for diagnosing TBI. Therefore, there is an unmet clinical need for accessible biomarkers that can accurately and reliably diagnose TBI. NRGN is a small neuronal protein, and it makes for an ideal candidate as a circulating biomarker of TBI. We demonstrated that NRGN is significantly elevated in an acute TBI cohort (p < 0.0001) with an ROC for diagnosing TBI of 0.72. However, NRGN did not discriminate severe injury with intracranial hemorrhage. Additional studies are needed to determine whether NRGN is associated with TBI outcomes, and whether elevated serum levels result from cellular injury or necrosis coupled with damage to the blood brain barrier, resulting from a combination of vascular shear and hypoxic injury. If found to be associated with TBI outcomes, measuring NRGN will provide a novel approach for objectively determining the prognosis of TBI in millions of patients. The glial/astrocyte proteins S100B and GFAP [31,32] and the neuronal protein UCHL-1 [33,34] have been studied extensively as circulating biomarkers of brain injury. S100B is also a calcium binding protein and abundant in astrocytes/glial cells [35], but has limited diagnostic specificity as it is also abundant in non-CNS tissues/cells, limiting its diagnostic utility [36,37]. GFAP, an abundant intermediate filament protein in astrocytes, has demonstrated significant sensitivity and specificity for diagnosing TBI [38]. In contrast UCHL-1, a relatively new biomarker, is an abundant neuronal protein, and also a sensitive and specific circulating measure of TBI [39]. Although these proteins have significant diagnostic sensitivity and specificity expected of a CNS protein, they all take hours to be detected in blood after TBI. It is hoped that identification of smaller, brain specific proteins such as NRGN, would increase the ability to detect brain injury faster. Another unexplored possibility, considering NRGNs' role in neuronal signaling and learning, is longitudinal measurements of NRGN as a measure of recovery.
Traumatic brain injury (TBI) is a heterogeneous disease; the basis for classification is clinical severity, injury type and pathophysiology. However, clinical assessment of TBI based on these factors does not provide sufficient information for targeting and precise treatment plan development. Biomarker measures that reflect injury status with easy access are urgently needed for clinical practice. The simple and sensitive ELISA described here provides a valuable tool to further validate the value of NRGN as a biomarker for TBI in larger TBI studies and possibly for future clinical diagnostics.
Validation of NRGN in different cohorts is necessary for qualifying NRGN as a clinically useful TBI biomarker. A major limitation of this study is the difference in processing and storage of serum samples between cases and control cohorts. Serum samples from cases were excess clinical samples that were initially stored at 4 °C prior to transferring to a −80 °C freezer, whereas serum samples for controls were processed and stored in −80 °C within 2 h of sampling. We have shown previously that NRGN is stable for 5 days at room temperature in blood, thus the different sample processing procedures have minimal impact on NRGN values in this study [40].
Conclusion
We have developed a new and sensitive ELISA for the neuronal protein NRGN, that demonstrates significant TBI diagnostic ability. Considering NRGN's pivotal role in learning, NRGN may play an important role as a biomarker of acute TBI and recovery.
Acknowledgments
We thank the Monoclonal Antibody Core Facility (MACF) at Johns Hopkins University for generating the monoclonal anti-neurogranin antibody. Under a licensing agreement between ImmunArray and the Johns Hopkins University, Drs. Everett, Yang and Korley are entitled to royalties on an invention described in this article.
Abbreviations
- NRGN
Neurogranin
- CaM
Calmodulin
- CaMKII
CaM kinase II
- TBI
Traumatic brain injury
- ACEP
American College of Emergency Physicians
- ED
Emergency Department
- AUC
Area under the receiver operator curve
References
- 1.Watson JB, Battenberg EF, Wong KK, Bloom FE, Sutcliffe JG. Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein. J Neurosci Res. 1990;26:397–408. doi: 10.1002/jnr.490260402. [DOI] [PubMed] [Google Scholar]
- 2.Javier Díez-Guerra F. Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity. IUBMB Life. 2010;62:597–606. doi: 10.1002/iub.357. [DOI] [PubMed] [Google Scholar]
- 3.Huang K, Huang F, Chen H. Characterization of a 7. 5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch Biochem Biophys. 1993;305:570–80. doi: 10.1006/abbi.1993.1463. [DOI] [PubMed] [Google Scholar]
- 4.Baudier J, Deloulme J, Van Dorsselaer A, Black D, Matthes H. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem. 1991;266:229–37. [PubMed] [Google Scholar]
- 5.Baudier J, Bronner C, Kligman D, Cole RD. Protein kinase C substrates from bovine brain. Purification and characterization of neuromodulin, a neuron-specific calmodulin binding protein. J Biol Chem. 1989;264:1824–8. [PubMed] [Google Scholar]
- 6.Deloulme JC, Sensenbrenner M, Baudier J. A rapid purification method for neurogranin, a brain specific calmodulin-binding protein kinase C substrate. FEBS Lett. 1991;282:183–8. doi: 10.1016/0014-5793(91)80473-g. [DOI] [PubMed] [Google Scholar]
- 7.Gerendasy DD, Herron SR, Jennings PA, Sutcliffe JG. Calmodulin stabilizes an amphiphilic alpha-helix within RC3/neurogranin and GAP-43/neuromodulin only when Ca2+ is absent. J Biol Chem. 1995;270:6741–50. doi: 10.1074/jbc.270.12.6741. [DOI] [PubMed] [Google Scholar]
- 8.Coggins PJ, Stanisz J, Nagy A, Zwiers H. Identification of a calmodulin-binding B-50 immunoreactive C-kinase substrate (BICKS) in bovine brain. Neurosci Res Commun. 1995;8:49–56. [Google Scholar]
- 9.Chang JW, Schumacher E, Coulter PM, Vinters HV, Watson JB. Dendritic translocation of RC3/neurogranin mRNA in normal aging, Alzheimer disease and fronto-temporal dementia. J Neuropathol Exp Neurol. 1997;56:1105–18. doi: 10.1097/00005072-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kubota Y, Putkey JA, Waxham MN. Neurogranin controls the spatiotemporal pattern of postsynaptic Ca2+/CaM signaling. Biophys J. 2007;93:3848–59. doi: 10.1529/biophysj.107.106849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez de Arrieta C, Morte B, Coloma A, Bernal J. The human RC3 gene homolog, NRGN contains a thyroid hormone-responsive element located in the first intron. Endocrinology. 1999;140:335–43. doi: 10.1210/endo.140.1.6461. [DOI] [PubMed] [Google Scholar]
- 12.Pak JH, Huang FL, Li J, Balschun D, Reymann KG, Chiang C, et al. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci U S A. 2000;97:11232–7. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li GL, Farooque M, Lewen A, Lennmyr F, Holtz A, Olsson Y. MAP2 and neurogranin as markers for dendritic lesions in CNS injury. An immunohistochemical study in the rat. APMIS. 2000;108:98–106. doi: 10.1034/j.1600-0463.2000.d01-32.x. [DOI] [PubMed] [Google Scholar]
- 14.Jin Z, Fu Z, Yang J, Troncosco J, Everett AD, Van Eyk JE. Identification and characterization of citrulline-modified brain proteins by combining HCD and CID fragmentation. Proteomics. 2013;13:2682–91. doi: 10.1002/pmic.201300064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korley FK, Schulman SP, Sokoll LJ, DeFilippis AP, Stolbach AI, Bayram JD, et al. Troponin elevations only detected with a high-sensitivity assay: clinical correlations and prognostic significance. Acad Emerg Med. 2014;21:727–35. doi: 10.1111/acem.12417. [DOI] [PubMed] [Google Scholar]
- 16.Martínez de Arrieta C, Pérez Jurado L, Bernal J, Coloma A. Structure, organization, and chromosomal mapping of the human neurogranin gene (NRGN) Genomics. 1997;41:243–9. doi: 10.1006/geno.1997.4622. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Bolado G, Rodríguez-Sánchez P, Tejero-Díez P, Fairén A, Díez-Guerra FJ. Neurogranin in the development of the rat telencephalon. Neuroscience. 1996;73:565–80. doi: 10.1016/0306-4522(96)00061-9. [DOI] [PubMed] [Google Scholar]
- 18.Represa A, Deloulme JC, Sensenbrenner M, Ben-Ari Y, Baudier J. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J Neurosci. 1990;10:3782–92. doi: 10.1523/JNEUROSCI.10-12-03782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnatenko DV, Dunn JJ, McCorkle SR, Weissmann D, Perrotta PL, Bahou WF. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood. 2003;101:2285–93. doi: 10.1182/blood-2002-09-2797. [DOI] [PubMed] [Google Scholar]
- 20.Singec I, Knoth R, Ditter M, Volk B, Frotscher M. Neurogranin is expressed by principal cells but not interneurons in the rodent and monkey neocortex and hippocampus. J Comp Neurol. 2004;479:30–42. doi: 10.1002/cne.20302. [DOI] [PubMed] [Google Scholar]
- 21.Guadaño-Ferraz A, Viñuela A, Oeding G, Bernal J, Rausell E. RC3/neurogranin is expressed in pyramidal neurons of motor and somatosensory cortex in normal and denervated monkeys. J Comp Neurol. 2005;493:554–70. doi: 10.1002/cne.20774. [DOI] [PubMed] [Google Scholar]
- 22.Bähler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–13. doi: 10.1016/s0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- 23.Gerendasy DD, Herron SR, Watson JB, Sutcliffe JG. Mutational and biophysical studies suggest RC3/neurogranin regulates calmodulin availability. J Biol Chem. 1994;269:22420–6. [PubMed] [Google Scholar]
- 24.Ramakers GM, Gerendasy DD, de Graan PN. Substrate phosphorylation in the protein kinase Cgamma knockout mouse. J Biol Chem. 1999;274:1873–4. doi: 10.1074/jbc.274.4.1873. [DOI] [PubMed] [Google Scholar]
- 25.Sheu FS, Mahoney CW, Seki K, Huang KP. Nitric oxide modification of rat brain neurogranin affects its phosphorylation by protein kinase C and affinity for calmodulin. J Biol Chem. 1996;271:22407–13. doi: 10.1074/jbc.271.37.22407. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney CW, Pak JH, Huang KP. Nitric oxide modification of rat brain neurogranin. Identification of the cysteine residues involved in intramolecular disulfide bridge formation using site-directed mutagenesis. J Biol Chem. 1996;271:28798–804. doi: 10.1074/jbc.271.46.28798. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Pak JH, Huang FL, Huang KP. N-methyl-D-aspartate induces neurogranin/RC3 oxidation in rat brain slices. J Biol Chem. 1999;274:1294–300. doi: 10.1074/jbc.274.3.1294. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Huang KP, Huang FL. Participation of NMDA-mediated phosphorylation and oxidation of neurogranin in the regulation of Ca2+- and Ca2+/calmodulin-dependent neuronal signaling in the hippocampus. J Neurochem. 2003;86:1524–33. doi: 10.1046/j.1471-4159.2003.01963.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang HM, Lee PH, Lim TM, Sheu FS. Neurogranin expression in stably transfected N2A cell line affects cytosolic calcium level by nitric oxide stimulation. Brain Res Mol Brain Res. 2004;129:171–8. doi: 10.1016/j.molbrainres.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 30.Kvartsberg H, Duits FH, Ingelsson M, Andreasen N, Öhrfelt A, Andersson K, et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer's disease. Alzheimers Dement. 2014:19. doi: 10.1016/j.jalz.2014.10.009. http://dx.doi.org/10.1016/j.jalz.2014.10.009 [pii:S1552-5260(14)02863-5] [DOI] [PubMed]
- 31.Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62:1303–10. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- 32.Bohmer AE, Oses JP, Schmidt AP, Peron CS, Krebs CL, Oppitz PP, et al. Neuron specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery. 2011;68:1624–30. doi: 10.1227/NEU.0b013e318214a81f. [DOI] [PubMed] [Google Scholar]
- 33.Papa L, Akinyi L, Liu MC, Pineda JA, Tepas JJ, III, Oli MW, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010;38:138–44. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mondello S, Linnet A, Buki A, Robicsek S, Gabrielli A, Tepas J, et al. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery. 2012;70:666–75. doi: 10.1227/NEU.0b013e318236a809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sedaghat F, Notopoulos A. S100 protein family and its application in clinical practice. Hippokratia. 2008;12:198–204. [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson RE, Hansson LO, Nilsson O, Liska J, Settergren G, Vaage J. Increase in serum S100A1-B and S100BB during cardiac surgery arises from extracerebral sources. Ann Thorac Surg. 2001;71:1512–7. doi: 10.1016/s0003-4975(01)02399-2. [DOI] [PubMed] [Google Scholar]
- 37.Gonçalves CA, Leite MC, Guerra MC. Adipocytes as an important source of serum S100B and possible roles of this protein in adipose tissue. Cardiovasc Psychiatry Neurol. 2010:790431. doi: 10.1155/2010/790431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okonkwo DO, Yue JK, Puccio AM, Panczykowski DM, Inoue T, McMahon PJ, et al. Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Investigators. GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J Neurotrauma. 2013;30:1490–7. doi: 10.1089/neu.2013.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz-Arrastia R, Wang KK, Papa L, Sorani MD, Yue JK, Puccio AM, et al. TRACK-TBI Investigators. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31:19–25. doi: 10.1089/neu.2013.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutierrez S, Everett A, Bembea M, Schwartz J. Impact of delayed plasma storage on brain injury biomarker stability [abstract 533] Crit Care Med. 2014;42(12 Suppl):A1488. [Google Scholar]