Abstract
Introduction
A substantial proportion of patients with Parkinson’s disease (PD) have concomitant cognitive dysfunction. Identification of biomarker profiles that predict which PD patients have a greater likelihood for progression of cognitive symptoms is pressingly needed for future treatment and prevention approaches.
Methods
Subjects were drawn from the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP) study, a large clinical trial that enrolled initially untreated PD patients. For the current study, Phase One encompassed trial baseline until just prior to levodopa administration (n=403), and Phase Two spanned the initiation of levodopa treatment until the end of cognitive follow-up (n=305). Correlations and linear mixed models were performed to determine cross-sectional and longitudinal associations between baseline amyloid β1-42 (Aβ42), total tau (t-tau), and phosphorylated tau (p-tau) in cerebrospinal fluid (CSF) and measures of memory and executive function. Analyses also considered APOE genotype and tremor- vs. rigidity-dominant phenotype.
Results
No association was found between baseline CSF biomarkers and cognitive test performance during Phase One. However, once levodopa treatment was initiated, higher p-tau and p-tau/Aβ42 predicted subsequent decline on cognitive tasks involving both memory and executive functions. The interactions between biomarkers and cognition decline did not appear to be influenced by levodopa dosage, APOE genotype or motor phenotype.
Conclusions
The current study has, for the first time, demonstrated the possible involvement of tau species, whose gene (MAPT) has been consistently linked to the risk of PD by genome-wide association studies, in the progression of cognitive symptoms in PD.
Keywords: Amyloid, Biomarkers, Cerebrospinal Fluid, Cognition, Neuropsychological Tests, Parkinson Disease, tau Proteins
Introduction
Identification of surrogate markers that herald neuropathologic changes associated with progression of cognitive symptoms represents an important focus in Parkinson’s disease (PD) research. In PD biomarker investigation, amyloid beta 1-42 (Aβ42) has been reported to be lower in cerebrospinal fluid (CSF) of PD patients, though to a lesser extent than in Alzheimer’s [1–3]. Distinctly different from Alzheimer’s, CSF total tau (t-tau) and phosphorylated tau181 (p-tau), also appear to be lower in patients with PD compared to controls in large-scale studies [1–3]. On the other hand, increased tau has been reported in some PD patients with dementia [4,5]. The relationship between CSF biomarkers and performance on specific cognitive tests in PD patients is likewise mixed [1,5–7]. However, cognition-related results reported to date have been primarily cross-sectional and based on small sample sizes with varying degrees of disease progression.
In a recent investigation, alterations in tau species were clearly associated with motor progression in the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP) trial,[8] the largest PD cohort assembled to date with longitudinal collection of CSF and clinical and cognitive assessments for close to eight years. The current study evaluates the relationship between CSF biomarkers and cognition in the DATATOP cohort with the following aims: 1) To determine cross-sectional correlations between CSF biomarkers and cognitive test scores in patients with early, untreated PD; and 2) To determine if CSF biomarkers predict subsequent rate of cognitive change.
Methods
Ethics Statement
The institutional review boards at all institutions that enrolled participants into the DATATOP study (see e-Mothods) approved the study, and all subjects or their legal surrogates provided written informed consent.
Participants
The DATATOP study was a multi-center randomized trial designed to measure the effectiveness of the monoamine oxidase type B inhibitor deprenyl (selegiline) and the antioxidant α-tocopherol (vitamin E) on delaying the progression of PD-related disability. Inclusion and exclusion criteria are published elsewhere [9,10]; briefly, 800 participants with de novo PD who did not require anti-parkinsonian medication upon study entry were enrolled during 1987–88. Study endpoint occurred when the participant required levodopa therapy. After ~14 months follow-up, the trial was discontinued due to the positive effects of selegiline on reducing PD-related disability; the study switched to open-label administration of selegiline for ~18 months. Cognitive performance was measured at baseline and every six months thereafter. Following the study endpoint or termination of the open-label phase, participants continued to undergo cognitive testing every six months. Though very limited data are available regarding the status of known PD-related gene mutations in DATATOP subjects, the few observations strongly suggest that the DATATOP cohort is similar to typical sporadic cohorts [8,11,12].
The longitudinal data for each patient were divided into two phases: the first phase spans study entry until just prior to levodopa initiation (an average of 2 years) (Phase 1), and the second phase spans time from initiation of levodopa until end of follow up (average 4.3 years, maximum 6.9 years) (Phase 2). Clinical data and CSF samples were collected at the beginning of Phases 1 and 2. Participants excluded from the current study included 110 with follow up time of less than six months, 34 who withdrew, 45 whose initial PD diagnoses were determined to be incorrect, and 212 who had missing CSF or UPDRS data at the beginning of Phase 1 (n=63) or Phase 2 (n=189). The remaining 403 subjects were included in the current analyses that examined data from Phase 1. Of these subjects, a total of 305 continued in the trial after starting levodopa and were included in Phase 2 analyses.
Study procedures
Cognitive measures
Longitudinal cognitive data were available across both Phases 1 and 2 for tests of verbal learning and memory (Selective Reminding Test [SRT] total and delayed recall) [13], visuospatial working memory/processing speed (Symbol Digit Modalities Test [SDMT]) [14], and visuospatial working memory (New Dot Test). The Mini-Mental Status Examination (MMSE)[15] and other cognitive measures were administered throughout Phase 1 [9], but not during Phase 2.
CSF biomarkers
CSF Aβ42, t-tau, and p-tau[181P] (p-tau) levels were obtained at the beginning of Phases 1 and 2. Assays were measured using the INNO-BIA AlzBio3 kit (Innogenetics, Gent, Belgium) as described previously [3]. We have extensively utilized the assay platform and quality control procedures in our laboratory [3,8,16,17]. We found that the concentrations of CSF Aβ42 and tau in the DATATOP CSF samples, frozen for more than two decades, were comparable with CSF samples collected recently [8]. There were no apparent molecular CSF signatures of Alzheimer’s changes (e.g., elevated tau, low Aβ42) in this population at baseline and during the first two years of investigation [8].
APOE genotype
Genomic DNA was available from 199 DATATOP participants included in Phase 2 analyses. APOE genotyping was performed as previously described (see e-Methods).[18]
PD Motor Subtypes
Participants were categorized according to whether their dominant motor features at baseline were related to postural instability and gait disturbance (PIGD), tremor (TD), or “intermediate” (referring to those who were neither PIGD- nor TD) based on previously published methods (see e-Methods).[19]
Analyses
Preliminary descriptive analyses found that during Phase 1 more than half of the subjects showed improvement on cognitive measures, likely related to practice effects. In contrast, during Phase 2 very few subjects improved on cognitive measures and most declined, consistent with the practice effect having been exhausted during Phase 1. Because of these observations, analyses were conducted separately for Phases 1 and 2. To create a more homogenous population in terms of stage of disease and drug treatment status, Phase 2 analyses included only those who had reached the endpoint by the end of the open-label trial. Levodopa equivalent daily dose (LEDD) was calculated as described previously [20]. Descriptive statistics were calculated for demographic, clinical, and CSF measurements at the beginning of Phases 1 and 2. CSF biomarker analysis included the following: t-tau, p-tau, p-tau/t-tau ratio, Aβ42, t-tau/Aβ42, and p-tau/Aβ42. All biomarkers were log-transformed due to non-normality with the exception of Aβ42 and p-tau/t-tau.
Cross-sectional partial correlations between biomarkers and cognitive tests measured at both Phase 1 and Phase 2 baseline visits controlling for age, sex, and education were performed. Linear mixed effects models were used to determine whether CSF biomarkers at the start of Phase 1 or Phase 2 predict subsequent longitudinal change of cognitive scores. For Phase 1 analyses, repeated measures of cognitive test scores were entered as dependent variables; sex, education, age, MMSE, UPDRS total, cognitive score, CSF biomarker at the beginning of Phase 1, follow-up time, CSF biomarker × follow-up time interaction were entered as covariates. For Phase 2 analyses, repeated measures of cognitive test scores during Phase 2 were entered as dependent variables; sex, education, age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total score and average cognitive score over six months prior to the beginning of Phase 2, CSF biomarker at the beginning of Phase 2, follow-up time, CSF biomarker × follow-up time interaction were entered as covariates. The models included random intercepts and slopes.
Statistical tests are two-tailed; p-value significance was set at below the 0.05 level. In order to protect against spurious results due to the somewhat large number of multiple comparisons being done, the cohort was divided into discovery and validation sets and only the findings that could be confirmed in the validation set were considered to be valid. All analyses were performed using IBM SPSS 20.0 (IBM, Chicago, IL).
Results
Cohort characteristics
Demographic data, clinical characteristics, and mean CSF biomarker levels at the start of Phases 1 and 2 are provided (Tables 1 and 2). Subjects were randomly split into discovery (Phase 1 n=201; Phase 2 n=154) and validation (Phase 1 n=202; Phase 2 n=151) sets; the groups did not differ significantly on demographic characteristics, clinical ratings, or biomarker levels.
Table 1.
Demographics and CSF biomarker values of the discovery and validation cohorts at the start of Phase 1 in the DATATOP sample
| Discovery (n=201) | Validation (n=202) | Total (n=403) | ||
|---|---|---|---|---|
| Sex, F/M (% male) | 71/130 (54.6%) | 71/131(54.2%) | 142/261 (54.4%) | |
|
| ||||
| Age (yrs) | Mean ± SD | 60.94 ± 9.55 | 60.93 ± 8.82 | 60.93 ± 9.18 |
| Range | 36 – 78 | 34 – 79 | 34 – 79 | |
|
| ||||
| Disease Duration (yrs) | Mean ± SD | 1.99 ± 1.33 | 2.08 ± 1.38 | 2.04 ± 1.36 |
| Range | 0 – 6 | 0 – 7 | 0 – 7 | |
|
| ||||
| MMSE | Mean ± SD | 28.78 ± 1.48 | 28.97 ± 1.41 | 28.87 ± 1.45 |
| Range | 23 – 30 | 23 – 30 | 23–30 | |
|
| ||||
| UPDRS total | Mean ± SD | 25.77 ± 11.10 | 22.91 ± 12.17 | 24.34± 11.72 |
| Range | 7 – 61 | 0 – 63 | 0 – 63 | |
|
| ||||
| UPDRS motor | Mean ± SD | 17.15 ± 8.46 | 15.47 ± 9.10 | 16.31± 8.82 |
| Range | 3 – 50 | 0 – 46 | 0–50 | |
|
| ||||
| Hoehn and Yahr | Median | 1.5 | 1.5 | 1.5 |
| Range | 1 – 3 | 1 – 3 | 1 – 3 | |
|
| ||||
| CSF Aβ42 (pg/mL) | Mean ± SD | 233.98 ± 78.63 | 238.29 ± 71.32 | 236.14 ± 74.99 |
| Range | 52.14 – 670.08 | 74.77 – 435.72 | 52.14–670.08 | |
|
| ||||
| CSF t-tau (pg/mL) | Mean ± SD | 47.27 ± 25.90 | 46.79 ± 25.07 | 47.03 ± 25.45 |
| Range | 13.97 – 146.91 | 10.72 – 213.58 | 10.72 – 213.58 | |
|
| ||||
| CSF p-tau (pg/mL) | Mean ± SD | 23.23 ± 11.66 | 23.44 ± 12.14 | 23.34 ± 11.89 |
| Range | 4.56 – 88.02 | 3.99 – 93.11 | 3.99 – 93.11 | |
|
| ||||
| CSF p-tau/t-tau | Mean ± SD | 0.54 ± 0.21 | 0.54 ± 0.21 | 0.54 ± 0.21 |
| Range | 0.14 – 1.06 | 0.14 – 1.06 | 0.14 – 1.06 | |
|
| ||||
| CSF t-tau/Aβ42 | Mean ± SD | 0.21 ± 0.11 | 0.21 ± 0.13 | 0.21 ± 0.12 |
| Range | 0.08 – 0.71 | 0.09 – 1.21 | 0.08 – 1.21 | |
|
| ||||
| CSF p-tau/Aβ42 | Mean ± SD | 0.11 ± 0.06 | 0.11 ± 0.07 | 0.11 ± 0.06 |
| Range | 0.03 – 0.42 | 0.02 – 0.55 | 0.02 – 0.55 | |
UPDRS: Unified Parkinson Disease Rating Scale; MMSE Mini Mental State Examination; Aβ1-42: amyloid beta peptide 1-42; CSF: cerebrospinal fluid; p-tau: phosphorylated tau; t-tau: total tau
Table 2.
Demographics and CSF biomarker values of the discovery and validation cohorts at the start of Phase 2 in the DATATOP sample
| Discovery (n=154) | Validation (n=151) | Total (n=305) | ||
|---|---|---|---|---|
| Sex, F/M (% male) | 48/106 (45.3%) | 54/97(55.7%) | 102/203 (50.2%) | |
|
| ||||
| Age (yrs) | Mean ± SD | 62.80 ± 9.57 | 62.86 ± 9.05 | 62.83 ± 9.3 |
| Range | 36 – 79 | 35 – 80 | 35 – 80 | |
|
| ||||
| Disease Duration (yrs) | Mean ± SD | 3.82 ± 1.58 | 3.83 ± 1.42 | 3.82± 1.5 |
| Range | 1 – 8 | 1 – 9 | 1 – 9 | |
|
| ||||
| MMSE | Mean ± SD | 28.72 ± 2.30 | 28.85 ± 2.10 | 28.79 ± 2.20 |
| Range | 8 – 30 | 9 – 30 | 8 – 30 | |
|
| ||||
| UPDRS total | Mean ± SD | 45.12 ± 13.37 | 43.84 ± 14.72 | 44.49 ± 14.05 |
| Range | 10 – 83 | 8.5 – 88 | 8.5 – 88 | |
|
| ||||
| UPDRS motor | Mean ± SD | 30.22 ± 10.30 | 29.61 ± 10.91 | 29.92 ± 10.59 |
| Range | 6 – 59 | 4.5 – 62 | 4.5– 62 | |
|
| ||||
| Hoehn and Yahr | Median | 2 | 2 | 2 |
| Range | 1 – 3 | 1 – 4 | 1 – 4 | |
|
| ||||
| CSF Aβ42 (pg/mL) | Mean ± SD | 233.37 ± 80.06 | 231.01 ± 72.14 | 232.21 ± 76.14 |
| Range | 12.13 – 631.25 | 15.53 – 464.04 | 12.13 – 631.25 | |
|
| ||||
| CSF t-tau (pg/mL) | Mean ± SD | 47.67 ± 25.18 | 46.65 ± 24.94 | 47.16 ± 25.02 |
| Range | 13.38 – 171.80 | 12.29 – 191.42 | 12.29 – 191.42 | |
|
| ||||
| CSF p-tau (pg/mL) | Mean ± SD | 22.95 ± 11.11 | 24.22 ± 13.06 | 23.58 ± 12.11 |
| Range | 3.85 – 62.90 | 4.35 – 96.93 | 3.85 – 96.93 | |
|
| ||||
| CSF p-tau/t-tau | Mean ± SD | 0.52 ± 0.21 | 0.56 ± 0.24 | 0.54 ± 0.23 |
| Range | 0.16 – 1.09 | 0.16 – 1.83 | 0.16 – 1.83 | |
|
| ||||
| CSF t-tau/Aβ42 | Mean ± SD | 0.24 ± 0.33 | 0.22 ± 0.13 | 0.23 ± 0.26 |
| Range | 0.10 – 4.14 | 0.10 – 0.81 | 0.10 – 4.14 | |
|
| ||||
| CSF p-tau/Aβ42 | Mean ± SD | 0.12 ± 0.20 | 0.12 ± 0.13 | 0.12 ± 0.17 |
| Range | 0.03 – 2.45 | 0.03 – 1.45 | 0.03 – 2.45 | |
MMSE:Pre-washout Mini Mental State Examination prior to the beginning of Phase 2; UPDRS: Unified Parkinson Disease Rating Scale; CSF: cerebrospinal fluid; Aβ42: amyloid beta peptide 1-42; p-tau: phosphorylated tau; t-tau: total tau.
Cross-sectional correlations
Cross-sectional correlation of CSF biomarkers and cognitive test performance at the beginning of each phase, controlling for age, sex, and education, did not meet our criteria of producing significant cross-sectional correlations in the discovery cohort with confirmation in the validation sample in either Phase 1 or Phase 2 (Tables e1 and e2).
Longitudinal analyses of Phase 1
CSF biomarkers measured at baseline did not predict longitudinal change of performance on any cognitive measures during the course of Phase 1.
Longitudinal analyses of Phase 2
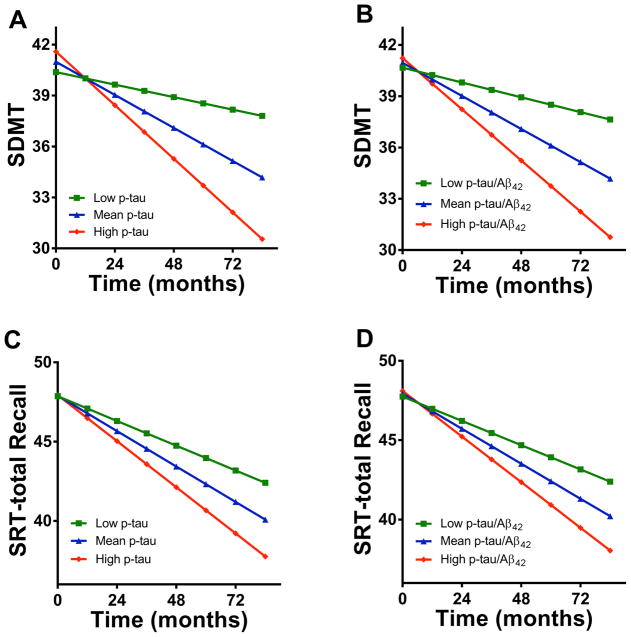
The predictive values of the CSF biomarkers measured at the beginning of Phase 2 on longitudinal change in cognition for the discovery and validation subjects are shown (Table 3). In both the discovery set and confirmed in the validation set, p-tau and the ratio p-tau/Aβ42 measured at the start of Phase 2 predicted subsequent decline on verbal learning (SRT-Total Recall) and on a measure of visuospatial working memory/processing speed (SDMT). The magnitude of this interaction is illustrated in Figure 1, in which those with higher levels of the biomarkers measured prior at the start of Phase 2 have a considerably faster decline of the cognitive measurements than those with lower levels of the biomarker.
Table 3.
CSF biomarkers at the start of Phase 2 as predictors of longitudinal cognitive change during Phase 2
| Cognitive Test | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRT-Total Recall | SRT-Delayed Recall | New Dot Test | SDMT | ||||||||||
|
| |||||||||||||
| Cohort | Biomarker | coef. | SE | p | coef. | SE | p | coef. | SE | p | coef. | SE | p |
| Discovery | Aβ42 | 0.0002 | 0.0002 | 0.137 | 0.00004 | 0.00005 | 0.354 | 0.00003 | 0.00003 | 0.332 | 0.00007 | 0.0002 | 0.732 |
| t-tau | −0.043 | 0.061 | 0.482 | −0.014 | 0.018 | 0.452 | −0.017 | 0.012 | 0.135 | −0.136 | 0.073 | 0.064 | |
| p-tau | −0.103 | 0.051 | 0.045 | −0.022 | 0.015 | 0.148 | −0.008 | 0.010 | 0.415 | −0.221 | 0.059 | 0.0003 | |
| p-tau/ t-tau | −0.093 | 0.059 | 0.122 | −0.010 | 0.018 | 0.584 | 0.010 | 0.011 | 0.366 | −0.157 | 0.070 | 0.027 | |
| t-tau/ Aβ42 | −0.070 | 0.058 | 0.231 | −0.014 | 0.018 | 0.427 | −0.018 | 0.012 | 0.117 | −0.109 | 0.071 | 0.125 | |
| p-tau/ Aβ42 | −0.106 | 0.046 | 0.023 | −0.019 | 0.014 | 0.173 | −0.008 | 0.009 | 0.393 | −0.171 | 0.055 | 0.002 | |
|
| |||||||||||||
| Validation | Aβ42 | 0.00009 | 0.0002 | 0.625 | −0.00003 | 0.00005 | 0.632 | −0.00001 | 0.00003 | 0.887 | 0.0002 | 0.0002 | 0.519 |
| t-tau | −0.096 | 0.065 | 0.146 | −0.020 | 0.018 | 0.277 | −0.039 | 0.011 | 0.0004 | −0.183 | 0.087 | 0.038 | |
| p-tau | −0.141 | 0.059 | 0.019 | −0.019 | 0.017 | 0.258 | −0.024 | 0.010 | 0.018 | −0.230 | 0.078 | 0.004 | |
| p-tau/ t-tau | −0.037 | 0.055 | 0.504 | 0.008 | 0.015 | 0.957 | 0.017 | 0.009 | 0.073 | −0.054 | 0.073 | 0.462 | |
| t-tau/ Aβ42 | −0.127 | 0.069 | 0.068 | −0.016 | 0.019 | 0.405 | −0.036 | 0.011 | 0.002 | −0.238 | 0.090 | 0.009 | |
| p-tau/ Aβ42 | −0.108 | 0.050 | 0.031 | −0.010 | 0.014 | 0.473 | −0.013 | 0.009 | 0.136 | −0.179 | 0.066 | 0.008 | |
|
| |||||||||||||
| Total | Aβ42 | 0.0002 | 0.0001 | 0.169 | 0.00001 | 0.00004 | 0.778 | 0.00001 | 0.00002 | 0.52 | 0.0001 | 0.0002 | 0.502 |
| t-tau | −0.071 | 0.045 | 0.116 | −0.017 | 0.013 | 0.186 | −0.027 | 0.008 | 0.001 | −0.159 | 0.056 | 0.005 | |
| p-tau | −0.122 | 0.038 | 0.002 | −0.021 | 0.011 | 0.067 | −0.014 | 0.007 | 0.051 | −0.219 | 0.048 | <0.0001 | |
| p-tau/ t-tau | −0.061 | 0.040 | 0.131 | −0.003 | 0.012 | 0.784 | 0.015 | 0.007 | 0.036 | −0.093 | 0.050 | 0.066 | |
| t-tau/ Aβ42 | −0.097 | 0.045 | 0.031 | −0.014 | 0.013 | 0.261 | −0.026 | 0.008 | 0.001 | −0.166 | 0.056 | 0.003 | |
| p-tau/ Aβ42 | −0.108 | 0.033 | 0.001 | −0.014 | 0.010 | 0.146 | −0.009 | 0.006 | 0.144 | −0.171 | 0.042 | <0.0001 | |
Data shown are from mixed models with repeated measures of cognition scores as dependent variables, and sex, education, age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total score and average cognitive score over 6 months prior to the beginning of Phase 2, CSF biomarker at the beginning of Phase 2, follow-up time, CSF biomarker × follow-up time interaction as covariates. The intercept and the regression coefficients for the follow-up time were treated as random effects. Results for CSF biomarkers are for variable × follow-up time interaction term. Coef.: the regression coefficient, or the estimated association between the baseline biomarkers and annual change in cognitive scores; SE: standard error; SRT: Selective Reminding Test; SDMT: Symbol Digit Modalities Test; Aβ42: amyloid beta peptide 1-42; p-tau: phosphorylated tau; t-tau: total tau. Bold values indicate results that met the pre-specified criteria of having a significant p-value in both the discovery and validation cohorts.
Figure 1. Change of predicted cognitive test scores over time in PD patients during Phase 2.
Data shown are the mean cognitive scores predicted from CSF biomarkers levels (low, mean, high) at the beginning of Phase 2 based on output from a mixed linear model; sex, education, and age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total and cognitive scores over 6 months prior to the beginning of Phase 2 were controlled as covariates. Blue lines indicate predicted cognitive scores by the mean levels of CSF biomarkers, green lines indicate predicted scores by low levels (mean – 1 SD) of CSF biomarkers, and red lines indicate predicted scores by high levels (mean + 1 SD) of CSF biomarkers. A: CSF p-tau vs SDMT, low p-tau can predict a 0.37/year decline of SDMT, mean p-tau can predict a decline of 0.97/year, high p-tau can predict a decline of 1.58/year; B: CSF p-tau/Aβ42 vs SDMT (low – 0.43/year, mean – 0.97/year, high – 1.50/year); C: CSF p-tau vs SRT-total Recall (low – 0.78/year, mean – 1.12/year, high – 1.45/year); D: CSF p-tau/Aβ42 vs. SRT-total Recall (low – 0.77/year, mean –1.10/year, high –1.44/year). SRT: Selective Reminding Test; SDMT: Symbol Digit Modalities Test
None of the other CSF biomarkers met our criterion of being significant in the discovery set and confirmed in the validation set, although there is some weaker evidence of an association between measures of p-tau and t-tau with visuospatial working memory (New Dot Test) and with t-tau and visuospatial working memory/processing speed (SDMT).
The linear mixed model was run for those with LEDD data available (n=254) both with LEDD and LEDD x follow up time interaction included as covariates, and without the LEDD data included in the model. The analyses produced similar results whether or not the LEDD data were included (Table e3).
Although several of the biomarkers correlated with rate of change in cognitive measures for the APOE ε4- group only, a time × biomarker × APOE ε4 genotype interaction was not found. Because the significant correlations in subgroups may be a result of disparate sample sizes, additional analyses were not pursued. Similarly, a time × biomarker × motor subtype (PIGD vs. TD vs. intermediate) interaction was not found (Table e4 and data not shown).
Discussion
The current study is the largest to date relating CSF biomarkers to longitudinal cognitive function in PD patients. Here, we report new findings concerning the predictive value of CSF biomarkers with respect to decline in cognitive function, including: 1) there was a lack of association between Aβ42 and cognitive function and subsequent rate of decline in cognitive function in PD patients, and 2) higher p-tau and p-tau/Aβ42 predicted subsequent decline on cognitive tasks involving both memory and executive functions, among patients who have just started levodopa therapy.
The absence of an association between Aβ42 and cognitive performance and decline in our data differs from several other reports with respect to PD patients. Cross-sectional reports have shown that lower values of multiple Aβ species (including Aβ42) correlate with impaired performance on both memory and non-memory measures in PD patients [1,6]. Similar to the current study, Siderowf et al.[7] failed to detect any baseline correlations between cognitive function and either Aβ42 or tau in a cohort of 45 patients with PD, yet they did report a strong association between lower baseline Aβ42 and subsequent decline on a global cognitive measure. However, participants in that cohort had a longer mean illness duration, thus it is possible that reduced Aβ42 may indicate the onset of subsequent cognitive decline in patients with more advanced disease than those evaluated in this cohort. Further, differences in sample size, stage of disease, cognitive measures incorporated, and included covariates may all contribute to the disparate findings with regard to Aβ42. Any of these (or additional unknown) factors might have contributed to some type of cohort bias in the DATATOP sample.
Clearly, the most important results coming out of this study center on alterations in tau species, whose gene (MAPT) has been consistently associated with PD risk, that may be associated with cognitive decline in PD. Several reports indicate cross-sectional reductions in CSF t-tau and p-tau in PD patients as compared to controls [1,2,21,22]. Reduced tau in the substantia nigra has been demonstrated in both animals and in human brain; further, reductions in tau were noted to facilitate neurodegeneration in mouse models of PD [23]. Recent studies have demonstrated a negative association between both CSF t-tau and p-tau and motor symptoms in PD patients [24] as well as greater presynaptic dopaminergic involvement in PD patients with lower CSF p-tau and t-tau [25]. In recently published DATATOP results [8], we reported that higher baseline p-tau as a proportion of total baseline tau predicted slower motor progression, suggesting that perhaps higher p-tau early in PD serves some type of protective function.
Intriguingly, the current study demonstrates that higher levels of p-tau at the end of Phase 1 predicted increased cognitive decline in subsequent years. Thus, a potential alternate explanation is that there is initial sequestration of soluble t-tau and p-tau as a protective mechanism in reaction to the earliest disease processes (thus resulting in lower tau cleared into the CSF), with subsequent loss of this protection occurring with more rapid disease progression. The associations of tau species with motor symptom worsening and with cognitive decline might also be different, and may reflect underlying differential mechanisms for cognitive versus motor symptoms. Support for this is found in recent studies that demonstrate that, while CSF tau levels may be reduced in nondemented PD patients as compared to controls, levels are higher in those with PD-related dementia.[4,5,26] An important caveat is that the role of tau phosphorylation in areas other than the tubulin-binding domains on tau is largely unknown, and our studies are purely correlational and do not provide mechanistic explanations as to how and why tau species are important in cognitive decline in PD patients.
Our findings differ from those presented in a recent follow up study by Parnetti et al.[27], which found no association between CSF levels of tau and subsequent cognitive decline, as measured by two global cognitive screening tests. However, the DATATOP study implemented more sensitive cognitive measures designed to measure performance in specific cognitive domains. Unlike the global tau pathology expected in Alzheimer’s disease, tau pathology in early PD is likely to be limited to the striatum [28]. As a result, the associations between tau and progression of specific measures of verbal learning (rather than recall) and executive functioning in the DATATOP sample are not entirely unexpected, as performance on these tasks may be impeded as a result of disruptions in the fronto-striatal pathways, often first affected in PD patients.
It is possible that the study medications (selegiline and or α-tocopherol) had some unknown or unmeasured effects that may interfere with the current analyses. Of note, prior analyses of the DATATOP cohort yielded no effects of the study medications on cognitive test performance [29]. Thus, we felt comfortable including all participants in the current set of analyses. A further limitation exists in terms of the range of cognitive tests and clinical data available for analysis. Not all of the cognitive tests administered at baseline were consistently measured for the duration of the study, and thus we were limited in our ability to detect the influence of CSF biomarkers on certain areas of cognitive test performance (e.g., visuospatial and language abilities). Further, information concerning participants who developed dementia or mild cognitive impairment during the course of the trial was not collected. To address this concern, we controlled for MMSE scores prior to the start of Phase 2; however, this measure does not necessarily correlate well with clinical cognitive diagnosis. Thus, future studies should carefully collect data related to cognitive impairment and dementia. Additionally, we did not have access to longitudinal biomarkers beyond Phase 2, the presence of which may have helped to further elucidate the relationship between tau species and cognitive change over time. Also, during Phase 2, all participants who had reached the designated endpoint were placed on levodopa or other anti-parkinsonian medications, and this coincided with cognitive decline in the sample. In the current analyses, we controlled for LEDD, which did not substantially affect the results; thus we surmise that the cognitive decline noted in Phase 2 may be more likely to be related to disease progression rather than the commencement of anti-parkinsonian medications. However, we were unable to control for levodopa onset and dose a priori, thus, there remains the possibility that levodopa treatment may have had an impact on cognition in this study. Additional studies in large cohorts that stringently control for dose and duration of anti-parkinsonian medications will be important contributions. Advancing age may have also played a role in the cognitive decline noted during Phase 2. Despite our control of this variable during analyses, the effects of age and other variables on CSF biomarkers independent of PD may have impacted our results; additional studies that incorporate non-PD control groups will be important to address this issue. Finally, we did not detect an association between APOE and cognition nor an interaction between APOE, CSF biomarkers, and cognition. We surmise that, given the reduced number of participants with available APOE data in combination with a limited cognitive test battery, our ability to detect such a relationship was reduced, particularly when testing three-way interaction effects.
In summary, the current study provides novel data concerning the potential ability of CSF tau species to predict subsequent decline in cognitive test performance in the largest sample of PD patients assembled to date. These findings are not only entirely consistent with GWAS studies linking tau to PD risk, but also suggest that tau may be involved in cognitive decline in PD patients. This is particularly important, given the recent realization that even at the time of PD diagnosis, many PD patients already have at least subtle cognitive difficulties. If reproduced, in addition to pathogenic roles of tau, these findings will move the field closer to possible identification of PD signature biomarkers in CSF that may herald the onset of cognitive decline.
Supplementary Material
Table e1. Cross-sectional correlations of CSF biomarkers with cognitive scores at the beginning of Phase 1
Data shown are results from partial correlations, adjusted for sex, education, and age. SRT: Selective Reminding Test; SDMT: Symbol Digit Modalities Test; Aβ42: amyloid beta peptide 1-42; p-tau: phosphorylated tau; t-tau: total tau.
Table e2. Cross-sectional correlations of CSF biomarkers with cognitive scores at the beginning of Phase 2
Data shown are results from partial correlations, adjusted for sex, education, and age. SRT: Selective Reminding Test; SDMT: Symbol DigitModalities Test; Aβ42: amyloid beta peptide 1-42; p-tau: phosphorylated tau; t-tau: total tau.
Table e3. CSF biomarkers as predictors of longitudinal cognitive change in phase 2.
Data shown are from type III tests in the mixed model with repeated measures of cognition scores as dependent variable, and sex, education, age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total score and average cognitive score over 6 months prior to the beginning of Phase 2, CSF biomarker at the beginning of Phase 2, follow-up time, CSF biomarker × follow-up time interaction as covariates; in Model 1, LEDD and LEDD × follow-up time interaction were also included. The intercept and the regression coefficients for the follow-up time were treated as random effects. Results for CSF biomarkers are for variable × follow-up time interaction term. LEDD: Levodopa Equivalent Daily Dose; SE: standard error; SDMT: Symbol Digit Modalities Test; SRT: Selective Reminding Test.
Table e4. CSF biomarkers as predictors of longitudinal change of cognition in PD patients with known APOE genotype and motor subgroups during Phase 2.
A, B and C: Data shown are from type III tests in the mixed model with repeated measures of cognition scores as dependent variable in Tremor-dominant, PIGD-dominant and Intermediate subgroups, and sex, education, age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total score, average cognitive score over 6 months prior to the beginning of Phase 2, CSF biomarker at the beginning of Phase 2, follow-up time, CSF biomarker × follow-up time interaction as covariates. The intercept and the regression coefficients for the follow-up time were treated as random effects. Results for CSF biomarkers are for variable × follow-up time interaction term. Results from the three way interaction test suggest the differences of coefficients of CSF biomarkers between Tremor-dominant PIGD-dominant and Intermediate subgroups were not significant (data not shown). SE: standard error; SRT: Selective Reminding Test; SDMT: Symbol Digit Modalities Test.
A, B and C: Data shown are from type III tests in the mixed model with repeated measures of cognition scores as dependent variable in Tremor-dominant, PIGD-dominant and Intermediate subgroups, and sex, education, age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total score, average cognitive score over 6 months prior to the beginning of Phase 2, CSF biomarker at the beginning of Phase 2, follow-up time, CSF biomarker × follow-up time interaction as covariates. The intercept and the regression coefficients for the follow-up time were treated as random effects. Results for CSF biomarkers are for variable × follow-up time interaction term. Results from the three way interaction test suggest the differences of coefficients of CSF biomarkers between Tremor-dominant PIGD-dominant and Intermediate subgroups were not significant (data not shown). SE: standard error; SRT: Selective Reminding Test; SDMT: Symbol Digit Modalities Test.
Figure e1. Change of cognitive test scores over time during Phase 1 and phase 2 in the DATATOP cohort. Phase 1, Endpointed = the participant’s disease had advanced so levodopa therapy was required; censored = the participant didn’t reach endpoint at the end of Phase 1. Phase 2, only the subjects who reached endpoint at the end of Phase 1 were included.
Highlights.
CSF amyloid β1-42 and tau were examined in relation to cognition in Parkinson’s.
No relationship between cognition and biomarkers in untreated Parkinson’s disease.
Higher CSF tau predicts cognitive decline once levodopa treatment is required.
Acknowledgments
The study is supported by generous grants from the Michael J. Fox Foundation as well as NIH (AG10124, ES004696-5897, ES007033-6364, AG033398, ES016873, ES019277, NS057567, NS062684-6221 and NS082137). We thank Karen Marder, Lorraine Clark, and the Parkinson’s Disease Foundation Weill Family Fund for providing the APOE data for this study.
Footnotes
Supplemental methods and data, in the form of tables and figures, are provided (ms_DATATOP_cognition_supplement_2_14)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Changqin Liu, Email: liuchangqin@126.com.
Brenna Cholerton, Email: bchol@uw.edu.
Min Shi, Email: mshi70@uw.edu.
Carmen Ginghina, Email: ginghina@uw.edu.
Kevin C. Cain, Email: cain@uw.edu.
Peggy Auinger, Email: peggy.auinger@chet.rochester.edu.
References
- 1.Alves G, Bronnick K, Aarsland D, Blennow K, Zetterberg H, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 2.Montine TJ, Shi M, Quinn JF, Peskind ER, Craft S, et al. CSF Abeta(42) and tau in Parkinson's disease with cognitive impairment. Mov Disord. 2010;25:2682–2685. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollenhauer B, Trenkwalder C, von Ahsen N, Bibl M, Steinacker P, et al. Beta-amlyoid 1–42 and tau-protein in cerebrospinal fluid of patients with Parkinson's disease dementia. Dement Geriatr Cogn Disord. 2006;22:200–208. doi: 10.1159/000094871. [DOI] [PubMed] [Google Scholar]
- 5.Compta Y, Marti MJ, Ibarretxe-Bilbao N, Junque C, Valldeoriola F, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord. 2009;24:2203–2210. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 6.Leverenz JB, Watson GS, Shofer J, Zabetian CP, Zhang J, et al. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson's disease. Parkinsonism Relat Disord. 2011;17:61–64. doi: 10.1016/j.parkreldis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, et al. CSF amyloid {beta} 1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Mattison HA, Liu C, Ginghina C, Auinger P, et al. Longitudinal assessment of tau and amyloid beta in cerebrospinal fluid of Parkinson disease. Acta Neuropathol. 2013;126:671–82. doi: 10.1007/s00401-013-1121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ParkinsonStudyGroup. DATATOP: a multicenter controlled clinical trial in early Parkinson's disease. Arch Neurol. 1989;46:1052–1060. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- 10.Shoulson I. Deprenyl and tocopherol antioxidative therapy of parkinsonism (DATATOP). Parkinson Study Group. Acta Neurol Scand Suppl. 1989;126:171–175. doi: 10.1111/j.1600-0404.1989.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 11.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67:1116–1122. doi: 10.1001/archneurol.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marder KS, Tang MX, Mejia-Santana H, Rosado L, Louis ED, et al. Predictors of parkin mutations in early-onset Parkinson disease: the consortium on risk for early-onset Parkinson disease study. Arch Neurol. 2010;67:731–738. doi: 10.1001/archneurol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 14.Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 1973. [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aasly JO, Shi M, Sossi V, Stewart T, Johansen KK, et al. Cerebrospinal fluid amyloid beta and tau in LRRK2 mutation carriers. Neurology. 2012;78:55–61. doi: 10.1212/WNL.0b013e31823ed101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghebranious N, Ivacic L, Mallum J, Dokken C. Detection of ApoE E2, E3 and E4 alleles using MALDI-TOF mass spectrometry and the homogeneous mass-extend technology. Nucleic Acids Res. 2005;33:e149. doi: 10.1093/nar/gni155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 20.Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12:37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 21.Kang J, Caspell C, Coffey C, Taylor P, Frasier M, et al. Association between CSF biomarkers and clinical phenotype of early Parkinson's disease in the Parkinson's Progression Marker Initiative (PPMI) Movement Disorders. 2012;27 (suppl 1):S34–35. [Google Scholar]
- 22.Shi M, Zhang J. CSF alpha-synuclein, tau, and amyloid beta in Parkinson's disease. Lancet Neurol. 2011;10:681. doi: 10.1016/S1474-4422(11)70130-2. author's reply 681–683. [DOI] [PubMed] [Google Scholar]
- 23.Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- 24.Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, et al. Association of cerebrospinal fluid beta-amyloid 1-42, T-tau, P-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013;70:1277–1287. doi: 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiaravalloti A, Stefani A, Fiorentini A, Lacanfora A, Stanzione P, et al. Do CSF levels of t-Tau, p-Tau and beta1-42 amyloid correlate with dopaminergic system impairment in patients with a clinical diagnosis of Parkinson disease? A (123)I-FP-CIT study in the early stages of the disease. Eur J Nucl Med Mol Imaging. 2014;41:2137–2143. doi: 10.1007/s00259-014-2841-4. [DOI] [PubMed] [Google Scholar]
- 26.Vranova HP, Henykova E, Kaiserova M, Mensikova K, Vastik M, et al. Tau protein, beta-amyloid(1)(-)(4)(2) and clusterin CSF levels in the differential diagnosis of Parkinsonian syndrome with dementia. J Neurol Sci. 2014;343:120–124. doi: 10.1016/j.jns.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 27.Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, et al. Differential role of CSF alpha-synuclein species, tau, and Abeta42 in Parkinson's Disease. Front Aging Neurosci. 2014;6:53. doi: 10.3389/fnagi.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wills J, Jones J, Haggerty T, Duka V, Joyce JN, et al. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson's disease brains with and without dementia. Exp Neurol. 2010;225:210–218. doi: 10.1016/j.expneurol.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieburtz K, McDermott M, Como P, Growdon J, Brady J, et al. The effect of deprenyl and tocopherol on cognitive performance in early untreated Parkinson's disease. Parkinson Study Group. Neurology. 1994;44:1756–1759. doi: 10.1212/wnl.44.9.1756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table e1. Cross-sectional correlations of CSF biomarkers with cognitive scores at the beginning of Phase 1
Data shown are results from partial correlations, adjusted for sex, education, and age. SRT: Selective Reminding Test; SDMT: Symbol Digit Modalities Test; Aβ42: amyloid beta peptide 1-42; p-tau: phosphorylated tau; t-tau: total tau.
Table e2. Cross-sectional correlations of CSF biomarkers with cognitive scores at the beginning of Phase 2
Data shown are results from partial correlations, adjusted for sex, education, and age. SRT: Selective Reminding Test; SDMT: Symbol DigitModalities Test; Aβ42: amyloid beta peptide 1-42; p-tau: phosphorylated tau; t-tau: total tau.
Table e3. CSF biomarkers as predictors of longitudinal cognitive change in phase 2.
Data shown are from type III tests in the mixed model with repeated measures of cognition scores as dependent variable, and sex, education, age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total score and average cognitive score over 6 months prior to the beginning of Phase 2, CSF biomarker at the beginning of Phase 2, follow-up time, CSF biomarker × follow-up time interaction as covariates; in Model 1, LEDD and LEDD × follow-up time interaction were also included. The intercept and the regression coefficients for the follow-up time were treated as random effects. Results for CSF biomarkers are for variable × follow-up time interaction term. LEDD: Levodopa Equivalent Daily Dose; SE: standard error; SDMT: Symbol Digit Modalities Test; SRT: Selective Reminding Test.
Table e4. CSF biomarkers as predictors of longitudinal change of cognition in PD patients with known APOE genotype and motor subgroups during Phase 2.
A, B and C: Data shown are from type III tests in the mixed model with repeated measures of cognition scores as dependent variable in Tremor-dominant, PIGD-dominant and Intermediate subgroups, and sex, education, age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total score, average cognitive score over 6 months prior to the beginning of Phase 2, CSF biomarker at the beginning of Phase 2, follow-up time, CSF biomarker × follow-up time interaction as covariates. The intercept and the regression coefficients for the follow-up time were treated as random effects. Results for CSF biomarkers are for variable × follow-up time interaction term. Results from the three way interaction test suggest the differences of coefficients of CSF biomarkers between Tremor-dominant PIGD-dominant and Intermediate subgroups were not significant (data not shown). SE: standard error; SRT: Selective Reminding Test; SDMT: Symbol Digit Modalities Test.
A, B and C: Data shown are from type III tests in the mixed model with repeated measures of cognition scores as dependent variable in Tremor-dominant, PIGD-dominant and Intermediate subgroups, and sex, education, age at the beginning of Phase 2, final Phase 1 MMSE score, average UPDRS total score, average cognitive score over 6 months prior to the beginning of Phase 2, CSF biomarker at the beginning of Phase 2, follow-up time, CSF biomarker × follow-up time interaction as covariates. The intercept and the regression coefficients for the follow-up time were treated as random effects. Results for CSF biomarkers are for variable × follow-up time interaction term. Results from the three way interaction test suggest the differences of coefficients of CSF biomarkers between Tremor-dominant PIGD-dominant and Intermediate subgroups were not significant (data not shown). SE: standard error; SRT: Selective Reminding Test; SDMT: Symbol Digit Modalities Test.
Figure e1. Change of cognitive test scores over time during Phase 1 and phase 2 in the DATATOP cohort. Phase 1, Endpointed = the participant’s disease had advanced so levodopa therapy was required; censored = the participant didn’t reach endpoint at the end of Phase 1. Phase 2, only the subjects who reached endpoint at the end of Phase 1 were included.