Abstract
Objective:
We investigated the relationship of diabetes and prediabetes with cognitive performances, assessed through raw test and z scores and according to neurocognitive impairment (NCI) classification in a cohort of individuals infected with HIV.
Methods:
The ANRS CO3 Aquitaine cohort is a prospective hospital-based cohort of HIV-1–infected patients under routine clinical management in 6 public hospitals in southwestern France. Between 2007 and 2009, an ancillary study consisted of a neuropsychological battery of 10 tests at baseline and 2-year follow-up. The severity of NCI (normal, asymptomatic, mild, HIV dementia) was assessed according to international guidelines.
Results:
At baseline (400 patients, 33 with prediabetes, 39 with diabetes), in cross-sectional multivariable analyses, patients with diabetes performed significantly worse on 9 neuropsychological tests that assessed memory, executive functions, attention, psychomotor speed, language, and manual dexterity. Participants with prediabetes had worse performances compared with those who had normal glycemia in 5 tests. The longitudinal analysis of the association between glycemia status at baseline and change in cognitive performances over 2-year follow-up (n = 283) suggested that patients with diabetes also showed a slightly higher decline on 5 of the 10 tests, those involving executive functions and memory functioning. Glycemia status at baseline was not significantly associated with NCI severity in cross-sectional (p = 0.44) and longitudinal (p = 0.64) analyses.
Conclusions:
In this hospital-based cohort of people living with HIV, diabetes, but not the other cardiovascular risk factors, is associated with worse cognitive performances in several cognitive domains and with larger decline in fewer domains over the short term.
Neurocognitive impairment (NCI) is a frequent condition in patients infected by HIV, even when their infection is well controlled by antiretroviral treatment (ART). Prevalence estimates of NCI in the era of wide use of ART vary from 20% to more than 50%.1–4 Etiology of NCI is likely to be multifactorial but is still not well understood.5 Associations with both HIV-related determinants, ART characteristics and other factors not directly HIV-related, such as smoking, hypertension, and metabolic syndrome (MetS), have been described with inconsistent evidence across studies.6–8
Among MetS components, prevalence of diabetes may be as high as 10% in midlife HIV populations.9,10 In studies of HIV-uninfected elderly persons, effects of diabetes on dementia risk and accelerated cognitive decline have well been established11,12 whereas data in middle-aged individuals are scarce.13,14 In HIV-infected, middle-aged patients, the link of diabetes with cognitive performances remains largely unknown.8,15–17 Furthermore, robust studies that explore how impaired glycemia (prediabetes) could affect brain health are lacking, and no study has examined the impact of diabetes or prediabetes on cognitive decline over time.
Within a large and unselected cohort of patients with HIV infection in France, we estimated the association of diabetes and prediabetes with cognitive performances and change in cognitive performances over 2-year follow-up.
METHODS
Study design.
The Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) CO3 Aquitaine cohort is a prospective, open, hospital-based cohort of HIV-1–infected patients under usual clinical care in 6 public hospitals in southwestern France. Criteria for inclusion are as follows: adult inpatients or outpatients of the participating hospital wards who have an HIV-1 infection confirmed by Western blot testing; ≥1 follow-up visit after the first clinic visit or documented date of death; and signed informed consent. The cohort is considered to be representative of patients with HIV infection in clinical care in this region of France. A standardized collection of data is performed at cohort inclusion (age, sex, HIV transmission category) and at each contact, including: existence of medical events since last interview, laboratory markers assessment (including HIV plasma RNA, T-CD4 lymphocyte count, hemoglobin, serologic status for hepatitis B and C), and detailed recording of past and current medication intake (ART, prophylaxis, and others). The ICD-10 was used for coding of all medical events.18
There has been a consecutive enrollment invitation of Aquitaine cohort's participants in the longitudinal substudy COGLOC, which focused on cognitive and motor functions.1,19 Substudy took place in 5 clinical centers of Bordeaux University Hospital from June 2007 to November 2009, so that the expected 400 participants were recruited.1,19 A follow-up wave was performed on average 2 years after enrollment (n = 283).
The specific eligibility criteria for the COGLOC study included age at least 18 years and to have a stable heath status (i.e., no medical condition involving imminent care or hospitalization).
Standard protocol approvals, registrations, and patient consents.
The Biomedical Ethics Committee, Comité de Protection des Personnes Sud-Ouest et Outre Mer, gave its approval of the study protocol, and each person included signed a specific informed consent. The study was conducted following the standards of the ethics committee and the Helsinki Declaration.
Clinical examination included blood intake, detailed recording of cerebrovascular and cardiac diseases, and information on substance use and working activity histories. The administration of specific questionnaires captured cognitive performances, cognitive complaints, limitations in physical activities (Lawton and Brody scale20), and depressive symptoms (Center for Epidemiologic Studies–Depression [CES-D]21 scale). Information on history of smoking, IV and other recreational drug use, and all medical treatments was also collected.
Neuropsychological testing.
The neuropsychological battery (described in e-Methods on the Neurology® Web site at Neurology.org), administered by trained psychologists, explored several cognitive domains and was built to be consistent with the classification of HIV-associated NCI.22
Four categories of NCI were defined: normal, asymptomatic NCI, mild neurocognitive disorder, and HIV-associated dementia. For all neuropsychological tests, performances were compared with US norms adjusted for age, sex, and education.
Exposure of interest.
Glycemia status was determined based on available routine laboratory results from the Aquitaine cohort database. Diabetes was defined by ≥2 glycemia >7 mmol/L up or ≥1 glycemia >11.1 mmol/L or any use of antidiabetic drug (medication intake was extracted from the Aquitaine cohort database). Hyperglycemia (prediabetes) was defined by ≥2 measures of glycemia between 6.1 and 7 mmol/L up.
Covariates.
The following covariates were extracted from the Aquitaine cohort database: sex; age; high education level (if baccalaureate level attained); tobacco and cannabis consumption; body mass index (BMI); hypertension; hypercholesterolemia, hypertriglyceridemia; history of cardiovascular or cerebrovascular disease; HIV-1 RNA level; CD4 cell count and nadir; date of HIV diagnosis; AIDS stage according to US Centers for Disease Control and Prevention classification; hepatitis B and C coinfection status; ART history; and current use of efavirenz-based ART. Lifetime exposures to stavudine, didanosine, or indinavir were classified through a categorical variable (yes/no through the entire follow-up).
High cholesterol was defined by plasma cholesterol >6.24 mmol/L on ≥2 consecutive measures or any use of lipid-lowering drugs; high triglycerides were defined by plasma level >2.2 mmol/L at 2 consecutive measures. Hypertension was characterized by systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or antihypertensive drug intake. BMI was computed as the ratio of weight (kg) to the square of height (m2). Overweight was defined as BMI ≥25. Isolating obese people (n = 8) among those who were overweight was not possible for the sake of power. Participants with glomerular filtration rate <60 mL/min/1.73 m2 for 3 months were classified as having chronic kidney disease. Hepatitis B coinfection was defined by at least one positive hepatitis B virus surface antigen measurement and hepatitis C coinfection by at least one positive hepatitis C virus antibody measurement since inclusion in the cohort.
Statistical methods.
Differences between categories of glycemia and covariate distributions were assessed using χ2 test and analysis of variance or nonparametric methods when appropriate.
In cross-sectional (baseline) analyses, linear regressions were performed to explore the association between glycemia and the cognitive test scores using 3 adjustment strategies. Models 1 were adjusted for sex, education, HIV transmission groups, AIDS stage, and CES-D score. Models 2 were adjusted for models 1 covariates + cardiovascular risk factors (hypertension, hypercholesterolemia, BMI, smoking habits, history of cardiovascular events, and chronic kidney disease). Models 3 were additionally adjusted for HIV-1 RNA level, CD4 cell count and nadir, HIV diagnosis date, hepatitis B and C coinfection status, ART history, current efavirenz treatment, and lifetime exposure to stavudine, didanosine, and indinavir. Association between NCI categories and glycemia status at baseline was investigated in a polytomous regression model adjusting for sex, education, HIV transmission groups, AIDS stage, CES-D score, and cardiovascular risk factors.
For all tests and each participant, we computed z scores = (individual raw score − sample mean score)/sample SD, in order to provide an effect comparison across tests using the same models as above.
To investigate whether the associations with diabetes were also observed with other MetS components, we analyzed the cross-sectional relationships among hypertension, hypertriglyceridemia, hypercholesterolemia, overweight, and cognitive performances under model 1.
For longitudinal analyses, yearly changes in cognitive performances were computed as: (score at wave 2 − score at wave 1)/delay in years between waves, and linear regression models with full adjustments were computed (as in model 3 above). To assess whether patients who withdrew during follow-up might have biased the results, sensitivity analyses were undertaken by (1) applying inverse probability weighting (under the missing-at-random assumption), and (2) assuming extreme scenarios for dropouts (under the informative dropout assumption). Robustness of findings was also assessed by excluding participants with HIV-associated dementia and those with obesity and by restricting the analyses to men. All possible 2 by 2 interactions were tested but none was significant.
Analyses were performed with SAS software, version 9.3 (SAS Institute, Cary, NC).
RESULTS
Four hundred patients with HIV-1 infection participated in the baseline COGLOC study. They were on average 47.2 years old (SD = 10.2) and almost 80% were men. Only a quarter of patients had been diagnosed with HIV <7 years before the study, and 95% were treated with ART. The majority (84.5%) had an HIV-1 RNA level below 500 copies/mL. Table 1 shows the sample characteristics for HIV-related variables and also cardiovascular risk factors. The most frequent metabolic disturbance was hypertriglyceridemia (half of the sample) while 10% of patients had diabetes and 8.2% had impaired glycemia without diabetes. Characteristics significantly associated with a higher frequency of diabetes or impaired glycemia (table 1) were male sex, higher age, hypertension, hypercholesterolemia, and hypertriglyceridemia. Less frequent history of stavudine was significantly related to impaired glycemia status. None of the other HIV-related characteristics was significantly related to glycemia status.
Table 1.
Sample characteristics at baseline by glycemia status, ANRS CO3 Aquitaine cohort, 2007–2009

Cross-sectional analyses.
The mean cognitive test performances at baseline are given in table 2 (column 1). This table also shows the results of multivariable modeling of each of the cognitive test performances at baseline in relation to glycemia status using both raw and z scores. These analyses show a consistent significant trend for patients with diabetes to have lower cognitive performances on average as compared with patients who had normal glycemia. For the Trail Making Test, Part B, and Purdue Pegboard Test, patients with impaired glycemia had performances as low as those of patients with diabetes. For the Digit Symbol Substitution Test and copy score of the Rey-Osterrieth Complex Figure test, patients with impaired glycemia had performances that were intermediate to those of normal and diabetic patients. In the multivariable modeling strategy, the successive adjustments did not modify the findings but rather reinforced the differences across glycemia status groups. None of the other cardiovascular risk factors (and MetS components), i.e., hypertension, hypertriglyceridemia, hypercholesterolemia, and overweight, was significantly related to cognitive performances in fully adjusted models (table e-1).
Table 2.
Multivariable analyses of the associations between baseline glycemia status and cognitive performances, ANRS CO3 Aquitaine cohort, 2007–2009
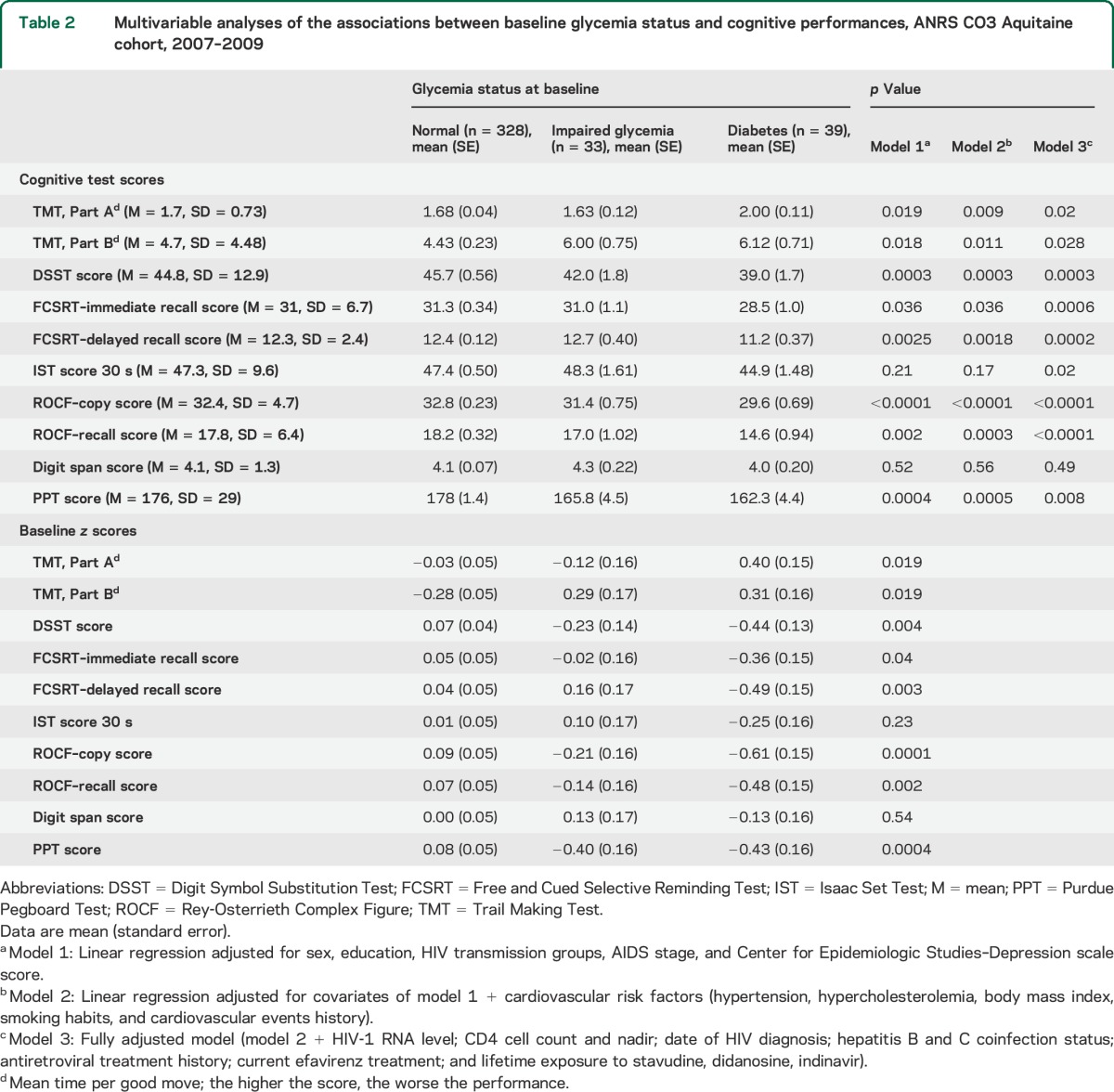
The multivariable association between glycemia status and severity of neurocognitive disorders according to Antinori et al.22 classification was not significant (p = 0.44) (table e-2). However, stratum-specific significant associations were observed: the odds of having mild neurocognitive disorder was significantly 3.4 times higher in those with impaired glycemia compared with those who had normal glycemia (p = 0.01), and the odds of having HIV-associated dementia was 6.5 times higher in those with diabetes compared with those who had normal glycemia (p < 0.001) (table e-2).
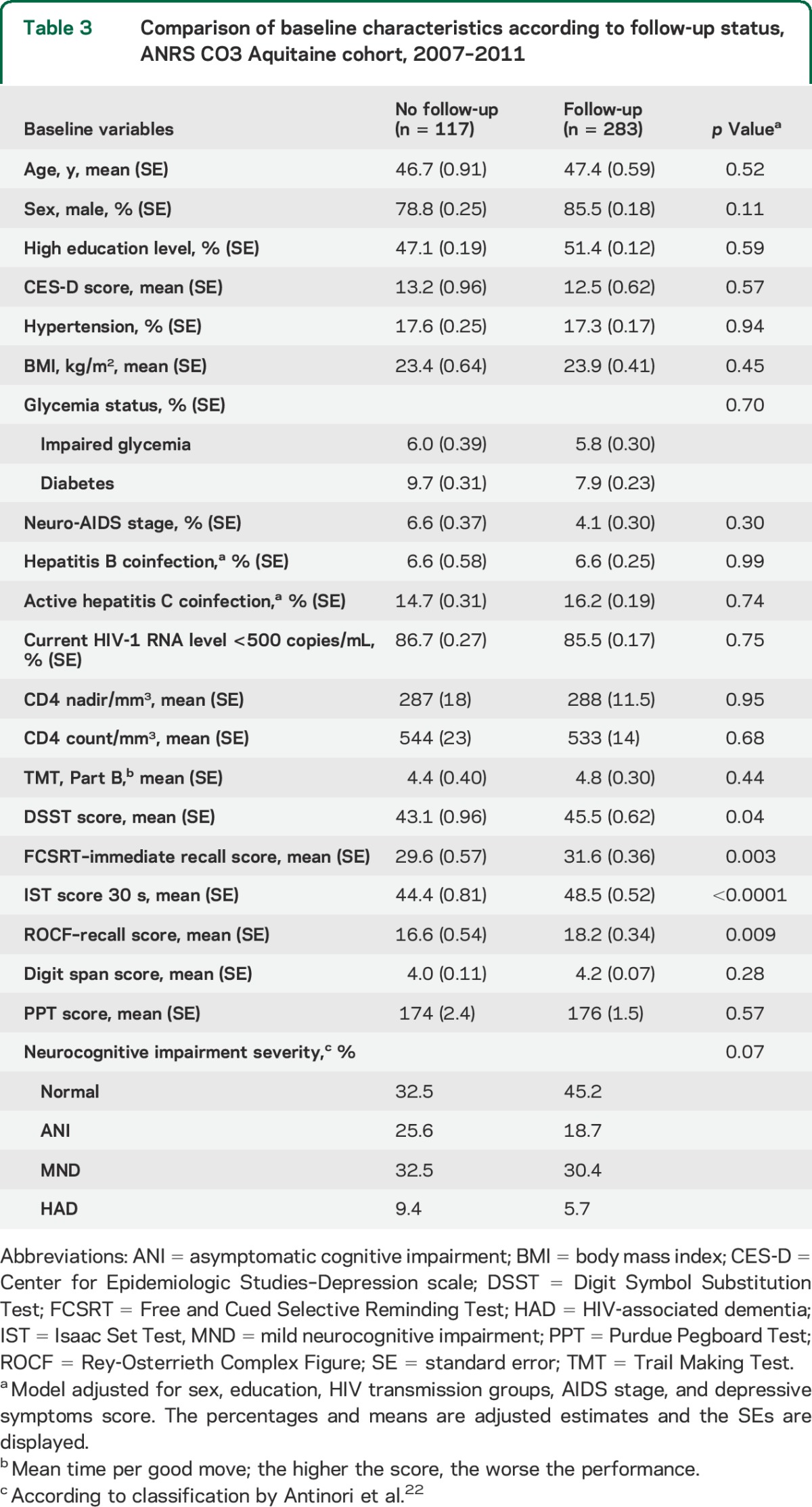
Among the COGLOC study participants, 283 had a follow-up examination as per protocol and they were compared with those who were not followed up (table 3). There were no substantial differences for demographic characteristics, cardiovascular risk factors (including glycemia status), or HIV-related characteristics but there were important differences in cognitive scores at baseline, the latter being worse in those who did not return.
Table 3.
Comparison of baseline characteristics according to follow-up status, ANRS CO3 Aquitaine cohort, 2007–2011
Longitudinal analyses.
The mean duration of follow-up was 2.1 (SD = 0.3) years with a range of 1 to 3.5 years. All mean scores but 2 (Rey-Osterrieth Complex Figure copy and Purdue Pegboard Test) improved over time, but increases were relatively small (table 4).
Table 4.
Multivariable analyses of the association between baseline glycemia status and change in cognitive performances, ANRS CO3 Aquitaine cohort, 2007–2011
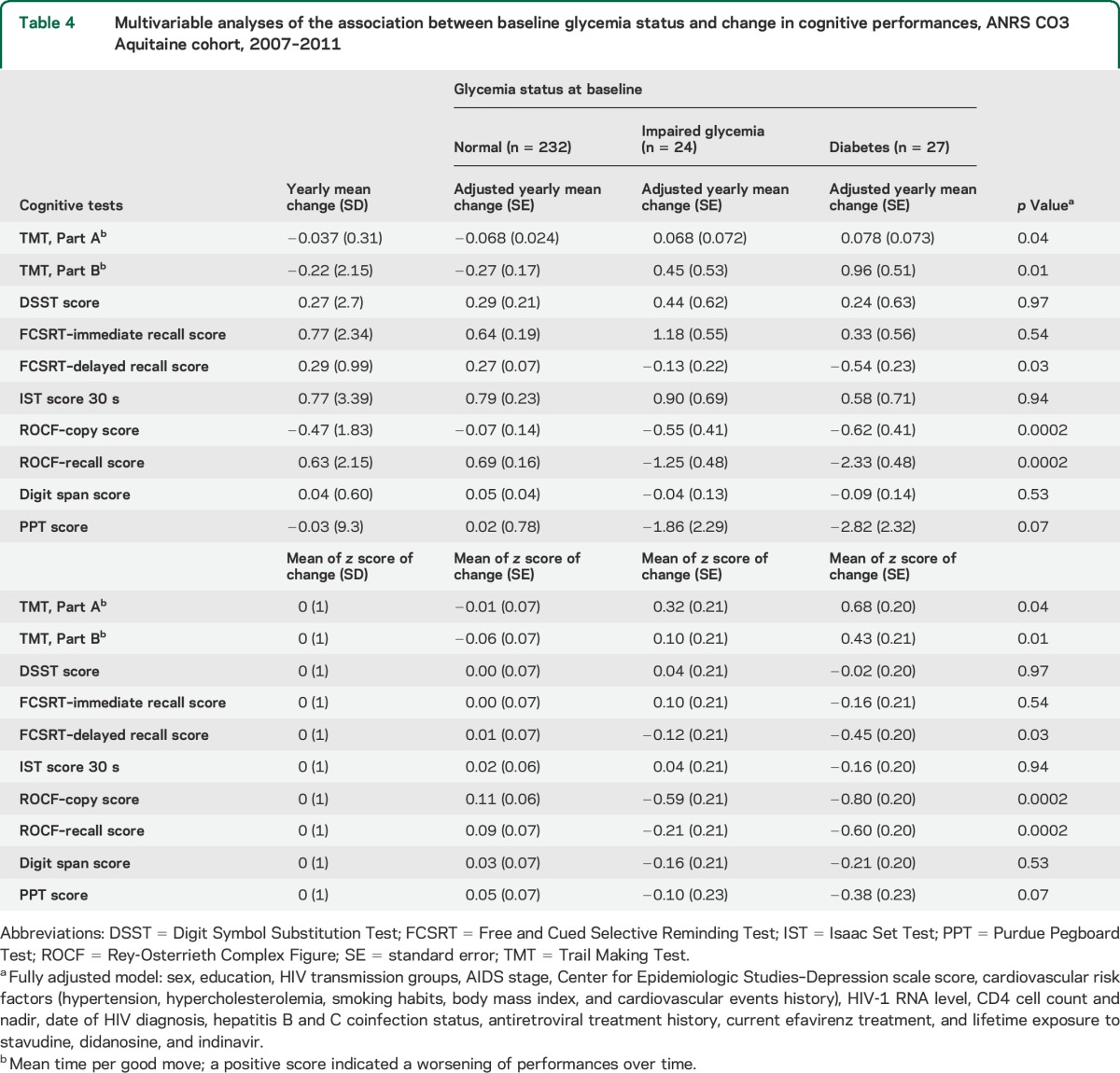
Multivariable longitudinal analyses showed that diabetes at baseline was related to higher decline of performances (both yearly change and z score of change) in Trail Making Test, Parts A and B, delayed recall of Free and Cued Selective Reminding Test, and Rey-Osterrieth Complex Figure test (table 4). None of the other cardiovascular risk factors (hypertension, hypertriglyceridemia, hypercholesterolemia, and overweight) was significantly related to change in cognitive performances over 2 years (results not shown).
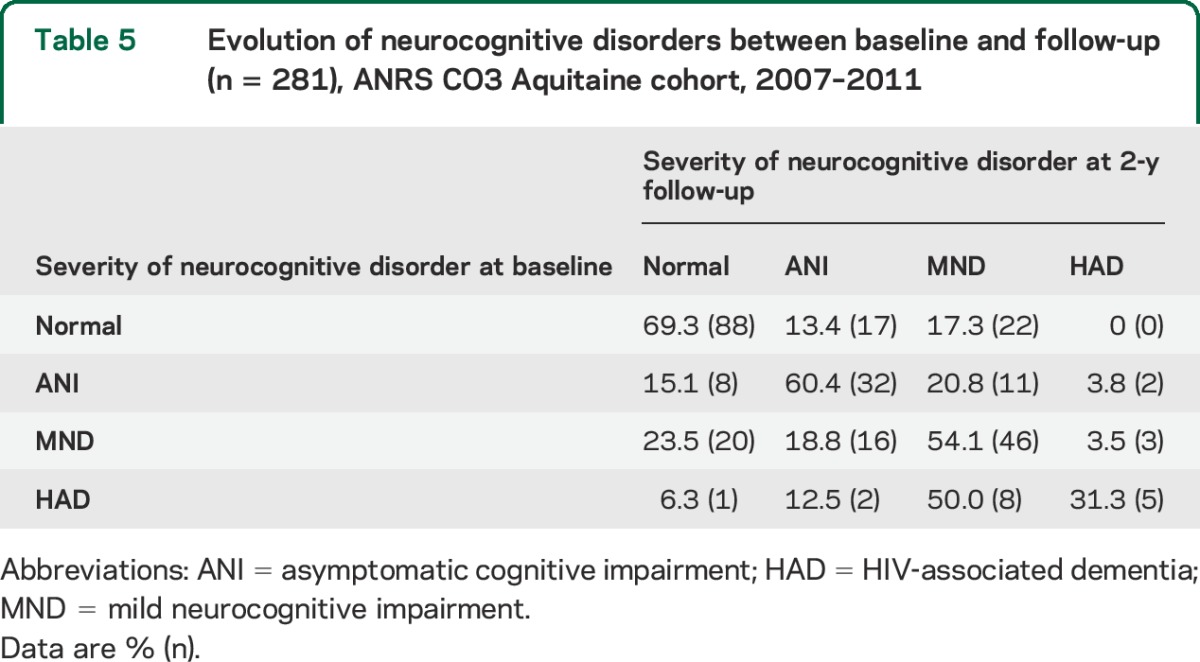
The association of glycemia status at baseline with severity of NCI at follow-up assessment was not significant (p = 0.64) (table e-3). Table 5 shows the evolution of NCI status between baseline and follow-up. Among patients with HIV-associated dementia at baseline, almost 70% had less severe NCI at follow-up. Similarly, 40% of patients with mild neurocognitive disorder at baseline had either normal cognition or asymptomatic cognitive impairment at follow-up.
Table 5.
Evolution of neurocognitive disorders between baseline and follow-up (n = 281), ANRS CO3 Aquitaine cohort, 2007–2011
We performed a series of sensitivity analyses to assess the robustness of our main findings (results not shown). We assessed whether the associations were driven by participants with HIV-associated dementia but excluding them did not modify the findings. Additional analyses restricted to men or excluding patients with obesity (n = 8) did not change the findings. We also performed weighted analyses by computing inverse probability weights under the missing-at-random assumption to assess the impact of dropouts on the longitudinal findings and we also tested extreme scenarios for those who dropped out, and the results remained robust to these scenarios (results not shown).
DISCUSSION
In this prospective cohort study of adults with HIV-1 infection in current clinical care in France, cognitive performances were lower in participants with diabetes. Lower performances in patients with diabetes were observed in several tests involving memory, executive functions, attention, psychomotor speed, language, and manual dexterity. They also presented over 2-year follow-up with a slightly greater worsening in a limited number of tests involving executive and memory functioning. Participants with prediabetes had worse performances compared with those who had normal glycemia in several tests in both cross-sectional and longitudinal analyses. Our findings suggest evidence for cognitive decline associated with diabetes and prediabetes independently of HIV-related factors, age, and other cardiovascular risk factors. The absence of association between other MetS components of the MetS with cognition suggests that diabetes has a specific effect on brain health. Our cohort is the first to date to investigate the consequences of prediabetes, a precursor of type 2 diabetes, and diabetes on cognitive aging measured longitudinally through a comprehensive cognitive test battery in individuals infected with HIV.
There are only a few reports on diabetes and cognition in patients with HIV, and their results are not always consistent. In a study that included 130 Americans living with HIV, diabetes was significantly associated with NCI23 in those aged 55 years and older.17 Consistent findings were reported in a study that showed an association of diabetes with prevalent dementia.15 By contrast, in the SMART (Strategies for Management of Antiretroviral Therapy) international study, diabetes was not significantly related to 2 indicators of neurocognitive disorders (a composite z score and NCI categories).8 The 292 participants were younger than those in COGLOC (median age 40 vs 47 years), prevalence of diabetes was lower (3.8% vs 10% in COGLOC), and patients with prediabetes were not considered as a separate category. Therefore, the SMART study might not have been powered enough to demonstrate an effect of diabetes on cognition.
Mechanisms by which diabetes could contribute to brain alterations are disputed. They include vascular damage, inadequate cerebral circulation that could lead to silent ischemic damage. More recent studies have focused on the possible role of insulin because of the neurotrophic properties of insulin in the brain. Hyperglycemia and hyperinsulinemia may accelerate brain aging and neurodegeneration by inducing tau hyperphosphorylation and amyloid oligomerization, as well as by leading to widespread brain microangiopathy.24,25 Other mechanisms specific to HIV could also act independently or in synergy. HIV could indeed contribute to the disruption of the blood–brain barrier since HIV proteins alter the tight junctions of the barrier, potentially increasing brain exposure to higher levels of glucose or other damaging molecules.15,26,27
The strengths of our study include its size, the multiple confounding factors controlled for in the multivariable analyses, and the longitudinal design. Our analyses assessed the impact of prediabetes and diabetes on both cognitive performances and NCI severity. The results are more consistent when cognitive scores are the outcomes of interests but the statistical effects observed might not be already clinically relevant for the participants. However, our findings also show the variability of NCI categories over 2-year follow-up, which could suggest that the current definitions of NCI are somewhat free-floating. Their value can be perceived as more conceptual than operational and therefore could mask the wide range of cognitive states encountered in aging patients living with HIV and in care.
This study has some limitations. The relatively short follow-up (2 years) prevents study of the long-term impact of diabetes on cognitive aging. In addition, the modeling of cognitive change from 2 measurements does not allow disentangling retest and aging effects and this might have underestimated the true cognitive change. Among the MetS components, we did not collect data on waist circumference or waist-hip ratio but the analyses were adjusted for BMI, which is a proxy measure of both potential confounders. Attrition in this cohort was related to cognitive performances but not to diabetic status, and the series of sensitivity analyses undertaken does not suggest that selection of participants could have biased our findings. Among diabetics, we did not collect data on insulin or glycated hemoglobin that could allow characterizing them regarding metabolic disturbances and better explain the impact of diabetes on cognition. Our data did not suggest that glycemia and cognitive levels were statistically related (results not shown), but the study was not sufficiently powered to study this association more specifically in patients with diabetes.
The absence of an HIV-uninfected control group prevents assessing whether the adverse effect of diabetes on brain functions is exacerbated or anticipated in patients infected with HIV compared with controls. Very few studies have investigated the impact of diabetes on cognitive health in middle-aged adults without HIV infection.13,14 In the Whitehall II study,13 analyses were performed when participants were 56 years on average (i.e., more than 10 years older on average than in our study): prevalence of diabetes was lower (5% vs 10%) and an association was found only between diabetes and lower memory performances. In the ARIC (Atherosclerosis Risk in Communities) study,28 diabetes (present in 14% of participants) or prediabetes, measured when participants were 57 years old on average, were related to both lower cognitive performances cross-sectionally and greater cognitive decline in the next 20 years.
Our longitudinal observations show that diabetes and, to a lesser extent, prediabetes were associated with worsening of cognitive performances, as early as middle age in a cohort of people living with HIV and well controlled for their infection. Because there is also evidence for an increased prevalence of diabetes,29 taken together, these results highlight the need for more stringent prevention, screening, and treatment of diabetes in the HIV-infected population. Further prospective cohort studies with long follow-up and monitoring of both changes in brain function (cognitive tests) and structure through large-scale cerebral imaging should aim at determining the mechanisms of diabetes in HIV-infected populations as well as its consequences on healthy brain aging.
Supplementary Material
ACKNOWLEDGMENT
The authors thank M.J. Blaizeau, M. Decoin, C. Hannapier, E. Lenaud A. Pougetoux, S. Delveaux, C. D'Ivernois, J. Delaune, O. Leleux, B. Uwamaliya-Nziyumvira, X. Sicard, S. Geffard, S. Lawson-Ayayi, I. Louis, G. Palmer, V. Conte, D. Touchard, J. Leray, and A. Frosch for contributing to data collection or providing technical support.
GLOSSARY
- ANRS
Agence Nationale de Recherches sur le Sida et les Hépatites Virales
- ART
antiretroviral treatment
- BMI
body mass index
- CES-D
Center for Epidemiologic Studies–Depression
- ICD-10
International Classification of Diseases, Tenth Revision
- MetS
metabolic syndrome
- NCI
neurocognitive impairment
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Noelle Bernard, Charles Cazanave, Carine Greib, Pierre Duffau, Michel Dupon, Hervé Dutronc, Hervé Fleury, Mojgan Hessamfar, Denis Lacoste, Marie-Edith Lafon, Estabaliz Lazaro, Denis Malvy, Patrick Mercié, Jean-François Moreau, Isabelle Pellegrin, Jean Luc Pellegrin, Thierry Pistone, Marie-Catherine Receveur, Pascale Trimoulet, Marie-Anne Vandenhende, and Jean-François Viallard
AUTHOR CONTRIBUTIONS
Carole Dufouil: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis, study supervision. Laura Richert: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision. Rodolphe Thiébaut: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Mathias Bruyand: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, study supervision. Hélène Amieva: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data. Frédéric Dauchy: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Jean-François Dartigues: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval. Didier Neau: study concept or design, accepts responsibility for conduct of research and will give final approval, acquisition of data. Philippe Morlat: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Patrick Dehail: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. François Dabis: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Fabrice Bonnet: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, contribution of vital reagents/tools/patients. Geneviève Chêne: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision, obtaining funding.
STUDY FUNDING
This work is supported by a grant of France Recherche Nord&Sud Sida-HIV Hépatites (ANRS) within the Coordinated Action no. 7 (AC7). Other sources of support include the Bordeaux University Hospital through the COREVIH Aquitaine, Inserm (U897), and the Bordeaux School of Public Health (ISPED).
DISCLOSURE
C. Dufouil, L. Richert, R. Thiébaut, M. Bruyand, H. Amieva, and F. Dauchy report no disclosures relevant to the manuscript. J. Dartigues received grant and personal fees from Novartis and IPSEN for activities outside the submitted work. D. Neau, P. Morlat, P. Dehail, and F. Dabis report no disclosures relevant to the manuscript. F. Bonnet received honoraria from Bristol-Myers Squibb, Gilead, Janssen Cilag, MSD, and ViiV Healthcare for activities outside the submitted work. G. Chêne reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bonnet F, Amieva H, Marquant F, et al. Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS 2013;27:391–400. [DOI] [PubMed] [Google Scholar]
- 2.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol 2011;17:176–183. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010;24:1243–1250. [DOI] [PubMed] [Google Scholar]
- 5.Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis 2013;13:976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamoto BK, Valcour VG, Kallianpur K, et al. Impact of cerebrovascular disease on cognitive function in HIV-infected patients. J Acquir Immune Defic Syndr 2011;57:e66–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valcour V, Maki P, Bacchetti P, et al. Insulin resistance and cognition among HIV-infected and HIV-uninfected adult women: the Women's Interagency HIV Study. AIDS Res Hum Retroviruses 2012;28:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright EJ, Grund B, Robertson K, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology 2010;75:864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobieszczyk ME, Hoover DR, Anastos K, et al. Prevalence and predictors of metabolic syndrome among HIV-infected and HIV-uninfected women in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr 2008;48:272–280. [DOI] [PubMed] [Google Scholar]
- 10.Data Collection on Adverse Events of Anti HIVDSG, Sabin CA, d'Arminio Monforte A, et al. Changes over time in risk factors for cardiovascular disease and use of lipid-lowering drugs in HIV-infected individuals and impact on myocardial infarction. Clin Infect Dis 2008;46:1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001;56:42–48. [DOI] [PubMed] [Google Scholar]
- 12.Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology 2004;63:1882–1891. [DOI] [PubMed] [Google Scholar]
- 13.Kumari M, Marmot M. Diabetes and cognitive function in a middle-aged cohort: findings from the Whitehall II study. Neurology 2005;65:1597–1603. [DOI] [PubMed] [Google Scholar]
- 14.Creavin ST, Gallacher J, Bayer A, Fish M, Ebrahim S, Ben-Shlomo Y. Metabolic syndrome, diabetes, poor cognition, and dementia in the Caerphilly prospective study. J Alzheimers Dis 2012;28:931–939. [DOI] [PubMed] [Google Scholar]
- 15.Valcour VG, Shikuma CM, Shiramizu BT, et al. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr 2005;38:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009;73:1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012;78:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiebaut R, Morlat P, Jacqmin-Gadda H, et al. Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. Groupe d'Epidemiologie du SIDA en Aquitaine (GECSA). AIDS 2000;14:971–978. [DOI] [PubMed] [Google Scholar]
- 19.Richert L, Dehail P, Mercie P, et al. High frequency of poor locomotor performance in HIV-infected patients. AIDS 2011;25:797–805. [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 22.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004;26:307–319. [DOI] [PubMed] [Google Scholar]
- 24.Biessels GJ. Unraveling the puzzle of dementia risk in diabetes. J Diabetes Complications 2012;26:359–360. [DOI] [PubMed] [Google Scholar]
- 25.Hassing LB, Hofer SM, Nilsson SE, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing 2004;33:355–361. [DOI] [PubMed] [Google Scholar]
- 26.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006;7:41–53. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz AA, Berman JW, Lyman WD. The role of the blood-brain barrier in HIV infection of the central nervous system. Adv Neuroimmunol 1994;4:249–256. [DOI] [PubMed] [Google Scholar]
- 28.Rawlings AM, Sharrett AR, Schneider AL, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 2014;161:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012;26:303–314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


