Summary
Backround
The aim of the study was to assess the impairment of the selected white matter tracts within normal appearing white matter (NAWM) in multiple sclerosis (MS) patients using diffusion tensor imaging (DTI).
Material/Methods
Thirty-six patients (mean age 33.4 yrs) with clinically definite, relapsing-remitting MS and mild disability (EDSS – Expanded Disability Status Scale 1–3.5) and 16 control subjects (mean age 34.4 yrs) were enrolled in the study. DTI examinations were performed on a 1.5T MR scanner. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) values were obtained with a small ROI method in several white matter tracts within NAWM including: the middle cerebellar peduncles (MCP), the inferior longitudinal fasciculi (ILF), inferior frontooccipital fasciculi (IFOF), genu (GCC) and splenium of the corpus callosum (SCC), posterior limbs of the internal capsules (PLIC), superior longitudinal fasciculi (SLF) and posterior cingula (CG). There were no demyelinative lesions within the ROIs in any of the patients.
Results
A significant decrease in FA was found in MS patients in both the ILFs and IFOFs (p<0.001) and in the left MCP and right SLF (p<0.05), compared to the normal subjects. There were no significant differences in FA values in the remaining evaluated ROIs, between MS patients and the control group. A significant increase in ADC (p<0.05) was found only in the right PLIC and the right SLF in MS subjects, compared to the control group.
Conclusions
The FA values could be a noninvasive neuroimaging biomarker for assessing the microstructural changes within NAWM tracts in MS patients.
MeSH Keywords: Diffusion Tensor Imaging, Magnetic Resonance Imaging, Multiple Sclerosis
Background
Magnetic resonance imaging (MRI) is the imaging technique of choice in establishing the diagnosis of multiple sclerosis (MS) as well as in disease monitoring. However, histopathological studies have proven that white matter (WM) of the brain is often damaged in MS, even in areas that appear normal on standard MRI sequences [1–5]. Microscopic abnormalities within so-called normal appearing white matter (NAWM), undetectable by conventional MR imaging techniques, have drawn much attention in the recent years, as they allow a better insight in the background of MS as well as commonly stated divergences between the radiological findings and clinical manifestation of the disease.
Diffusion tensor imaging (DTI) is an advanced MR technique based on the detection of micron scale water molecules movements in the examined tissues. In addition to diffusion weighted imaging (DWI) which is already widely used in clinical practice, DTI allows not only investigation of the magnitude of water diffusion in the tissues but also its direction. This is important, especially in highly organised tissues with the preferred orientation such as central nervous system (CNS) white matter, where water molecules motion occurs predominantly in one direction, along the long axis of the fibers. Hypothetically, even minor WM architecture disruption can interfere with the dominant direction of movement of water particles, and therefore may be detected by means of DTI, making it an extremely sensitive diagnostic tool. DTI measures that are most often used to quantify diffusion disturbances include fractional anisotropy (FA) and apparent diffusion coefficient (ADC) or mean diffusivity (MD). FA reflects the degree of directionality of water molecule motion and varies between 0 (for isotropic diffusion – equal in all directions, for example within fluid spaces) and 1 (diffusion occurring in only one direction – complete anisotropic diffusion). MD represents the average diffusion in a voxel volume.
The aim of this study was to assess the FA and ADC values of the selected white matter tracts within normal appearing white matter (NAWM) in mildly impaired MS patients using the DTI technique.
Material and Methods
Population
The study included thirty-six patients (23 women and 13 men, mean age 33.4 yrs, range from 24 to 56 years) with clinically definite MS according to McDonald’s diagnostic criteria [6] and sixteen control subjects (3 women and 13 men, mean age 34.4 yrs, range from 26 to 43 years) with no clinical evidence of neurological disease and with normal conventional brain MR imaging.
The inclusion criteria for MS patients were: relapsing-remitting course of the disease and mild disability, assessed with the use of Expanded Disability Status Scale (EDSS –score up to 3.5). The patients were followed up at the Department of Neurology of our University.
The study followed ethical rules and was approved by the Wrocław University Ethics Committee. All participants gave their informed consent before entering the study.
MR imaging acquisition
The imaging examinations were performed on 1.5T MRI unit (Signa Hdx, GE Medical Systems) using a sixteen-channel coil dedicated for head and spine imaging. All patients were submitted to the conventional MR imaging protocol, including sagittal and coronal T2 FRFSE sequences (5-mm slice thickness, 1-mm gap, TR/TE 3900/81, field of view 240×240 mm, matrix 384×224 mm, number of excitations: 2 and 5-mm slice thickness, 1-mm gap, TR/TE 4940/91, field of view 240×180 mm, matrix 384×256 mm, number of excitations: 2), axial T1 SE (5-mm slice thickness, 1-mm gap, TR/TE 500/8, field of view 240×180 mm, matrix 320×192 mm, number of excitations: 2), T2 FSE (5-mm slice thickness, 1-mm gap, TR/TE 5600/93.5, field of view 240×240 mm, matrix 320×320 mm, number of excitations: 1.5) and FLAIR sequences (5-mm slice thickness, 1-mm gap, TR/TE 8000/104.8, field of view 240×240 mm, matrix 288×288 mm, number of excitations: 1), axial DWI SE/EPI sequence (5-mm slice thickness, no gap, TR/TE 8000/81.2, field of view 260×208 mm, matrix 128×128 mm, number of excitations: 1) and gadolinium-enhanced 3D-FSPGR sequence (2-mm slice thickness, no gap, TR/TE 9.2/4.2, field of view 240×180 mm, matrix 320×192 mm, number of excitations: 1).
DTI acquisitions were performed using an axial single-shot spin-echo echo-planar imaging (SE/EPI) sequence along 25 different diffusion-encoding directions. For each direction, two b-values were used: 0 and 1000s/mm2 (4-mm slice thickness, no gap, TR/TE 8500/100.8, field of view of 260×208 mm, matrix 128×128 mm and number of excitations: 2). The acquisition time of DTI in each participant was 7.31 min. All patients and controls were fully cooperative, as a result no motion artefacts occurred.
Image post-processing, analysis and statistics
DTI data were transferred to GE Advantage Workstation 4.6 and post-processed using Ready View software, provided by the manufacturer. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) values were obtained by placing round-shaped ROIs of 51 mm2 area in FA and ADC maps in selected white matter tracts within NAWM including:
middle cerebellar peduncles (MCP) (Figure 1);
inferior longitudinal fasciculi (ILF) (Figure 2);
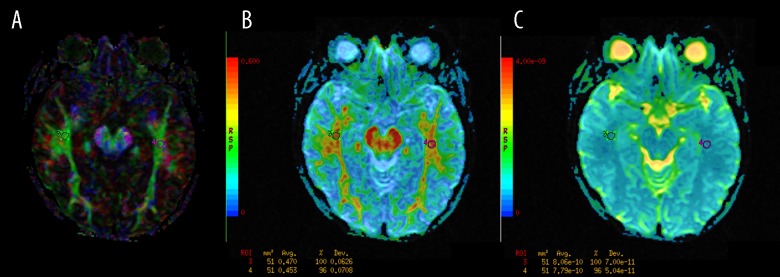
inferior frontooccipital fasciculi (IFOF) (Figure 3);
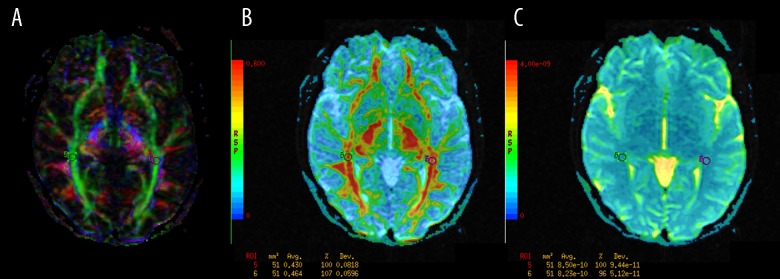
genu (GCC) and splenium (SCC) of the corpus callosum (Figure 4);
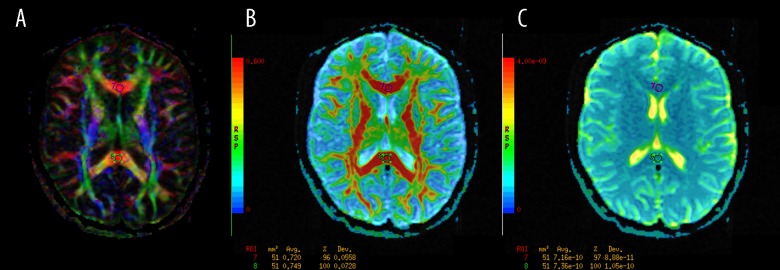
posterior limbs of the internal capsules (PLIC) (Figure 5);
superior longitudinal fasciculi (SLF) (Figure 6);
posterior cingula (CG) (Figure 7).
Figure 1.
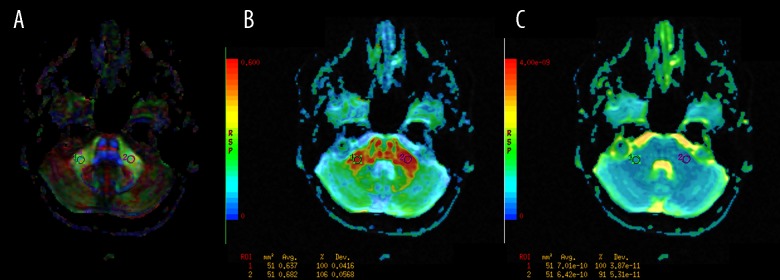
The regions of interest (ROI) positioning in the middle cerebellar peduncles (MCP). (A): Color-coded directional map weighted with FA; (B): FA map; (C): ADC map.
Figure 2.
The regions of interest (ROI) positioning in the inferior longitudinal fasciculi (ILF). (A): Color-coded directional map weighted with FA; (B): FA map; (C): ADC map.
Figure 3.
The regions of interest (ROI) positioning in the inferior frontooccipital fasciculi (IFOF). (A): Color-coded directional map weighted with FA; (B): FA map; (C): ADC map.
Figure 4.
The regions of interest (ROI) positioning in the genu (GCC) and splenium (SCC) of the corpus callosum (A): Color-coded directional map weighted with FA; (B): FA map; (C): ADC map.
Figure 5.
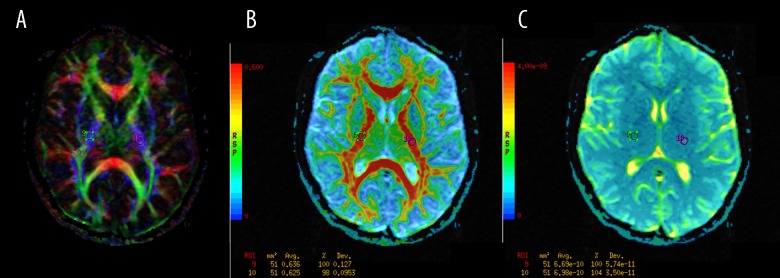
The regions of interest (ROI) positioning in the posterior limbs of the internal capsules (PLIC). (A) Color-coded directional map weighted with FA; (B): FA map; (C): ADC map.
Figure 6.
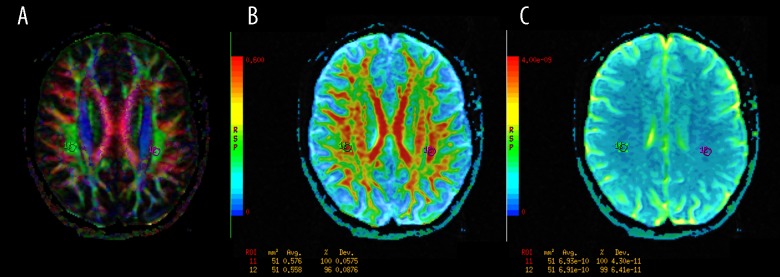
The regions of interest (ROI) positioning in the superior longitudinal fasciculi (SLF). (A): Color-coded directional map weighted with FA; (B): FA map; (C): ADC map.
Figure 7.
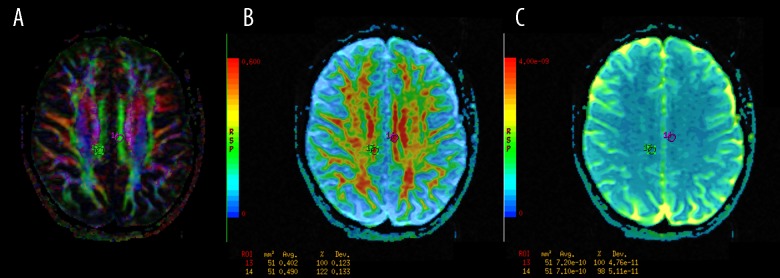
The regions of interest (ROI) positioning in the posterior cingula (CG). (A): Color-coded directional map weighted with FA; (B): FA map; (C): ADC map.
In symmetrical fibre bundles one ROI was placed on each side, as shown in Figures 1–3 and 5–7. Simultaneously, plain MR images were evaluated to ensure that there were no demyelinative lesions within or nearby the ROIs in any of the patients included in the study. The total number of 14 ROIs were placed in each DTI study, all by the same observer.
Statistical analysis was performed by employing the Statistica10 software package. Comparisons between the patients and control group of ROIs were performed using a t-test. Any P value lower than 0.05 was considered statistically significant.
Results
Table 1 presents the comparison of averaged FA values measured in selected white matter tracts between patients and controls. Table 2 shows comparison of averaged ADC values measured in the same locations (ROIs) between patients and controls.
Table 1.
Comnparison of averaged FA values measured in selected white matter tracts between patients and controls. Statistically significant changes (p<0.05) are highlighted in bold.
| FA mean values | MCP | ILF | IFOF | CC | PLIC | SLF | CG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R ROI 1 | L ROI 2 | R ROI 3 | L ROI 4 | R ROI 5 | L ROI 6 | GCC ROI 7 | SCC ROI 8 | R ROI 9 | L ROI 10 | R ROI 11 | L ROI 12 | R ROI 13 | L ROI 14 | |
| Patients | 0.687 | 0.712 | 0.543 | 0.592 | 0.592 | 0.564 | 0.771 | 0.792 | 0.652 | 0.680 | 0.591 | 0.627 | 0.570 | 0.623 |
| Controls | 0.703 | 0.750 | 0.619 | 0.634 | 0.634 | 0.652 | 0.760 | 0.830 | 0.670 | 0.685 | 0.646 | 0.617 | 0.600 | 0.641 |
| p value | 0.388 | 0.021 | 0.000 | 0.046 | 0.000 | 0.000 | 0.194 | 0.077 | 0.196 | 0.686 | 0.003 | 0.654 | 0.172 | 0.395 |
FA – fractional antisotropy; MCP – the middle cerebellar peduncles; ILF – the inferior lognitudinal fasciculi; IFOF – inferior frontooccipital fasciculi; CC – corpus callosum; GCC – genu of the corpus callosum; SCC – splenium of the corpus callosum; PLIC – posterior limbs of the internal capsules; SLF – superior lognitudinal fasciculi; CG – posterior cingula.
Table 2.
Comnparison of averaged ADC values measured in selected white matter tracts between patients and controls. Statistically significant changes (p<0.05) are highlighted in bold.
| FA mean values [×10−2 mm/s2] | MCP | ILF | IFOF | CC | PLIC | SLF | CG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R ROI 1 | L ROI 2 | R ROI 3 | L ROI 4 | R ROI 5 | L ROI 6 | GCC ROI 7 | SCC ROI 8 | R ROI 9 | L ROI 10 | R ROI 11 | L ROI 12 | R ROI 13 | L ROI 14 | |
| Patients | 0.686 | 0.874 | 0.879 | 0.799 | 0.881 | 0.827 | 0.845 | 0.789 | 0.730 | 0.715 | 0.740 | 0.698 | 0.753 | 0.750 |
| Controls | 0.690 | 0.688 | 0.840 | 0.790 | 0.859 | 0.818 | 0.821 | 0.761 | 0.693 | 0.717 | 0.696 | 0.683 | 0.739 | 0.734 |
| p value | 0.772 | 0.298 | 0.126 | 0.688 | 0.453 | 0.692 | 0.203 | 0.225 | 0.001 | 0.840 | 0.002 | 0.335 | 0.364 | 0.204 |
ADC – apparent diffusion coefficient; MCP – the middle cerebellar peduncles; ILF – the inferior lognitudinal fasciculi; IFOF – inferior frontooccipital fasciculi; CC – corpus callosum; GCC – genu of the corpus callosum; SCC – splenium of the corpus callosum; PLIC – posterior limbs of the internal capsules; SLF – superior lognitudinal fasciculi; CG – posterior cingula.
As can be seen in Table 1, lower FA values in the MS patients compared to the control subjects were observed in all examined white matter tracts except for the left SLF. However significant decrease in FA was found in MS patients only in both ILFs and IFOFs (P<0.001) and in the left MCP and right SLF (P<0.05). Significant increase in ADC (P<0.05) was found only in the right PLIC and the right SLF in MS subjects, compared to the control group.
There were no significant differences in FA and ADC values according to age and gender in both patients and controls.
Discussion
Multiple sclerosis is a disease causing multifocal damage to the central nervous system, involving an inflammatory-demyelinative process as well as axonal loss. Typical disseminated lesions in the WM can be visualized in standard MR imaging. However, previous studies of patients with MS found only a modest correlation between the MRI-visible changes and symptoms of neurological deficit or clinical course of the disease [4,7–10]. The reason for this paradox may be associated with the presence of additional abnormalities in the so-called normal-appearing white matter [4,10,11]. Two mechanisms have been proposed as the principal causes of NAWM damage in MS patients: microscopic demyelinating lesions [12,13] and neurodegeneration of axons and neurons [4,5,12,13]. Early demyelinating process may result in axonal degeneration due to the lack of trophic support from oligodendroglia and myelin [1]. Acute inflammatory damage within WM plaques leads to axonal transection and secondary Wallerian-like degeneration in the fibers emerging from plaques [13–17]. According to different studies, this process is most prominent in the peri-plaque NAWM [1,4,18]. In the NAWM distant from plaques, pronounced microglial activation without axonal pathology or myelin loss was reported [4,5]. It is interesting to note that microglial activation was also observed in subcortical NAWM in the neighborhood of demyelinating cortical lesions [4]. The clinical significance of NAWM abnormalities is still the subject of debate, yet there are strong indications that they show relationships with physical disability and cognitive dysfunction in multiple sclerosis patients [19].
Several previous studies employing the DTI method have shown the involvement of NAWM in MS patients [20–29]. Most researchers investigated NAWM damage according to plaque location, finding reduced FA and sometimes increased ADC values in peri-plaque NAWM and, to varying degrees, in NAWM remote from plaques.
In the presented study, we investigated the integrity of the selected white matter tracts within non-lesional WM in patients with MS using the DTI technique. We found lower FA values in MS patients compared to the control subjects in almost all examined fiber bundles. However, a significant decrease in FA was detected in MS patients only in both ILFs and IFOFs (P<0.001) and in the left MCP and right SLF (P<0.05). Significant increase in ADC (P<0.05) was found only in the right PLIC and the right SLF in MS subjects, compared to the control group.
In previous studies diffusion differences between control subjects and MS patients were detected in the corpus callosum [19,30–32], corticospinal or pyramidal tracts [19,30,31], inferior longitudinal fasciculus [19,31], fornix [33], cingulum [33], superior longitudinal fasciculus [33] and uncinate fasciculus [33]. These studies differed in terms of DTI data post-processing and analysis methods as well as in sampling of MS patients’ groups. In a recent study, using an optimized voxel based analysis (VBA) approach, Van Hecke et al. [34] found a significant decrease in FA values in moderately impaired MS patients (EDSS 4–7), in the inferior longitudinal fasciculus, capsula externa, and forceps major of the corpus callosum. Researchers did not observe significant differences in FA between the control group and mildly affected MS patients (EDSS 1–3). In opposition we found a significant decrease in FA value in mildly impaired (EDSS 1–3.5) MS patients in some of the investigated WM tracts, particularly the inferior longitudinal fasciculi (ILF) and inferior fronto-occipital fasciculi (IFOF). ILF is a direct pathway between the occipital cortex and temporal lobe, whereas the IFOF is a fiber bundle connecting the occipital cortex to the frontal brain. These two tracts spatially overlap along a part of their pathways and probably share a similar function. According to Chanraud et al., ILF is functionally correlated with thought disorders, visual emotion and cognitive impairments [35]. Interestingly, Dineen et al., in a study investigating the correlations between FA values and cognitive dysfunction in MS patients found that FA values in ILF correlated with the Paced Auditory Serial Addition Test (PASAT) scores [19]. PASAT is a commonly used experimental paradigm to evaluate sustained attention, working memory and speed of information processing in MS [34].
The significant decrease of FA value in the selected WM tracts was not connected with simultaneous increase in ADC in our study. This finding may indicate that those two indices do not necessarily reflect the same microstructural changes. Moll et al. [4] investigated pathology-imaging correlations in multiple sclerosis NAWM through the examination of autopsy brain tissue with MRI, including MTR (magnetization transfer ratio) and DTI measures. The study results suggest that in non-lesional WM increase in MD (ADC) may be indicative of axonal loss, whereas decrease in FA may signal microglial activation [4]. However, in DTI studies performed in vivo it is impossible to connect observed changes in DTI measures to the exact micro-structural pathophysiology. Despite its high sensitivity, the DTI technique is characterized by a low specificity of the underlying changes in WM tissue organization [34].
It is of paramount importance to detect microstructural alterations of NAWM in MS patients, since it may contribute to the prognosis and the course of the disease. We believe that examination of the specific fibre bundles instead of random NAWM location could help to better connect DTI study findings with patients’ symptoms, making it more useful in clinical practice. Further studies correlating DTI measures obtained from the selected WM tracts with clinical status (including cognitive performance) of the MS patients are warranted and we plan to continue investigation in this field.
Conclusions
FA values could be a non-invasive neuroimaging biomarker for assessing the microstructural changes within NAWM tracts in patients with multiple sclerosis. In the future, quantitative evaluation of impairment of specific WM tracts by means of DTI measures, could help in better understanding the MS pathology underlying subtle clinical deficits and diversity of the course of the disease.
Footnotes
Source of support: Supported by Wrocław Medical University Grant ST-869
References
- 1.Kornek B, Storch MK, Weissert R, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–76. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen IJ, McKeown SR. A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci. 1979;41:81–91. doi: 10.1016/0022-510x(79)90142-4. [DOI] [PubMed] [Google Scholar]
- 3.Evangelou N, Esiri MM, Smith S, et al. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol. 2000;47:391–95. [PubMed] [Google Scholar]
- 4.Moll NM, Riietsch AM, Thomas S, et al. Multiple sclerosis NAWM: pathology-imaging correlations. Ann Neurol. 2011;70:764–73. doi: 10.1002/ana.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fillippi M, Rocca M, Barkhof F, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11:349–60. doi: 10.1016/S1474-4422(12)70003-0. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain. 1998;121:3–24. doi: 10.1093/brain/121.1.3. [DOI] [PubMed] [Google Scholar]
- 8.McDonald WI, Miller DH, Barnes D. The pathological evolution of multiple sclerosis. Neuropathol Appl Neurobiol. 1992;18:319–34. doi: 10.1111/j.1365-2990.1992.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson VL, Miller DH, Rovaris M, et al. Primary and transitional progressive MS: a clinical and MRI cross-sectional study. Neurology. 1999;52:839–45. doi: 10.1212/wnl.52.4.839. [DOI] [PubMed] [Google Scholar]
- 10.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. 2002;15:239–45. doi: 10.1097/00019052-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Vrenken H, Geurts JJ, Knol DL, et al. Normal-appearing white matter changes vary with distance to lesions in multiple sclerosis. Am J Neuroradiol. 2006;27:2005–11. [PMC free article] [PubMed] [Google Scholar]
- 12.Raz E, Cercignani M, Sbardella E, et al. Clinically isolated syndrome suggestive of multiple sclerosis: voxelwise regional investigation of white and gray matter. Radiology. 2010;254:227–34. doi: 10.1148/radiol.2541090817. [DOI] [PubMed] [Google Scholar]
- 13.Allen IV, McQuaid S, Mirakhur M, Nevin G. Pathological abnormalities in the normal-appearing white matter in multiple sclerosis. Neurol Sci. 2001;22:141–44. doi: 10.1007/s100720170012. [DOI] [PubMed] [Google Scholar]
- 14.Evangelou N, Konz D, Esiri MM, et al. Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain. 2000;123:1845–49. doi: 10.1093/brain/123.9.1845. [DOI] [PubMed] [Google Scholar]
- 15.Evangelou N, Konz D, Esiri MM, et al. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain. 2001;124:1813–20. doi: 10.1093/brain/124.9.1813. [DOI] [PubMed] [Google Scholar]
- 16.Ciccarelli O, Werring DJ, Barker GJ, et al. A study of the mechanisms of normal-appearing white matter damage in multiple sclerosis using diffusion tensor imaging: evidence of Wallerian degeneration. J Neurol. 2003;250:287–92. doi: 10.1007/s00415-003-0992-5. [DOI] [PubMed] [Google Scholar]
- 17.Dziedzic T, Metz I, Dallenga T, et al. Wallerian degeneration: a major component of early axonal pathology in multiple sclerosis. Brain Pathol. 2010;20:976–85. doi: 10.1111/j.1750-3639.2010.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo AC, Jewells VL, Provenzale JM. Analysis of normal-appearing white matter in multiple sclerosis: comparison of diffusion tensorMR imaging and magnetization transfer imaging. Am J Neuroradiol. 2001;22:1893–900. [PMC free article] [PubMed] [Google Scholar]
- 19.Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132:239–49. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- 20.Guo AC, MacFall JR, Provenzale JM. Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal-appearing white matter. Radiology. 2002;222:729–36. doi: 10.1148/radiol.2223010311. [DOI] [PubMed] [Google Scholar]
- 21.Werring DJ, Clark CA, Barker GJ, et al. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–32. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 22.Waxman SG. Demyelinating diseases: new pathological insights, new therapeutic targets. N Engl J Med. 1998;338:323–25. doi: 10.1056/NEJM199801293380610. (editorial) [DOI] [PubMed] [Google Scholar]
- 23.Ciccarelli O, Werring DJ, Wheeler-Kingshott CAM, et al. Investigation of normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001;56:926–33. doi: 10.1212/wnl.56.7.926. [DOI] [PubMed] [Google Scholar]
- 24.Rocca MA, Iannucci G, Rovaris M, et al. Occult tissue damage in patients with primary progressive MS is independent of T2-visible lesions. J Neurol. 2003;250:456–60. doi: 10.1007/s00415-003-1024-1. [DOI] [PubMed] [Google Scholar]
- 25.Kealey SM, Kim Y, Provenzale JM. Redefinition of multiple sclerosis plaque size using diffusion tensor MRI. Am J Roentgenol. 2004;183:497–503. doi: 10.2214/ajr.183.2.1830497. [DOI] [PubMed] [Google Scholar]
- 26.Fillippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304–11. doi: 10.1212/wnl.56.3.304. [DOI] [PubMed] [Google Scholar]
- 27.Rocca MA, Filippi M. Diffusion tensor and magnetization transfer MR imaging of early-onset multiple sclerosis. Neurol Sci. 2004;25:344–45. doi: 10.1007/s10072-004-0338-9. [DOI] [PubMed] [Google Scholar]
- 28.Andrade RE, Gasparetto EL, Cruz LC, Jr, et al. Evaluation of white matter in patients with multiple sclerosis through diffusion tensor magnetic resonance imaging. Arq Neuropsiquiatr. 2007;65:561–64. doi: 10.1590/s0004-282x2007000400002. [DOI] [PubMed] [Google Scholar]
- 29.Ceccarelli A, Rocca M, Falini A, et al. Normal-appearing white and grey matter damage in MS. a volumetric and diffusion tensor MRI study at 3.0 Tesla. J Neurol. 2007;254:513–18. doi: 10.1007/s00415-006-0408-4. [DOI] [PubMed] [Google Scholar]
- 30.Mesaros S, Rocca MA, Riccitelli G, et al. Corpus callosum damage and cognitive dysfunction in benign MS. Hum Brain Mapp. 2009;30:2656–66. doi: 10.1002/hbm.20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roosendaal SD, Geurts JJ, Vrenken H, et al. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2008;44:1397–403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Cercignani M, Bozzali M, Iannucci G, et al. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875–83. doi: 10.1007/s00415-002-0752-y. [DOI] [PubMed] [Google Scholar]
- 33.Rocca MA, Valsasina P, Ceccarelli A, et al. Structural and functional MRI correlates of Stroop control in benign MS. Hum Brain Mapp. 2009;30:276–90. doi: 10.1002/hbm.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Hecke W, Nagels G, Leemans A, et al. Correlation of cognitive dysfunction and diffusion tensor MRI measures in patients with mild and moderate multiple sclerosis. J Magn Reson Imaging. 2010;31:1492–98. doi: 10.1002/jmri.22198. [DOI] [PubMed] [Google Scholar]
- 35.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20:209–25. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


