Abstract
Background
Asiaticoside is one of the main functional components of the natural plant Centella asiatica urban. Studies have reported it has several functions such as anti-depression and nerve cell protection. Asiaticoside can reduce the cerebral infarct size in acute focal cerebral ischemia in a mouse model and asiatic acid glycosides can significantly improve neurobehavioral scores. Currently, there is a lack of understanding of asiaticoside in regard to its neural protective mechanism in cerebral ischemia. This study aimed to solve this problem by using an ischemia-hypoxia cell model in vitro.
Material/Methods
An in vitro ischemia hypoxia cell model was successfully established by primary cultured newborn rat cortical neurons. After being treated by asiaticoside for 24 h, cell survival rate, lactate dehydrogenase release quantity, and B-cell lymphoma gene-2 (BCL-2), Bax, and caspase-3 protein expressions was detected.
Results
After 10 nmol/L or 100 nmol/L of asiaticoside were given to the cells, cell survival rate increased significantly and presented concentration dependence. Asiaticoside can reduce lactate dehydrogenase release. Lactate dehydrogenase release in model cells is gradually reduced with the increase of asiaticoside concentration. The lactate dehydrogenase release in asiaticoside 10 nmol/L group, asiaticoside 100 nmol/L group and ischemia hypoxia group were 26.75±1.05, 22.36±2.87 and 52.35±5.46%, respectively (p<0.05). It was also found that asiaticoside could modulate the expression of apoptotic factors, including bcl-2, Bax, and caspase-3.
Conclusions
Asiaticoside helps to protect in vitro ischemia hypoxia neurons. This nerve cell protection may be mediated by the BCL-2 protein.
MeSH Keywords: Abstracting and Indexing as Topic; Dilazep; Hypoxia-Ischemia, Brain
Background
Centella asiatica urban, also known as Centella asiatica, is a type of Zhuang medicine that originated from Guangxi, China. Clinically, Centella asiatica urban is used in the treatment of diseases such as epidemic cerebrospinal meningitis [1]. At present, there were more than 20 kinds of Centella worldwide, mainly distributed in tropical and subtropical regions. In 1971, Chasseaud et al. [2] identified asiaticoside as one of the main functional components of the natural plant Centella asiatica by chemical refining [1–3]. Since then, some experiments indicated that asiaticoside may have an anti-depressive effect and protect nerve cells [4]. Specifically, asiaticoside can reduce the infarct size, decrease brain water, and improve the corresponding neurobehavioral scores in acute focal cerebral ischemia in a mouse model [5]. On the other hand, foreign scholars reported that oral asiaticoside can promote nerve cells axon elongation in Wistar rats. Axon regeneration has a vital role in the recovery of neural function damage. These results contribute to promote asiaticoside clinical application in neurological diseases [6,7]. Understanding of asiaticoside’s neural protective mechanism in cerebral ischemia has been lacking. This study aimed to investigate the mechanism of the neuroprotective effect by use of an ischemia-hypoxia cell model in vitro.
Material and Methods
Reagents and instruments
Asiaticoside was bought from Modern Pharmaceutical Co., LTD (Shanghai, China); ECL chemiluminescence reagent kit was purchased from Amersham; CO2 incubator was purchased from BD Company; and B-cell lymphoma gene-2 (BCL-2) and β-actin monoclonal antibodies were from Santa Cruz (USA).
Establishment of the in vitro ischemia-hypoxia neurons cell model
Temporal and frontal cortexes were collected from the newborn SD rat brain in aseptic condition. The brain tissue was first cleaned by Neurobasal medium and cut up. After being screened with stainless steel mesh (5×5 mm), the tissue was digested by 0.25% trypsin for 10–20 min at 37°C. The digested tissue went through the stainless steel sieve again and the filtrate was centrifuged at 1200 r/min at 37°C for 5 min. After removing the nutrient solution, Neurobasal medium containing 10% fetal bovine serum (FBS) was used to make the single-cell suspension. The cells were then seeded in a 10-mu g/ml poly-L-lysine-coated dish and maintained in a 5% CO2 incubator. The medium was changed every other day. Four days later, Neurobasal medium with 10% FBS was removed and the cells were moved to the hypoxia (1%) incubator to simulate hypoxia-ischemia condition for 24 h.
Grouping
The cells were randomly divided into 4 groups: 1. Normal control, normal cultured cortical neurons; 2. Hypoxia ischemia group; 3. Asiaticoside treatment group (10 nmol/L) and asiaticoside intervention group (100 nmol/L). Cells in the hypoxia incubator had different concentrations of asiaticoside (soluble in the medium) added and were incubated with serum-free medium for 24 h.
MTT assay
We seeded 5×104 neurons into 96-well plates according to the experimental grouping. After cultivation for 48 h, 20-μl MTT solutions (5 g/L) was added to the cells and incubation was continued for 4 h. We added 200 μl dimethyl sulfoxide to the cells after removing the supernatant and were vibrated for 10 min at low speed to make the crystals dissolve. Absorbance at 570 nm was read by microplate reader.
Apoptosis detection
After being treated with asiaticoside for 24 h, the cells were fixed and stained according to the instructions of the In Situ Cell Death fluorescein kit. Apoptotic cells were quantified under a fluorescent microscope.
Lactate dehydrogenase (LDH) test
The supernatant was collected from different groups and the detection was performed according to the LDH kit instructions. The principle of the kit is enzyme-linked immunosorbent assay (ELISA). Firstly, the supernatant, standard sample, and detection antibodies were added to the capture antibody pre-coated wells. The wells were washed with phosphate-buffered saline (PBS) after incubation and colored by substrate 3,3′,5,5′-tetramethylbenzidine (TMB). Absorbance (OD value) at 450 nm wavelength was detected by microplate reader. The LDH release level from cells was expressed as the ratio against total LDH (cytosolic plus medium levels). All experiments were repeated at least 3 times.
Western blot
Total protein was separated by denaturing 15% SDS–polyacrylamide gel electrophoresis and transferred to PVDF membrane. After being incubated with primary antibody against bax (1:1 000), capase-3 (1:1000) or β-actin (1:2000), and washed with TBST, membrane detection was performed with ECL chemiluminescence. Antibody dilutions were 1:500 for BCL-2 and 1:2000 for β-actin. Protein levels were normalized to β-actin and changes were determined by use of a CM-2000B type biomedical image analysis system.
Statistical analysis
All statistical analyses were performed using SPSS17.0 software (Chicago, IL). Numerical data are presented as mean ± standard deviation (SD). Differences between means were analyzed using one-way ANOVA. P<0.05 was considered as statistically significant difference.
Results
The effect of asiaticoside on ischemia hypoxia cell survival
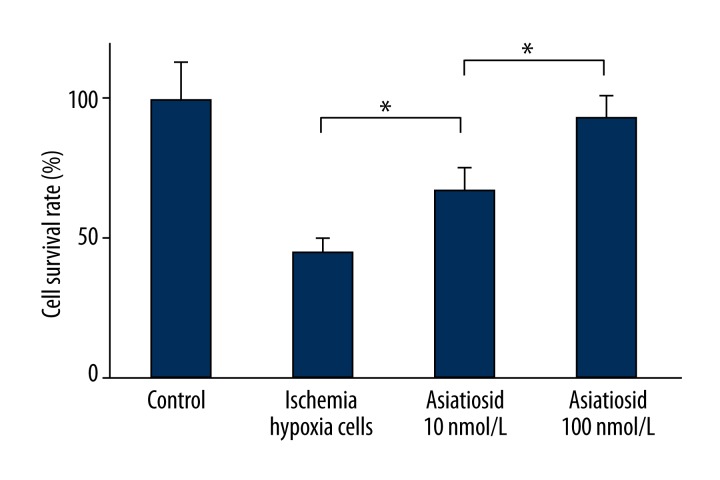
The MTT test results showed that, compared with ischemic hypoxia cell group, asiaticoside treatment group exhibited higher cell survival rate (P<0.05). Moreover, the survival rate significantly increased following the dose augmentation. This indicates that asiaticoside treatment obviously enhances the proliferation capacity of ischemia-hypoxia cells (Figure 1).
Figure 1.
MTT test showed ischemia-hypoxia cell survival rate.
The effect of asiaticoside on LDH release in the ischemia-hypoxia cell model
Lactate dehydrogenase release in model cells gradually reduced with the increase of asiaticoside concentration. The lactate dehydrogenase release in asiaticoside 10 nmol/L group, asiaticoside 100 nmol/L group, and ischemia-hypoxia group were 26.75±1.05, 22.36±2.87 and 52.35±5.46%, respectively (P<0.05).
The effect of asiaticoside on ischemia hypoxia cell apoptosis
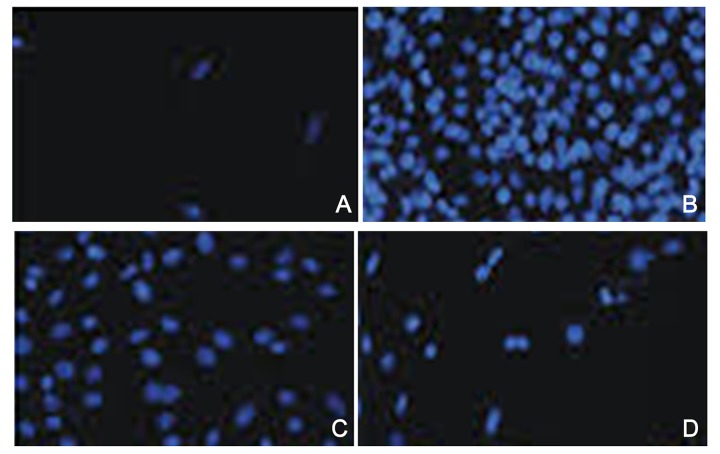
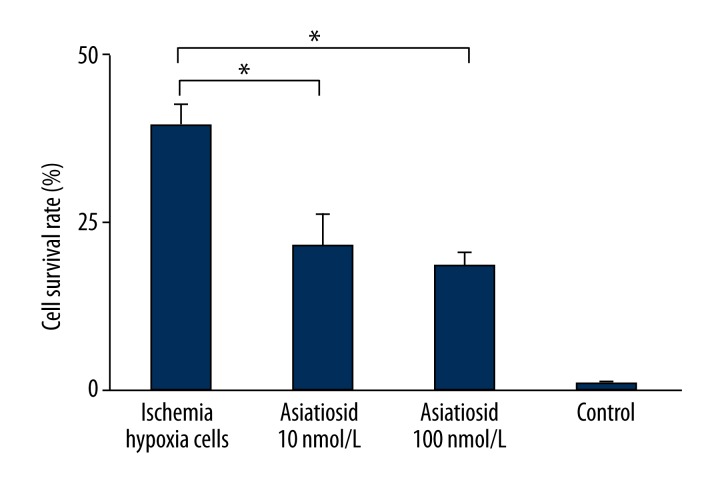
As shown in Figures 2 and 3, cell apoptosis rates in the ischemia-hypoxia group increased significantly to 39.87±2.87%. After different concentrations of asiaticoside treatment, the cell apoptosis rate reduced markedly, with gradually increased of asiaticoside concentration, at 22.53±4.98% in the asiaticoside treatment group (10 nmol/L) and 18.87±2.32% in the asiaticoside intervention group. The apoptosis rate in the normal group was 1.01±0.43%, and the data were statistically significant (P<0.05).
Figure 2.
The protective effect of asiaticoside on model cell apoptosis. (A) Control; (B) ischemia hypoxia group; (C) asiaticoside treatment group; (D) asiaticoside intervention group. The concentration of asiaticoside was 10 nM in C and 100 nM in D.
Figure 3.
Asiaticoside effect on model cell apoptosis.
The effect of asiaticoside on bcl-2, bax and caspase-3 protein expression
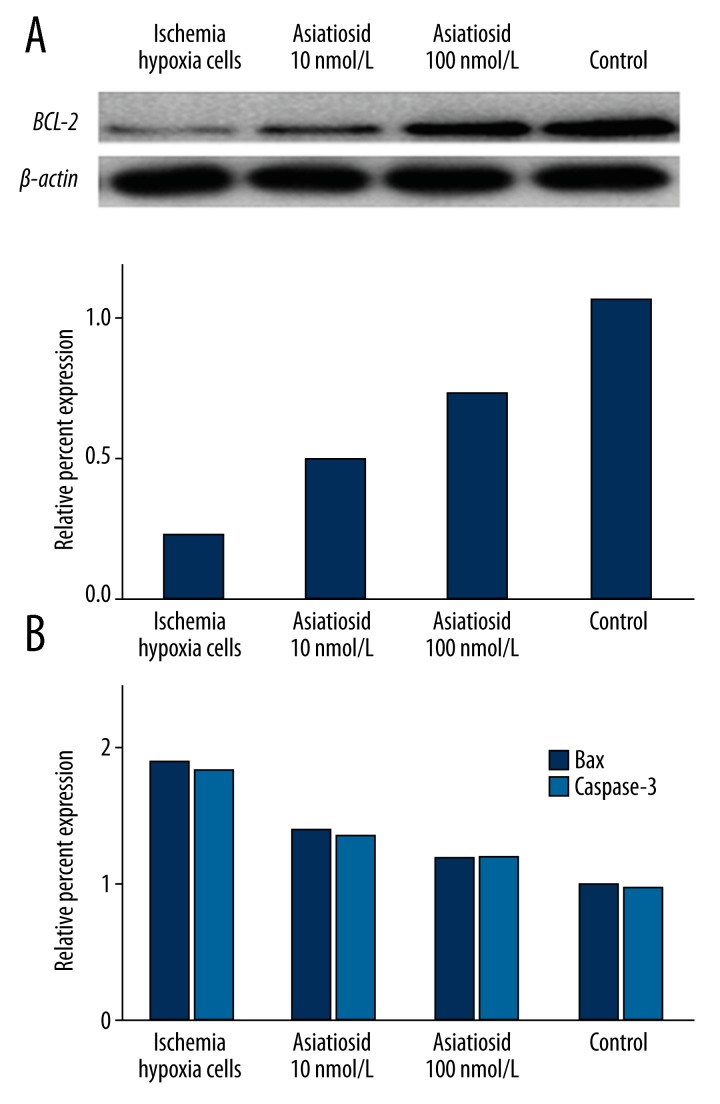
Compared with normal neurons, bcl-2 protein expression decreased significantly in ischemia-hypoxia cells, while bax and caspase-3 expressions increased. After asiaticoside treatment, however, cytosolic bcl-2 expression was increased while bax and caspase-3 expression was significantly suppressed (P<0.05, Figure 4).
Figure 4.
Western blot of protein expression in ischemia-hypoxia cells. (A) shows bcl-2 protein expression; (B) shows bax and caspase-3 protein expression.
Discussion
In recent years, many studies have confirmed that asiaticoside has various pharmacological effects and that it also exhibited functions in cardiovascular and cerebral ischemia protection [8–16].
Our results suggest that asiaticoside has a protective effect on ischemia-hypoxia neural cells in vitro. In addition, MTT testing found that asiaticoside application in vitro can increase the ischemia-hypoxia nerve cell survival rate and reduce cell apoptosis, which may associated with asiaticoside concentration.
The integrity of the neuron membrane is damaged under the ischemia-hypoxia condition, resulting in large release of lactate dehydrogenase. The increased lactate dehydrogenase content in cell supernatant shows that it may be related to the cell membrane lipid peroxidation caused by ischemia-hypoxia. Lactate dehydrogenase detection found that asiaticoside can inhibit lactate dehydrogenase release by ischemia hypoxic cells. Our results further confirmed that asiaticoside may inhibit membrane lipid peroxidation, thereby reducing neuron necrosis.
Our Western blot results confirmed that asiaticoside can increase BCL-2 protein expression. BCL-2 belongs to a membrane protein that is coded by BCL-2 proto-oncogenes [17] to promote cell survival [18]. Numerous in vitro [19] and in vivo experiments [20,21] proved that the BCL-2 protein is a key apoptosis regulatory factor and acts as a switch for apoptosis and can prevent cell apoptosis. Apoptosis refers to a type of programmed cell death in certain physiological and pathological conditions, in order to keep the body’s internal environment stable. Apoptosis is regulated by multiple genes and proteins, and Bcl-2 is the first gene identified to be related to apoptosis. In vitro experiments demonstrated that Bcl-2 plays an important regulatory role by adjusting mitochondrial outer membrane permeability and integrity. Bcl-2 embedded on mitochondrial membranes regulate related intracellular molecules, such as the release of cytochrome C and other apoptosis-induced proteins, finally inhibiting cell apoptosis [16,17]. BCL-2 protein is also an important marker for nerve cell apoptosis in an ischemic animal model [20,21].
Our results confirmed that the ischemia-hypoxia model can reduce BCL-2 protein expression and lead to decreased cell survival, which is similar with previous reports. However, we also found that the application of asiaticoside can up-regulate BCL-2 protein expression, thus increasing the survival rate of nerve cells. The abovementioned experiments further confirmed the protective effect of asiaticoside on ischemia and hypoxia neurons, and this protection may be related to the regulation of the BCL-2 protein [16,17].
In contrast, as a member of the bcl-2 gene family, bax is known to be closely related to cell apoptosis because it can bind with bcl-2 protein to form a complex. The over-expression of bax can facilitate the apoptosis. Caspase-3, on the other hand, is a hallmark molecule of the death receptor-inducing apoptotic pathway. Our study showed that asiaticoside can up-regulate the expression of bcl-2 protein and down-regulate the expression of bax and caspase-3 proteins. These data confirmed that asiaticoside plays a role in nerve cell apoptosis.
In 2006, Western medicine found that asiaticoside can improve the body’s resistance to various nonspecific stimulations and that it has an antidepressant effect [14]. Subsequently, scientists focused on asiaticoside’s role in neural field. Recent animal [13] and cell experiments [11,12] showed that asiaticoside may also improve memory and protect nerve cells [15]. It was also reported that it has the ability to selectively dilate blood vessels, and can prevent the formation of cerebral thrombus [14,15]. Recent animal experiments presented that asiaticoside treatment of the acute cerebral ischemia mice model for 3 consecutive days can obviously improve the mouse behavior status after ischemia. It can narrow the ischemic area and obviously alleviate brain tissue water content, cerebral index, and cerebral infarction volume in acute cerebral ischemia (P<0. 01) [5]. The nerve-protective effect of asiaticoside on acute focal cerebral ischemia mice revealed its potential medicinal value for alleviating acute cerebral ischemia. Our in vitro experiments also confirmed the view of recent animal reports [5]. Furthermore, our research also found that under the in vitro ischemia hypoxia condition, the nerve-protective effect of asiaticoside is dose-dependent. Larger doses of asiaticoside have stronger nerve protective effects and can improve the survival rate. Further clinical experiments are needed to explore the adverse effects of asiaticoside in large doses. Finding a safe and effective dose will help asiaticoside play a positive role in stroke.
Recent studies have shown that asiaticoside can alleviate hippocampal neuron damage by improving the anti-oxidative stress and inhibiting neuronal anti-inflammatory effects [7]. We found that the key apoptosis regulator BCL-2 protein is regulated by asiaticoside in vitro. Our experimental results suggest that an antiapoptotic effect is also one of the mechanisms by which asiaticoside acts.
Certain limitations and weakness exist in the current study; however, only explored the in vitro protective effect of asiaticoside in cultured ischemia-hypoxia rat neurons, without further explanation in in vivo models. A future plan therefore will be the application of asiaticoside in cerebral ischemia model animals to elucidate the clinical effect of this traditional medicine. The adverse effects of asiaticoside are currently poorly understood. In subsequent research, we plan to explore the adverse effects of asiaticoside in animals to provide information for clinical trials.
Conclusions
Our results further prove that asiaticoside has good nerve-protective effects in vitro. It can increase the survival rate of ischemia-hypoxia neurons, with some degree of concentration dependence. This effect may be mediated by the bcl-2, bax, and caspase-3 proteins. In recent years, following research on asiaticoside’s pharmacological effects on nerves, it was found asiaticoside protectives neurons, inhibits neuron apoptosis, and improves memory. We believe it will play a greater role in stroke treatment, with broad prospects for development.
Footnotes
Source of support: Departmental sources
References
- 1.Belcaro G, Maquart FX, Scoccianti M, et al. TECA (Titrated Extract of Centella asiatica): new microcirculatory, biomolecular, and vascular application in preventive and clinical medicine. A status paper. Panminerva Med. 2011;53:105–18. [PubMed] [Google Scholar]
- 2.Chassaud LF, Fry BJ, Hawkins DR, et al. The metabolism of asiatic acid,-madecassic acid and asiaticoside in the rat. Arzneimittelforschung. 1971;21:1379–84. [PubMed] [Google Scholar]
- 3.Shinomol GK, Muralidhara, Bharath MM. Exploring the role of “Brahmi” (Bocopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:33–49. doi: 10.2174/187221411794351833. [DOI] [PubMed] [Google Scholar]
- 4.James JT, Dubery IA. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules. 2009;14:3922–41. doi: 10.3390/molecules14103922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabassum R, Vaibhav K, Shrivastava P, et al. Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. Neurol Sci. 2013;34:925–33. doi: 10.1007/s10072-012-1163-1. [DOI] [PubMed] [Google Scholar]
- 6.Soumyanath A, Zhong YP, Gold SA, et al. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J Pharm Pharmacol. 2005;57:1221–29. doi: 10.1211/jpp.57.9.0018. [DOI] [PubMed] [Google Scholar]
- 7.Krishnamurthy RG, Senut MC, Zemke D, et al. Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res. 2009;87:2541–50. doi: 10.1002/jnr.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbosa NR, Pittella F, Gattaz WF. Centella asiatica water extract inhibits iPLA2 and cPLA2 activities in rat cerebellum. Phytomedicine. 2008;15:896–900. doi: 10.1016/j.phymed.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Ramanathan M, Sivakumar S, Anandvijayakumar PR, et al. Neuroprotective evaluation of standardized extract of Centella asciatica in monosodium glutamate treated rats. Indian J Exp Biol. 2007;45:425–31. [PubMed] [Google Scholar]
- 10.Yuan Y, Zhang H, Sun F, et al. Biopharmaceutical and pharmacokinetic characterization of asiatic acid in Centella asiatica as determined by a sensitive and robust HPLC-MS method. J Ethnopharmacol. 2015;163:31–38. doi: 10.1016/j.jep.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Y, Ding H, Xu M, Gao J. Protective effects of asiatic acid on rotenone- or H2O2-induced injury in SH-SY5Y cells. Neurochem Res. 2009;34:746–54. doi: 10.1007/s11064-008-9844-0. [DOI] [PubMed] [Google Scholar]
- 12.Xu MF, Xiong YY, Liu JK, et al. Asiatic acid, a pentacyclic triterpene in Centella asiatica, attenuates glutamate-induced cognitive deficits in mice and apoptosis in SH-SY5Y cells. Acta Pharmacol Sin. 2012;33:578–87. doi: 10.1038/aps.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhanasekaran M, Holcomb LA, Hitt AR, et al. Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer’s disease animal model. Phytother Res. 2009;23:14–19. doi: 10.1002/ptr.2405. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh MT, Peng WH, Wu CR, et al. Review on experimental research of herbal medicines with anti-amnesic activity. Planta Med. 2010;76:203–17. doi: 10.1055/s-0029-1240707. [DOI] [PubMed] [Google Scholar]
- 15.Gao J, Tang X, Dou H, et al. Hepatoprotective activity of Terminalia catappa L. leaves and its two triterpenoids. J Pharm Pharmacol. 2004;56:1449–55. doi: 10.1211/0022357044733. [DOI] [PubMed] [Google Scholar]
- 16.Veerendra Kumar MH, Gupta YK. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol. 2003;30:336–42. doi: 10.1046/j.1440-1681.2003.03842.x. [DOI] [PubMed] [Google Scholar]
- 17.Jonas EA, Porter GA, Alavian KN. Bcl-xL in neuroprotection and plasticity. Front Physiol. 2014;5:355. doi: 10.3389/fphys.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lossi L, Gambino G, Salio C, Merighi A. Autophagy regulates the post-translational cleavage of BCL-2 and promotes neuronal survival. ScientificWorldJournal. 2010;10:924–29. doi: 10.1100/tsw.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XQ, Sheng R, Qin ZH. The neuroprotective mechanism of brain ischemic preconditioning. Acta Pharmacol Sin. 2009;30:1071–80. doi: 10.1038/aps.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Mao W, Yi X, Qin J, et al. CXCL12 inhibits cortical neuron apoptosis by increasing the ratio of Bcl-2/Bax after traumatic brain injury. Int J Neurosci. 2014;124:281–90. doi: 10.3109/00207454.2013.838236. [DOI] [PubMed] [Google Scholar]