Abstract
Glutathione S-transferase P1-1 (GSTP) is one member of the family of GSTs and is ubiquitously expressed in human tissues. The literature is replete with reports of high levels of GSTP linked either with cancer incidence or drug resistance, and yet no entirely cogent explanation for these correlations exists. The catalytic detoxification properties of the GST isozyme family have been a primary research focus for the last four decades. However, it has become apparent that they have undergone structural and functional convergence where evolutionary selective pressures have favored the emergence of noncatalytic properties of GSTP that has imbued this isozyme with expanded biological importance. For example, GSTP has now been linked with two cell-signaling functions that are critical to survival. Through protein:protein interactions, GSTP can sequester c-jun N-terminal kinase (JNK) and act as a negative regulator of this stress kinase. Pharmacologically, this activity has been linked with the activity of GSTP inhibitors in stimulating myeloproliferation. In addition, GSTP is linked with the forward S-glutathionylation reaction, a post-translational modification that impacts the function/activity of a number of proteins. Catalytic reversal of S-glutathionylation is well characterized, but the role of GSTP in catalyzing the forward reaction contributes to the “glutathionylation cycle.” Moreover, GSTP is itself susceptible to S-glutathionylation, providing an autoregulatory loop for the cycle. Because oxidative stress regulates both S-glutathionylation and JNK-signaling pathways, such links may help to explain the aberrant patterns of GSTP expression in the cancer phenotype. As such, there is an ongoing preclinical and clinical platform of drug discovery and development around GSTP.
Keywords: Glutathione, glutathione S-transferase, glutathionylation, redox, anticancer drugs, cancer, stress response, stress kinases
Introduction
Sulfur has the essential chemical properties to exist in a biologically reduced sulfhydryl state where the pKa of the thiol group is ~9.65, accounting for the nucleophilicity of the predominant cellular reductant, reduced glutathione (GSH). GSH homeostasis is maintained in cells by a complex series of balanced pathways. De novo synthesis can occur through the γ-glutamyl cycle, where the three constituent amino acids (glu-cys-gly) are combined with rate-limiting catalysis through γ-glutamylcysteine synthetase (GCS). Salvage of GSH can occur through the cleavage activity of the membrane-associated γ-glutamyl transpeptidase (GGT) that can recycle constituents of the molecule. Whereas intracellular concentrations of GSH may vary considerably, 0.1–10 mM are not uncommonly found in mammalian cells (10–30 μM in plasma). Glutathione can occur in reduced (GSH), oxidized (GSSG), or in mixed disulfide forms, and its ubiquitous abundance is testament to its biological importance.
Whereas many small chemical electrophiles spontaneously react with GSH, glutathione S-tranferases (GSTs) can catalyze their thioether conjugation. In mammals, there are six different cytosolic GST iso-forms: alpha, mu, pi, theta, omega, and zeta. GST polymorphisms leading to altered catalytic activity have been linked to cancer susceptibility and prognosis (Townsend and Tew, 2003; Raimondi et al., 2006; Kellen et al., 2007; Voso et al., 2008; Kraggerud et al., 2009). Although <10% of the primary sequence is conserved, all GST isozymes have a similar topology and two domains responsible for protein folding. The N-terminal domain (residues 1–80) comprises one third of the protein and forms the G-site. It is composed of four β-sheets with three flanking α-helices, a structural motif common to thioredoxin, and other proteins that have evolved to bind GSH or cysteine (Armstrong, 1997). This region contains a catalytic tyrosine, serine, or cysteine residue that directly interacts with the thiol group of GSH (Armstrong, 1997).
GSTP (π) is of particular interest with regard to cancer, because many tumors and cancer cell lines are characterized by high GSTP expression. Further, increased expression of GSTP has also been linked to acquired resistance to cancer drugs (Tew, 1994). However, because most cancer drugs are not good substrates for GSTP, the reason(s) for the high levels of expression of this isozyme are not always clear. It is only recently that systematic investigations have suggested that GSTP has a diversity of functions in cancer cells, some of which are unrelated to its capacity to detoxify chemicals or drugs.
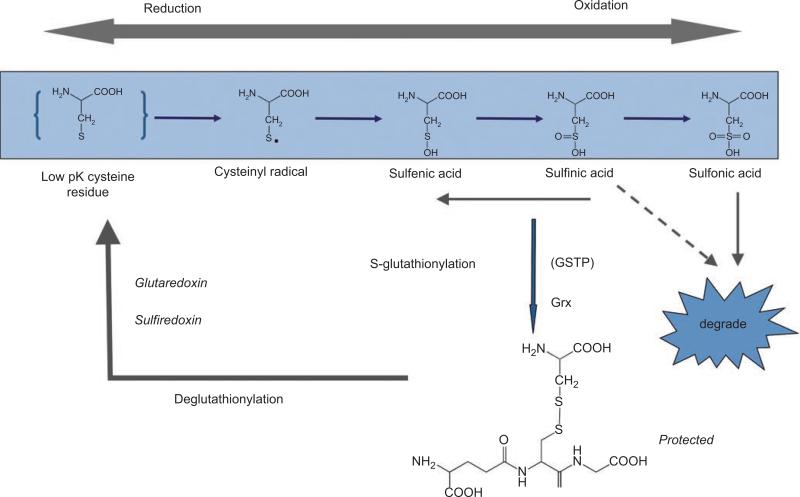
The balance between oxidation and reduction reactions determines cellular redox homeostasis and plays an essential role in numerous signaling cascades, including those associated with proliferation, inflammatory responses, apoptosis, and senescence. Reactive oxygen and nitrogen species (ROS; RNS) are invariable components of aerobic metabolism and are key contributors to cellular redox. Redox-sensitive cysteines are generally at various oxidation states and can be subject to post-translational modification through S-glutathionylation. More than 125 genes and signal-transduction proteins are directly affected by redox conditions. In context, because susceptible protein targets for S-glutathionylation are still appearing, this number will probably increase. In addition, signaling events may be further controlled by protein-protein interactions, exemplified by the interaction of GSTP with the MAPK c-jun NH2 terminal kinase (JNK). Oxidative stress can result in the reversal of GSTP-regulated intrinsic JNK-inhibitory activity via dissociation of the GSTP-JNK complex. In this regard, GSTP serves as a sensor of intracellular changes in redox potential and can directly regulate kinase pathways, perhaps reflecting a contributory component to the drug-resistance phenotypes of many GSTP-overexpressing cancers. S-glutathionylation occurs when a cysteine in a low-pK environment forms a disulfide bond with GS• (see Figure 1). This is reversible, and the resultant S-glutathionylation cycle has the potential to selectively regulate the function of a number of enzymes, receptors, structural proteins, transcription factors, and transport proteins and may alter a variety of protein-protein interactions.
Figure 1.
Scheme illustrating the forward and reverse reactions of the S-glutathionylation cycle. Modified from Tew (2007).
The following sections review some of the properties of GSTP unrelated to detoxification and place these in context of the cellular and physiological importance of this prevalent protein in oncologic diseases.
GSTP and cancer
Sulfur can exist in a number of different oxidation states from–2 in thiols to +6 in sulfates. This property and the flexible valence states of sulfur provide significant nucleophilicity and facilitate interaction with electrophilic oxidative metabolites. This property can enable intermediate oxidative states of sulfur to participate in regulating intracellular signaling events. Cysteine is one of the least common amino acids in mammalian proteins and is subject to a number of important post-translational modifications. GSH incorporates cysteine and provides the primary reductive potential in the cell. Whereas maintenance of GSH homeostasis is achieved by a series of de novo and salvage pathways (Tew, 1994), in cancer, aberrant redox balance is frequently manifested by increased levels of GSH, dysfunctional associated pathways, and an altered adaptive response to both ROS and RNS conditions. GSH homeostasis may also be dysregulated in cells that are entering apoptosis. Indeed, following cytotoxic drugs, tumor cells actively efflux GSH either as a cause or effect of the apoptotic process (Hammond et al., 2007).
High levels of GSTP are found in many tumors, but, in particular, ovarian, non-small-cell lung (NSCLC), breast, colon, pancreas, and lymphomas and in a wide range of drug-resistant cell lines and tumors (Tew, 1994). The reasons for such increased expression ratios (when compared to normal tissues or wild-type cell lines, respectively) are not always easily understandable. For example, one of the earliest reports of increased GST expression in drug-resistant cell lines involved chlorambucil, where evidence of a GST-catalyzed formation of the thioether conjugate was subsequently documented (Wang and Tew, 1985; Ciaccio et al., 1991) and could, in principle, explain a cause-effect relationship for the selection of GST overexpression. However, an MCF7 cell line resistant to adriamycin had ~50-fold more GSTP than the wild type, which had very low levels (Batist et al., 1986). This relationship was not easily explained by GSTP catalytic properties, because GSH conjugates of adriamycin do not occur under physiological conditions. In the years since these reports, tacit (and sometimes without justification) assumptions have linked GST-mediated detoxification with such acquired drug resistance. The importance of GST in kinase regulation and proliferation and the link of GSTP to the forward reaction of S-glutathionylation now open up plausible and different ways to view GSTP expression patterns. In other words, some tumors and drug-resistant cells may develop an acquired dependence on the protein. Because of the proliferative nature of tumor cells, kinase pathways are frequently dysregulated, and, consequently, tumor cells could attempt to compensate by enhancing the expression of GSTP to counterbalance increased kinase activity. Such “addiction” to overexpressed proteins is not without precedent in the transformed phenotype. Not inconsequentially, phosphatases, such as PTP1B (Barrett et al., 1999) and cdc25 (Sohn and Rudolph, 2003), are subject to regulation by S-glutathionylation of critical cysteine residues. The kinase/phosphatase cycle impacts many pathways critical to uncontrolled cell growth. As such, it may be tempting to speculate that the relative abundance of GSTP, in certain cancers, may indicate a role(s) unrelated to drug detoxification. Contextually, in the absence of exogenous electrophilic stress, it seems odd that GSTP can be such an abundant protein in many tumor cells. Selective pressures or conditions of convergent evolution could favor the emergence of the nondetoxifying properties of GSTP and could have provided them with significant biological importance.
There is at least one example of downregulation of GSTP expression in cancer. In prostate cancer, epigenetic silencing of the GSTP gene through methylation creates a phenotype characterized by low or absent expression of GSTP (Bakker et al., 2002). It has been speculated that reduced GSTP may alter the capacity to detoxify possible carcinogens and thus may be causal to malignant transformation and disease progression in the prostate. This conclusion inculcates the reader through the accepted dogma that GST isozymes are primarily catalytically detoxifying, but does not consider the alternative explanations, such as the altered capacity to regulate kinase-dependent proliferation pathways or to control protein S-glutathionylation, each (or both) of which could imbue the property of uncontrolled cell growth.
GSTP and kinase regulation
GST isozymes have additional regulatory roles via kinase interactions and subsequent downstream control of cellular stress response, apoptosis, and proliferation pathways. GSTP interacts with the mitogen-activated protein kinase (MAPK) JNK complex and is subject to further regulation by changes in redox conditions. Fluorescence resonance energy-transfer measurements with purified recombinant proteins showed that the C terminus of JNK is critical to its interaction with GSTP (Wang et al., 2001b). The apparent binding constant (Kd) for full-length JNK was 188 nm and 217 nm for a C-terminal fragment (residues 200–424). An N-terminal fragment (residues 1–206) did not bind to GSTP1-1. Increased expression of the C-terminal JNK fragment in transfected NIH3T3 cells produced a concentration-dependent increase in the kinase activity of JNK under normal, unstressed growth conditions, indicating a dominant-negative effect. This implied that the fragment can compete with endogenous full-length functional JNK, resulting in dissociation of the GSTP1-1–JNK interaction and concomitant JNK enzyme activation. By using an antibody to hemagglutinin-tagged C-JNK, a concentration-dependent coimmunoprecipitation of GSTP was achieved. These data provide evidence for direct interactions between the C-terminal of JNK and GSTP1-1 and a rationale for considering GSTP1-1 as a critical ligand-binding protein with a role in regulat ing kinase pathways. The stoichiometry of the GSTP-JNK interaction has been difficult to assess by standard procedures. In spite of the catalytic function of GSTP being dependent upon dimerization, in some instances, it was assumed that the monomeric form of GSTP would interact with JNK. A recent study supports the principle that the JNK-GSTP interaction occurs via the dimer of the latter. For example, the conformational stability of GSTP1-1 was examined by equilibrium folding and unfolding kinetics and did not demonstrate the existence of a stable monomer. Instead, unfolding of hGSTP1-1 proceeded via an inactive, native-like dimeric intermediate, in which the highly dynamic helix 2 unfolded. Molecular modeling showed a dimeric GSTP1-1 could bind JNK. As a consequence of these studies, this group concluded that formation of a complex between GSTP1-1 and JNK most likely involved the dimeric form of the GST and not its monomer (Gildenhuys et al., 2010).
Oxidative stress can destabilize the GSTP-JNK complex and cause an activation of the kinase cascade (Adler et al., 1999). Thus, GSTP serves as a sensor of intracellular changes in redox potential and has the capacity to regulate kinase pathways, perhaps contributing to the GSTP overexpressing, drug-resistance phenotype. JNK phosphorylation and subsequent transactivation of c-Jun transcription factors has been linked to cell proliferation (Shaulian and Karin, 2001). GSTP also has a role in modulating ERK and p38 activation (Yin et al., 2000), and of consequence to the possible involvement of GSTP in pathways relevant to myeloproliferation, the regulation of p38 is important in the maintenance of human stem cell (HSC) self-renewal and hematopoiesis (Jang and Sharkis, 2007).
Adding a further layer of complexity is the understanding that proteins do not act in isolation in a cellular milieu. Rather, essential protein-protein interactions govern how cellular events unfold (Golemis et al., 2002). This process has proved to be significant to the regulation of JNK signaling by GSTP (Adler et al., 1999; Wang et al., 2001b). This same paradigm seems to hold for thioredoxin and apoptosis signal-regulating kinase ASK1 (Saitoh et al., 1998), implying the possible existence of a general regulatory mechanism for kinases that may involve GSH, small redox active proteins, and associated pathways (Davis et al., 2001).
The S-glutathionylation cycle
One of the more interesting conundrums to emerge from the completion of the genome project is the realization that humans are a composite of <30,000 genes, and yet, complexity of protein structure/function seems distinctly more layered. In the burgeoning era of proteomics, it becomes clear that the central dogma of genetic determinism can be influenced by a number of processes that can include polymorphic variants, gene-splicing events, exon shuffling, protein domain rearrangements, and the large number of post-translational modifications that contribute to alterations in tertiary and quaternary protein structure. Among these, phosphorylation, glycosylation, methylation, and acetylation can account for a large proportion of modifications. However, the addition of GSH to available cysteine residues has now been shown to be of considerable consequence. The importance of modifying cysteine residues is not necessarily restricted to redox regulation, but now seems to be a plausible event that can lead to changes in signaling processes, particularly as a response to a divergent number of stress stimuli. By adding the GSH tripeptide to a target protein, ~305 Da and an additional negative charge are introduced (as a consequence of the glu residue), and a change in protein conformation is made likely. The implication from these facts is that cells actively participate in the stochastic production of multiple protein building blocks with the intent of realizing functional nonredundancy.
Cysteine residues found in basic environments (e.g., vicinal to lys, arg, or his) can have low pKa values, a property that makes them susceptible to the addition of GSH (i.e., can be S-glutathionylated). The last decade has produced a number of reports that emphasize the importance of S-glutathionylation as a post-translational modification of proteins involved in various critical cell functions. Figure 1 shows how S-glutathionylation of proteins may occur through a dynamic, cyclical process. The addition of GSH to the cysteine protects this site from further, perhaps irreversible, oxidative damage. It can also effect a change in conformation and/or charge that may alter protein function and/or subcellular localization. Critical in ascribing any regulatory function (i.e., extrapolation to phosphorylation/dephosphorylation) to this process is the reversibility of S-glutathionylation by small-molecule, cysteine-rich proteins, such as glutaredoxin, thioredoxin, and sulfiredoxin (Shelton et al., 2005; Findlay et al., 2006). Glutaredoxin (Grx) is reportedly involved in both the forward and reverse steps (Gravina and Mieyal, 1993). Protein disulfide isomerase (PDI) is another CXXC-motif–containing protein implicated as a possible deglutathionylation enzyme (Nakamura et al., 1996). Some PDI localizes to the endoplasmic reticulum (ER) and is involved in protein folding through disulfide bond formation and isomerization. Interestingly, within this family of small redox active proteins, Grx (Fratelli et al., 2002), GSTP (Townsend et al., 2009a), and PDI (Townsend et al., 2009b) are themselves each regulated by S-glutathionylation. Indeed, it is instructive to consider the modification of PDI and GSTP in more detail, because these provide insight into the biological importance of the S-glutathionylation process.
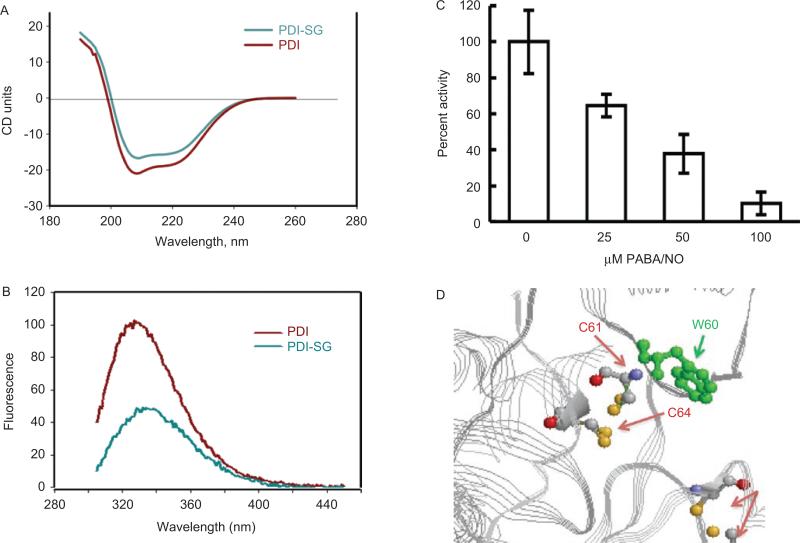
PDI is a ubiquitous, prevalent member of the thioredoxin superfamily and, at 57 kD, is organized into five domains (a, b, b’, a’, and c), with a C-terminal KDEL sequence that targets it to the ER. It has two active sites in the a and a’ domains, each with two conserved cysteine residues within the motif, CGHC, that cycle between oxidized and reduced states (Pihlajaniemi et al., 1987). PDI facilitates correct folding of proteins in the ER through isomerase activity that catalyzes the rearrangement of incorrectly formed disulfide bonds and oxidase activity that introduces disulfides into proteins. PDI can also be part of multimeric protein clusters as a subunit of prolyl-4-hydroxylase and microsomal triglyceride transfer protein. In addition, cell-surface PDI may be involved in cellular entry antigen processing (Park et al., 2006), complexes with integrin receptor in regulating cell adhesion (Swiatkowska et al., 2008), glioma cell invasion (Goplen et al., 2006), attachment and infectivity of Chlamydia (Conant and Stephens, 2007), to promote disulfide bond rearrangements in HIV-1 envelope protein that accompanies viral entry (Ou and Silver, 2006) and may even be a determinant of drug resistance in malignant B lymphocytes (Tager et al., 1997). When cells are exposed to drug-induced ROS, human full-length PDI is S-glutathionylated. In order to determine which cysteine residues were affected, LysC-digested fragments of native and S-glutathionylated PDI were analyzed under nonreducing conditions by tandem mass spectroscopy. Within two distinct peptides (residues 43–57 and 387–401), a mass increase of 305.6 was consistent with the S-glutathionylation of one cysteine residue in each fragment. Figure 2 illustrates the effects of S-glutathionylation of PDI on its secondary structure. The circular dichroism spectrum (200–240 nm) of S-glutathionylated PDI was quite similar to that of the native protein, but consistent with a small decrease in the α-helix content (206–220 nm) of the protein. Based on the crystal structure of PDI, the sulfhydryls of cysteine residues 61 and 64 are proximal to a tryptophan at position 60. Consistent with the known quenching effect of a disulfide bond on adjacent tryptophan fluorescence, a substantial fluorescence decrease was associated with S-glutathionylation of PDI. These results indicate the dynamic nature of S-glutathionylation, resulting in spatial disulfide/sulfhydryl equilibria. A hypsochromic shift of emission maximum approximately 4 nm after protein S-glutathionylation indicates some shielding of Trp38 from a polar environment through modification of Cys61 and/or Cys64. The possible disulfide that may form at positions Cys90 and Cys97 is too distal from the tryptophan residue to have an effect. These fluorescent analyses were used to confirm that S-glutathionylation alters the tertiary structure of PDI (Townsend et al., 2009a).
Figure 2.
S-glutathionylation of the active-site cysteines on PDI alters protein structure. Spectroscopic analysis of native (red) and PABA/NO+GSH-treated (green) PDI in vitro using circular dichroism (A) and tryptophanyl fluorescence (B) of purified protein. The enzymatic activity of PDI was assessed using the insulin turbidity assay (C). According to the published crystal structure (Tian et al., 2006) and (D), the relative positions of the PDI C61 and 64 and W60 are depicted using Ras Mol 2.7.4.2 (http://rasmol.org last accessed February 18 2011). From Townsend et al., (2009b).
The rate of protein S-glutathionylation is significantly enhanced by the presence of a catalytically active GSTP. Catalysis of this post-translational modification by a proximal donor of GSH and the low-pK cysteine thiol of target proteins is a novel property for GSTP and is an important forward reaction in the S-glutathionylation cycle (Townsend et al., 2009a). This GSTP function has also been identified in the ischemic heart, where aldose reductase (AR) is a target for the forward catalysis of S-glutathionylation by GSTP. In ischemic hearts, AR coimmunoprecipitates with GSTP, whereas in reperfused hearts, the association of AR with glutaredoxin (GRX) is increased (Wetzelberger et al., 2010). These results support the model that, upon reperfusion, AR-SOH is converted to AR-SSG via GSTP-assisted S-glutathionylation. AR-SSG is then reduced by GRX to AR-SH. Ultimately, the cycle would provide a general redox-switching mechanism that regulates the reduction of protein sulfenic acids to cysteines in this environment.
As part of this cycle, it appears that GSTP is itself auto-regulated by S-glutathionylation. For example, oxidative stress induces multimerization and inactivation of GSTP (Davis et al., 2001). Whether such oligomerization of GSTP is linked with functional redundancy or altered properties is not entirely clear. Recently, treatment of cells with an electrophilic prostanoid was shown to induce irreversible GSTP oligomerization, involving the cysteine residue at position 101 (Sanchez-Gomez et al., 2010). This residue is present in human, but not rodent, GSTs, implying possible species differences in the capacity to oligomerize; however, there are other cysteine, and even histidine, residues that are within the requisite structural domains that could also permit cross-linking to occur (Sanchez-Gomez et al., 2010).
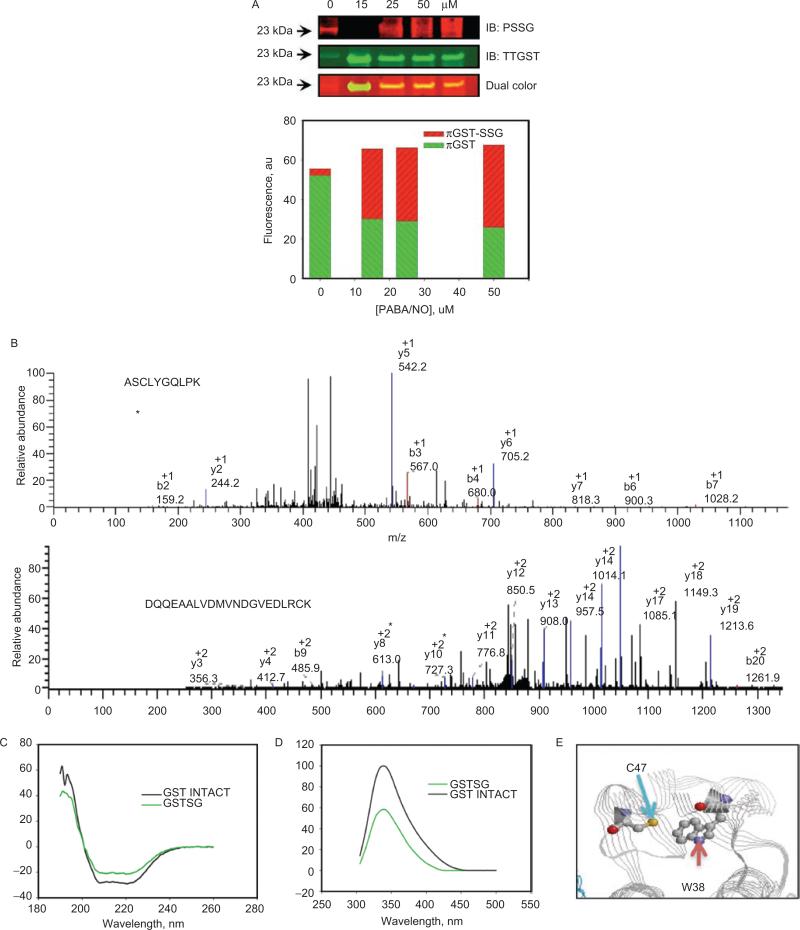
Tandem mass spectroscopy of S-glutathionylated GSTP determined that Cys47 and Cys101 were the modified residues, and that S-glutathionylation caused a reduction in the catalytic activity of GSTP (Townsend et al., 2009a). Using approaches similar to those for PDI, Figure 3 shows the evaluation of the effects of S-glutathionylation on protein secondary structure. The CD spectrum (206–220 nm) of S-glutathionylated GSTP was similar to native protein and consistent with a small decrease in the α-helical content (206–220nm) (Figure 3C). Based on the crystal structure, the sulfhydryl of Cys47 is proximal to Trp38 (~3 Å). Again, consistent with the known quenching effect of the disulfide, the intrinsic fluorescence of GSTP was substantially decreased upon S-glutathionylation (Figure 3D). These data indicate the dynamic nature of S-glutathionylation, resulting in spatial disulfide/sulfhydryl equilibrium. Moreover, a hypsochromic shift of emission maximum ~4 nm after protein S-glutathionylation indicates some shielding of Trp38 from a polar environment through Cys47 S-glutathionylation (Figure 3E). These observations confirm that S-glutathionylation of GSTP alters its tertiary structure.
Figure 3.
Autoregulation of GSTP occurs through the S-glutathionylation of Cys47 and Cys101. HEK293 cells were treated with 0–50 uM of PABA/NO for 1 hour (A). The proteins were separated by nonreducing sodium dodecyl sulfate polyacrylamide electrophoresis, and S-glutathionylation was evaluated by immunoblot with PSSG monoclonal primary antibody and GSTP polyclonal primary antibody and detected simultaneously with both red (antimouse) and green (antirabbit) fluorescent secondary antibodies. The dual-colored image was quantified, and the bars represent a relative input of red and green fluorescence in each band. Matrix-assisted laser desorption/ionization mass spectrometric analysis of purified, expressed GSTP treated with 50 μM of PABA/NO and 0.5 mM of GSH showed that peptides containing Cys47 and Cys101 are S-glutathionylated (B). S-glutathionylation of Cys47 and Cys101 on GSTP alters structure. Spectroscopic analysis of native (black) and PABA/NO+GSH-treated (green) GSTP in vitro was performed using CD (C) and tryptophanyl fluorescence (D) of purified protein. According to the published crystal structure (Ji et al., 1997), the relative positions of GSTPs C47 and W38 are depicted (E) using RasMol 2.7.4.2 (http://rasmol.org last accessed February 18 2011). From Townsend et al., (2009a).
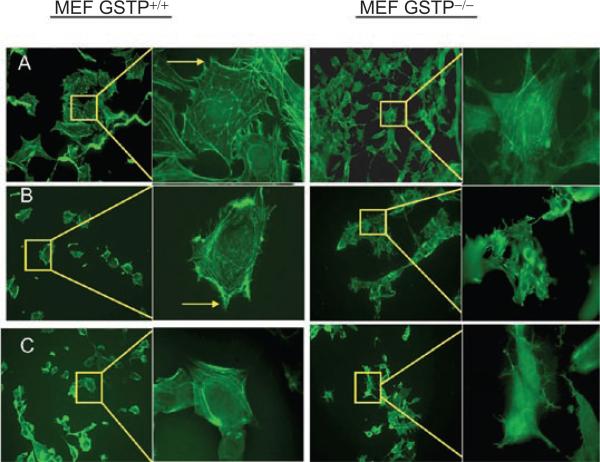
Relative to the proteome, the actual number of S-glutathionylated proteins is not large. The most common is actin. When cells are stimulated with growth factors, S-glutathionylation of actin can alter the ratio between its soluble and polymerized forms. Changes in microfilament structure/number, membrane ruffling, cellular adhesion, cell-cell interactions, and intracellular trafficking are the consequences of this actin modification (Fiaschi et al., 2006). Examples of some of these changes are shown in Figure 4. Individual globular actin (G-actin) subunits polymerize to form filamentous actin (F-actin). S-glutathionylation of G-actin subunits prevents its polymerization and alters both stress fiber and focal contact formation. Phalloidin staining showed that in mouse embryo fibroblasts from wild-type animals (MEF-GSTP+/+), there is a decrease in formation of both stress fibers and focal contacts, compared to knockout (MEF-GSTP−/−) cells. These results serve to emphasize the catalytic role of GSTP in regulating the forward S-glutathionylation reaction of cellular G-actin.
Figure 4.
S-glutathionylation of actin is elevated in mouse embryo fibroblast (MEF) from wild-type (GST+/+) cells. MEF-GST+/+ and −/− cells were seeded on glass coverslips and treated with 1) vehicle; 2) 300 μM of GSSG; or 3) 15 μM of PABA/NO for 1 hour and stained with phalloidin to visualize actin polymerization/stress fiber formation. From Townsend et al., (2009a).
In general, proteins susceptible to S-glutathionylation can be broken into specific clusters, which are summarized in Table 1. Although this list is expanding based on ongoing research, cytoskeletal constituents, energy metabolism, glycolysis-controlling proteins in mitochondria, signaling proteins, particularly kinases and phosphatases, calcium and redox homeostasis-regulating proteins, protein folding, and stability-controlling proteins all fall into the category of having cysteines subject to S-glutathionylation (Townsend, 2007). Like phosphorylation, cysteine modification is critical to cellular signaling, and its deregulation impacts multiple diseases. Because S-glutathionylation has a direct effect upon phosphatases and kinases, perhaps this is an evolutionarily conserved nexus between sulfur and phosphorus biochemistry. One direct connection is that Srx reverses S-glutathionylation of phosphatases with a functional impact on their catalytic activity (Findlay et al., 2006).
Table 1.
Examples of protein clusters subject to S-glutathionylation.
| Protein “cluster” | Selected examples | Functional significance | References |
|---|---|---|---|
| Cytoskeletal | Actin; spectrin; vimentin | Alters actin cycling, cell motility, morphology | (Wang et al., 2001a; Rossi et al., 2006) |
| Glycolysis, energy metabolism | Aldolase; pyruvate kinase; GAPDH | Inhibits enzyme activities | (Fratelli et al., 2002) |
| Mitochondrial | ATP synthase; succinyl CoA transferase | Inhibits enzyme activity | (Garcia et al.) |
| Calcium homeostasis | SERCA; ryanodine receptor; some S100 family members | Impacts Ca++ uptake, transport, and release | (Adachi et al., 2004; Hidalgo et al., 2006) |
| Protein folding | PDI; 20S proteosome; HSP65/70 | Influences processing of misfolded proteins | (Demasi et al., 2001; Townsend et al., 2009b) |
| Signaling | PTP1B; PTEN; PKC; creatine kinase; MEKK1; NfkB | A broad range of target proteins are in this category. The effects on phosphatases and kinases act as a second line of regulation. | (Findlay et al., 2004; Yu et al., 2005; Shelton et al., 2007) |
| Redox regulation | Thioredoxin; GSTP1-1; peroxiredoxins | Alters catalytic activity as well as binding affinity for partner proteins | (Haendeler, 2006; Noguera-Mazon et al., 2006) |
| ATP-sensitive K+ channels | Kir6.1/SUR2B | Regulates response to oxidative stress | (Yang et al., 2010) |
Human peroxiredoxins are nonselenium-dependent lipid peroxidases that convert lipid hydroperoxides to corresponding alcohols. They contain two conserved surface-located catalytic cysteines that are subject to oxidation to sulfinic acids and subsequently become substrates for sulfiredoxin (Rhee et al., 2007). Prdx6 has a single buried catalytic cysteine (Cys47), which, by virtue of structure, is not accessible to thioredoxin or sulfiredoxin. Reversible oxidative (sulfenic acid) inactivation of this enzyme is similar to peroxiredoxins (1-IV), but it is also not accessible to GSH. Reactivation of Prdx6 requires heterodimerization with GSH-loaded GSTP to overcome an accessibility barrier for GSH to regenerate sulfhydryls (Manevich et al., 2004). This exemplifies a further role for GSTP in the maintenance of redox homeostasis of proteins. Srx appears to target more than one intracellular protein, but is distinct, in that it is not itself S-glutathionylated during the removal of GSH. Srx is implicated specifically in the reductive deglutathionylation step, perhaps as a consequence of the presence of only one cysteine residue within the entire sequence. This differs from Grx, which can be involved in both the oxidative S-glutathionylation and reductive deglutathionylation steps. Thus, deglutathionylation seems to involve multiple cofactors that are presumably controlled by sets of kinetic constants that govern both the forward and reverse reactions.
Pharmaceutical targeting of GSTP
GSTP polymorphisms
As drug discovery and development move toward a targeted personalized platform, there is more incentive to design clinical trials around plausible pharmacogenetic principles. Because there are specific examples of GSTP-targeted agents, the polymorphic variants of this isozyme take on more significance. In reality, there are few correlative clinical trials that have used predictive analyses to understand response patterns to therapy. However, it seems likely that success of the sorts of trials described in this section may well be contingent upon population differences in GSTP expression. For example, for the GSTP class, in humans, a single gene located on chromosome 11q13 encodes for these proteins. The GSTP1 gene spans 3 kb and encodes 210 amino acids in seven exons (Cowell et al., 1988). Polymorphisms at the GSTP1 locus result in three alleles (GSTP1*A–D) that differ structurally and functionally. The allele frequencies for *A–C in Caucasian populations are 0.685, 0.262, and 0.068, respectively. The promoter region contains a TATA box, two SP1 sites, an insulin response element, and an antioxidant response element within an AP1 site (Lo and Ali-Osman, 2007). In mice, there are two GSTP genes, designated mGSTP1 and mGSTP2, that contain seven exons and are ~3 kb in length. These genes lie adjacent to one another on chromosome 19, a fact that was relevant to the targeting strategy in creating knockout animals (Henderson et al., 1998, 2000).
There is evidence to suggest that the polymorphic GSTP variants influence patient response to cancer therapy, particularly platinum-based regimens (Lo and Ali-Osman, 2007). Although these correlative results remain nascent, the principle that variation may play a role in population-based analysis of cancer susceptibility and response to treatment takes on a greater relevance in light of the connections between GSTP and kinase regulation and S-glutathionylation. Perhaps an individual's capacity to respond rapidly to oxidative or nitrosative stress may be dependent upon their GSTP phenotype. Moreover, population distribution patterns of GSTP polymorphisms may determine response to the GST-targeted drugs discussed below.
GSTP-activated prodrugs
TLK286 (Telcyta®; canfosfamide)
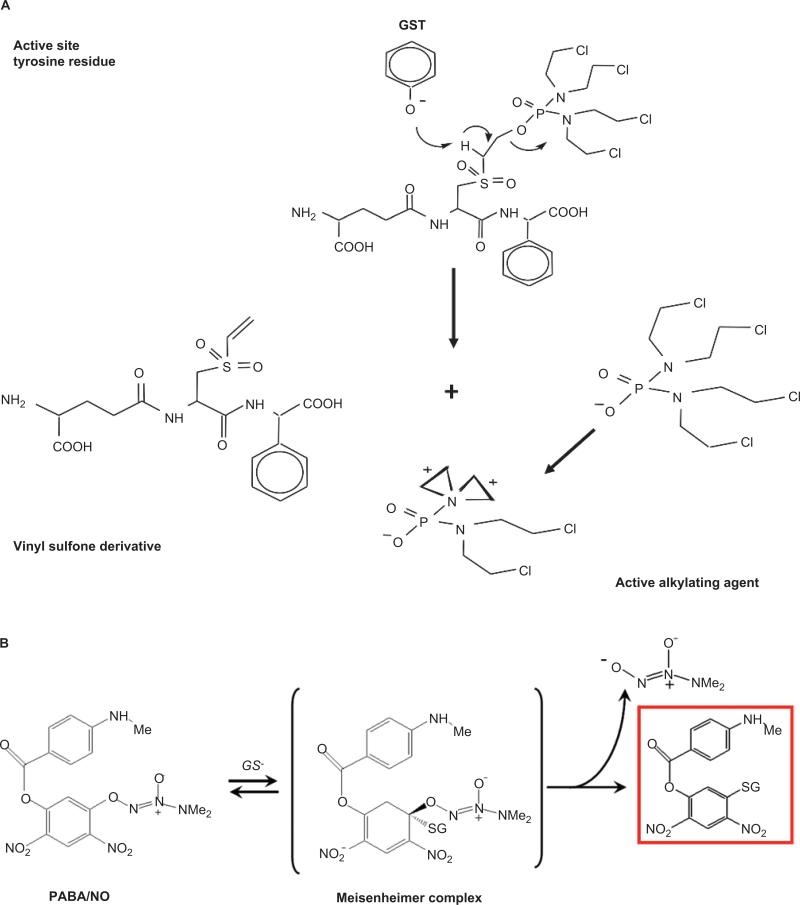
Pathways that involve proteins that are aberrantly expressed in cancer cells are preferential targets for drug intervention. The GSTP isozyme falls into this category. The rational design of TLK286 was premised on the principle that an enhanced therapeutic index may be accrued as a consequence of high GSTP expression in tumors and drug-resistant cells. The TLK286 design strategy relied on the principle that proton abstraction at the active site of GST would initiate a cleavage reaction, converting the inactive prodrug into a cytotoxic species (Lyttle et al., 1994). The histidine residue proximal to the G-binding site is integral to the removal of the sulfhydryl proton from the GSH cosubstrate, generating a nucleophilic sulfide anion. This moiety would be more reactive, with electrophiles in the absence of GSH (Mannervik and Danielson, 1988). Crystallographic analysis of GSTP later assigned the tyrosine hydroxyl residue as the proton-abstraction moiety (Reinemer et al., 1992). Figure 5A shows that proton abstraction results in the deprotonation of the α-carbon to yield a sulfone, which undergoes a β-elimination to give the active alkylating species. Unlike other standard anticancer nitrogen mustards, (e.g., nor-nitrogen mustard, melphalan, chlorambucil, and cyclophosphamide) TLK286 contains a tetrakis (chloroethyl) phosphorodiamidate moiety. Other compounds bearing this structure have been shown to be more cytotoxic than a similar structure with a single bis-(chloroethyl) amine group (Borch and Valente, 1991). As with other nitrogen mustards, the chlorines act as leaving groups, creating aziridinium ions with electrophilic characteristics. Although the temporal/sequential formation of the four possible chlorine-leaving events is vague, the assumption is that these species are cytotoxic through alkylation of target nucleophiles, such as DNA bases. Tetrafunctionality could result in the formation of cross-links with bonding distances greater than for bifunctional agents. However, a number of caveats apply to this interpretation. For example, alkylating agents, whether mono-, bi-, or putatively tetrafunctional, generally produce cumulative myelosuppression. In clinical trials, TLK286 hematological toxicities were not generally dose limiting, despite treatment periods that were greater than 1 year (Rosen et al., 2003, 2004). In addition, despite being a micromolar inhibitor of DNA-dependent protein kinase, TLK286 caused minimal direct damage to DNA (Townsend et al., 2002). GSTP activation also produced a vinyl sulfone derivative of the GSH backbone (Figure 5A). This could be a factor in chain reactions leading to lipid peroxidation and even production of hydrogen peroxide (Comporti, 1989), considerations that could explain the enhanced expression of catalase in an HL60 cell line selected for resistance to TLK286. Also notable, GSTP expression levels are lower in the TLK286-resistant cell line, a situation that is quite dissimilar to the frequency of reports where GSTP is upregulated in resistant cells (Rosario et al., 2000).
Figure 5.
Structure and activation of two GSTP-activated prodrugs. (A) TLK286 (Telcyta®; canfosfamide). (B) PABA/NO.
Initial phase II clinical trials evaluated TLK286 as a single agent in the salvage setting in ovarian, NSCLC, breast, and colorectal cancers (Kavanagh et al., 2005). In the absence of complications resulting from drug combinations, these trials demonstrated the tolerability and possible activity of TLK286 in each of these GSTP-expressing tumors, with objective responses and durable disease stabilization reported. Whereas a larger phase III randomized, clinical trial in platinum-resistant ovarian cancer did produce a slight increase in overall survival (Vergote et al., 2009), there is doubt as to whether the results will allow for U.S. Food and Drug Administration registration. As a consequence, the company continues to develop the drug in a phase II/III setting in other disease sites, such as NSCLC (Sequist et al., 2009).
There are other efforts underway to synthesize GST-activated drugs. For example, some GST isozymes can catalyze the GSH-mediated hydrolysis of sulfonamide bonds. A recent report provides the synthesis of novel chimeric sulfonamide derivatives of bombesin, activated by the human isoenzyme, GSTA1-1. These derivatives bear a peptidyl-moiety as a molecular recognition element for targeting the drug selectively to tumor cells. The released S-alkyl-glutathione has antitumor activity (Sau et al., 2010; Axarli et al., 2009).
PABA/NO
Nitric oxide (NO) has a diverse number of physiological functions, but, at high enough concentrations, can also be cytotoxic (Furchgott and Vanhoutte, 1989). As a consequence of interactions with metals, superoxides, oxygen, and glutathione, NO can also lead to S-glutathionylation (Wink et al., 1997; Findlay et al., 2004). Directed delivery (i.e., tumor cells with high GSTP) of a therapeutic concentration of NO was considered a relevant approach to drug design. In early development, Saavedra et al. used a strategy to derivatize the 02 position of a diazeniumdiolate with protective groups in order to convert them into substrates for GST (Saavedra et al., 2006). The resulting inactive prodrug becomes cytotoxic when localized in a cell that has high GSTP concentrations. PABA/NO (O2-{2,4-dinitro-5-[4-(N-methylamino)benzoyloxy]phenyl} 1-(N,N-dimethylamino)diazen-1-ium-1,2-diolate) has N-methyl-p-aminobenzoic acid bound via its carboxyl oxygen as a 5-substituent on the 2,4-dinitrophenyl ring (Saavedra et al., 2006). PABA/NO belongs to the O2-aryl diazeniumdiolates, electrophiles known to transfer their aryl groups to attacking nucleophiles with simultaneous production of ions that release NO at physiological pH. In the presence of GSH, PABA/NO is activated by GSTP (Saavedra et al., 2006), resulting in the formation of a Meisenheimer-complex intermediate, and subsequently, the leaving group of the reaction generates two moles of NO (Figure 5B). Elevated NO levels lead to cytotoxic effects by forming RNS/ROS intermediates. Evidence in publications suggests that PABA/NO-induced nitrosative stress results in limited levels of protein nitrosylation (i.e., nitration), but high levels of S-glutathionylation (Findlay et al., 2004; Townsend et al., 2006).
GSTP and drug resistance
There are examples of GSTP-mediated GSH thioether conjugation reactions with some anticancer drugs; however, the catalytic rate constants for these reactions are generally not impressive. Although the rate and extent of conjugation for some alkylating drugs can be enhanced by GSTP catalysis (Ciaccio et al., 1991), the description of protein-protein interactions between GSTP and JNK has delineated a specific, important ligand-binding property for the isozyme. Interactions between the proteins yield an apparent association constant in the nanomolar range (Wang et al., 2001b). Through this binding, GSTP was shown to be an endogenous regulator of JNK (Adler et al., 1999); this may be of consequence in consideration of the role that JNK might play in the initiation of apoptosis pathways following TLK286 or other drugs. In addition, the activation of TLK286 could, in principle, be affected by the JNK-GST association.
Recent reports suggest that human GSTP1 may be phosphorylated and functionally activated by either the epidermal growth factor, tyrosine kinase (Okamura et al., 2009), or the protein kinase C (PKC) class of serine/threonine kinases. For example, PKCα activation and associated phosphorylation of GSTP1 correlated with increased formation of a platinum-glutathione conjugate, decreased DNA interstrand cross-link formation, and increased resistance to cisplatin. Following PKC activation, the IC50 of cisplatin increased approximately 2-fold. As a consequence of these studies, the investigators conclude that a mechanism of cisplatin resistance may be mediated by PKCα-dependent serine phosphorylation of GSTP1 and its associated increased conjugation of cisplatin with glutathione (Singh et al., 2010).
HL60 cells made resistant to PABA/NO had a decreased growth rate, partly as a consequence of altered cellular differentiation patterns (increased expression of CD11b, decreased expression of CD14, decreased nuclear to cytoplasmic ratios, and a condensation of nuclear chromatin). This was accompanied by alterations in plasma and mitochondrial membrane potentials. Both GSTP expression and NO release were reduced 2-fold, whereas increased expression levels of genes involved in the unfolded protein response (UPR) were evident in resistant cells. Wild-type cells treated with PABA/NO had increased levels of protein S-glutathionylation and JNK activation, whereas JNK was constitutively active in resistant cells, and these cells also had reduced levels of S-glutathionylation. By removing PABA/NO from the growth medium, resistant cells reverted to sensitivity within 7 days, suggesting that resistance is not genetically stable. Mechanistically, PABA/NO resistance is mediated through reduced levels of GSTP, resulting in reduced NO release and its subsequent alterations in cellular response to nitrosative stress (Hutchens et al., 2010).
A human promyelocytic leukemia (HL60) TLK286-resistant cell line was selected by chronic, long-term exposure to the drug. Whereas resistance was not readily achieved, eventually, a 5-fold resistant clone was isolated. Cross-resistance to melphalan occurred, but not to doxorubicin or taxol. The protein, transcript levels, and enzymatic activity of GSTP were reduced in the selected resistant line. Other GST isozymes were either unchanged or undetectable. Although glutathione levels were elevated in the TLK286-resistant cells, no changes in the expression of thiol-related genes, including γ-glutamylcysteine synthetase, γ -glutamyl transpeptidase, or multidrug resistance protein were found. A 7-fold increase in catalase expression in the resistant cell line indicated an adaptive response to oxidative and electrophilic stress, and this was also reflected in the lower prevalence of drug-induced DNA single-strand breaks in the resistant cells. As a correlative approach, mouse embryo fibroblast GSTP null cells exhibited a 2-fold resistance to TLK286, compared with GSTP wild-type cells. Similar to PABA/NO resistance, these results indicate that adaptive lower levels of GSTP activate less of the drug and, consequently, have a lower sensitivity (Rosario et al., 2000). As a consequence, these general observations support the rationale that tumors expressing high levels of GSTP1-1 would be more sensitive to the cytotoxic effects of either TLK286 or PABA/NO.
GSTP and myeloproliferative pathways
The bone marrow produces all differentiated hematopoietic cells that populate the peripheral blood. Within the bone marrow environment, hematopoietic stem and progenitor cells (HSCs and HPCs) and mature plasma cells have their own anatomical niches, with specific characteristics that regulate their number and destiny. For example, committed HPCs localize to the central part of the bone marrow cavity (Lo Celso et al., 2009). Approximately 75% of HSCs are in the G0 phase of the cell cycle at any given time, remain quiescent (Cheshier et al., 1999), and reside at the bone–bone marrow interface (i.e., osteoblastic niche). A smaller proportion of cells localize closer to blood vessels in the vascular niche, where the microenvironment favors proliferation and differentiation (Nilsson et al., 2001; Calvi et al., 2003; Wilson et al., 2008; Iwasaki and Suda, 2009; Lo Celso et al., 2009). Each population can be defined by the expression of adhesive cytokines and chemokine-signaling molecules that maintain cells within a specific niche. The chemokine, CXCL12, can cause HSCs to migrate to the vascular niche. Depletion of CXCR4 reduces the number of HSCs in the vascular niche (Sugiyama et al., 2006; Arai et al., 2009). Osteoclast- and osteoblast-mediated bone remodeling results in a Ca2+ gradient in the endosteum, enabling calcium-sensitive HSCs to migrate appropriately (Adams et al., 2006).
The bone marrow is relatively hypoxic (1–2% O2) (Cipolleschi et al., 1993), although oxygen levels are higher closer to the vascular niche, presenting an oxygen gradient. Thus, both the oxygen and calcium gradients regulate HSC quiescence contingent upon the location within the osteoblastic or vascular niche. Migration to the vascular niche is a necessary prelude to eventual provision of myeloid and lymphoid hematopoietic cells to the peripheral blood supply (Iwasaki and Suda, 2009). There exists a possible role for redox signaling and thiols in HSC subpopulation migration and differentiation. Aging in mice is accompanied by accumulation of HSCs in the endosteum as well as increased levels of DNA damage (Rossi et al., 2007; Kohler et al., 2009). This correlates with an increased number, but decreased function, of older HSCs (Chambers et al., 2007), perhaps reflecting accumulated oxidative damage associated with aging.
It has been known, for some time, that cysteines and thiols are important in bone marrow proliferation (Baldini and Sacchetti, 1953). Long-term self-renewing HSCs have low levels of intracellular ROS, and mice deficient in ROS regulating genes have HSCs that do not maintain quiescence or self-renewal capabilities (Naka et al., 2007). Two subpopulations of HSCs have been indentified. ROSlow HSCs retain self-renewal capabilities in serial transplantations, whereas this capacity is diminished in ROShigh HSCs. Thiol-containing antioxidants rescue HSC self-renewal (Jang and Sharkis, 2007). As yet, there is no information of whether such subpopulations have niche preferences or migrate differently.
HSCs from Nrf2null mice are more sensitive to oxidative stress, implicating the redox-sensitive transcription factor in the regulation of HSC function. Moreover, Id1 is a helix-loop-helix transcription factor that also has a role in myeloid differentiation (Tanaka et al., 1998). The forkhead O (FoxO) family of transcription factors can protect quiescent HSC cells from oxidative stress via the upregulation of ROS-detoxifying genes, such as MnSOD, catalase, and GADD45. FoxOs are expressed with the transition from HSCs to myeloid progenitors, and conditional knockout of FoxO increases ROS and alters the repopulating capacities of HSCs. Treatment with the antioxidant, N-acetyl-cysteine (NAC), restores these defects and the FoxO transcriptional program (Tothova et al., 2007). Studies using FoxO3 germ-line knockout animals indicate that p38 MAPK may participate in these pathways (Miyamoto et al., 2007). These studies suggest that redox-sensitive transcriptional programs may be specific to certain subpopulations of HSCs. It is reasonable to speculate that the difference in ROS levels in myeloid progenitor and quiescent HSCs may act in intracellular-signaling events that drive HSC differentiation. The modulation of oxidizing proteins via redox-sensitive cysteines may have a key role in these events. The role of ROS in HSC function has been reviewed elsewhere (Naka et al., 2008). From our earlier published work (Gate et al., 2004), we observed that hematopoietic cells from GSTP-deficient animals proliferated faster than their wild-type counterparts. This was associated with an increase in JAK-STAT pathway activation in response to interleukin-3 and was possibly linked to a decrease in expression of SHP-1 and −2 or other phosphatases.
TLK199 (Telintra®; ezatiostat)
Telintra was designed to take advantage of the fact that GSTP is frequently overexpressed in some cancers and in drug-resistant cancer cells. It was designed and developed as a peptidomimetic inhibitor of GSTP and initially called TLK199 [γ-glutamyl-S-(benzyl)-cysteinyl-R-(-) phenyl glycine diethyl ester]. Whereas therapeutic utility initially focused on overcoming GSTP-associated drug resistance, preclinical studies in mice revealed the drug also caused increased circulating blood cells of all lineages (Ruscoe et al., 2001). Telintra increased peripheral white blood cell numbers in wild-type, as compared to GSTP-deficient, mice. Further, GSTP-null animals exhibited elevated numbers of circulating leukocytes, suggesting an increase in myeloid cell differentiation and proliferation. Subsequently, the phenotype was associated with an increased number of bone marrow progenitor cells that populated circulating mature blood cells (Gate et al., 2004). Mechanistically, these observations are not inconsistent with the fact that Telintra dissociates GSTP from the JNK-containing complex, allowing kinase phosphorylation and downstream activation of STAT proteins with resultant myeloproliferative effects. The importance of other GST isozymes in myeloproliferative events may be of additional interest. GSTA1 has been shown to suppress stress-induced activation of JNK signaling, suggesting that GST binding may be promiscuous (Romero et al., 2006). Increased GSTP also has the potential to mediate the S-glutathionylation of a number of proteins that may be involved in myeloproliferative events (e.g., JNK and SHP-1,-2, etc.).
Phase 1 testing of ezatiostat, for the treatment of myelodysplastic syndrome (MDS; a stem cell disorder characterized by ineffective blood cell production and an increased risk for transformation to acute leukemia) was conducted in a multidose escalation study. Patients received 10 dose levels (ranging from 200 to 6,000 mg) of ezatiostat tablets in divided doses on a 21-day cycle for a maximum of eight cycles. Safety and kinetics were evaluated in 45 patients with low to intermediate-2 International Prognostic Scoring System risk MDS. No dose-limiting toxicities were observed. The most common grade 1 or 2 adverse events were nonhematologic and included nausea, diarrhea, vomiting, abdominal pain, constipation, anorexia, and dyspepsia. Seventeen hematologic improvement (HI) responses were observed, with 11 HI responses at doses of 4,000–6,000 mg/day. HI responses occurred in all lineages, including three bilineage and one complete cytogenetic response. Decreased numbers of red blood cell and platelet transfusions and, in some cases, transfusion independence were attained. At this point, extended dose schedules of ezatiostat tablets are under investigation. The drug has also shown positive results in an ongoing phase II clinical trial for MDS (online company reference: http://www.telik.com/pr/2010/pr_2010_0608.html last accessed February 18 2011). Once again, treated patients with low to intermediate-1 risk MDS demonstrated multi-lineage hematologic improvements, including decreased requirements for red blood cell, platelet, and growth factor support. Additional clinical trials are focusing on the use of the drug in the treatment of chronic idiopathic neutropenia and additional blood disorders.
Summary
For metabolism of anticancer drugs, the catalytic importance of GSTP to form thioether conjugates is not substantial. Nevertheless, high levels of GSTP are commonly found in cancers and drug-resistant cell lines. Realistically, convergent evolution could have selected GSTP and other related redox proteins for multiple cellular functions. It is apparent that GSTP has established additional roles in the regulation of kinase activity and post-translational S-glutathionylation reactions. The biological importance of these reactions may have provided strong selection pressures. It would seem that GSH and GST have roles that extend further than simple detoxification reactions. Indeed, it does not seem unreasonable to predict that S-glutathionylation may provide regulatory control complementary to other well-studied, established post-translational modifications. Pharmaceutical discovery and development efforts have utilized the GSH and GSTP platform and produced some interesting lead candidates that are in varying stages of preclinical and clinical development.
Footnotes
Declaration of interest
KDT was a member of the SAB of Telik inc and holds stock in the company.
References
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, et al. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F, Yoshihara H, Hosokawa K, Nakamura Y, Gomei Y, Iwasaki H, et al. Niche regulation of hematopoietic stem cells in the endosteum. Ann N Y Acad Sci. 2009;1176:36–46. doi: 10.1111/j.1749-6632.2009.04561.x. [DOI] [PubMed] [Google Scholar]
- Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- Axarli I, Labrou NE, Petrou C, Rassias N, Cordopatis P, Clonis YD. Sulphonamide-based bombesin prodrug analogues for glutathione transferase, useful in targeted cancer chemotherapy. Eur J Med Chem. 2009;44:2009–2016. doi: 10.1016/j.ejmech.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Bakker J, Lin X, Nelson WG. Methyl-CpG binding domain protein 2 represses transcription from hypermethylated pi-class glutathione S-transferase gene promoters in hepatocellular carcinoma cells. J Biol Chem. 2002;277:22573–22580. doi: 10.1074/jbc.M203009200. [DOI] [PubMed] [Google Scholar]
- Baldini M, Sacchetti C. Effect of cystine and cysteine on human bone marrow cultured in medium deficient in amino acids [in French]. Rev Hematol. 1953;8:3–19. [PubMed] [Google Scholar]
- Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, et al. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- Batist G, Tulpule A, Sinha BK, Katki AG, Myers CE, Cowan KH. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986;261:15544–15549. [PubMed] [Google Scholar]
- Borch RF, Valente RR. Synthesis, activation, and cytotoxicity of aldophosphamide analogues. J Med Chem. 1991;34:3052–3058. doi: 10.1021/jm00114a014. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaccio PJ, Tew KD, LaCreta FP. Enzymatic conjugation of chlorambucil with glutathione by human glutathione S-transferases and inhibition by ethacrynic acid. Biochem Pharmacol. 1991;42:1504–1507. doi: 10.1016/0006-2952(91)90468-k. [DOI] [PubMed] [Google Scholar]
- Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- Comporti M. Three models of free radical-induced cell injury. Chem Biol Interact. 1989;72:1–56. doi: 10.1016/0009-2797(89)90016-1. [DOI] [PubMed] [Google Scholar]
- Conant CG, Stephens RS. Chlamydia attachment to mammalian cells requires protein disulfide isomerase. Cell Microbiol. 2007;9:222–232. doi: 10.1111/j.1462-5822.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- Cowell IG, Dixon KH, Pemble SE, Ketterer B, Taylor JB. The structure of the human glutathione S-transferase pi gene. Biochem J. 1988;255:79–83. doi: 10.1042/bj2550079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W, Jr., Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296:1–6. [PubMed] [Google Scholar]
- Demasi M, Shringarpure R, Davies KJ. Glutathiolation of the proteasome is enhanced by proteolytic inhibitors. Arch Biochem Biophys. 2001;389:254–263. doi: 10.1006/abbi.2001.2332. [DOI] [PubMed] [Google Scholar]
- Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, Tew KD. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66:6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay VJ, Townsend DM, Saavedra JE, Buzard GS, Citro ML, Keefer LK, et al. Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug. Mol Pharmacol. 2004;65:1070–1079. doi: 10.1124/mol.65.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- Garcia J, Han D, Sancheti H, Yap LP, Kaplowitz N, Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J Biol Chem. 2010;285:39646–39654. doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate L, Majumdar RS, Lunk A, Tew KD. Increased myeloproliferation in glutathione S-transferase pi-deficient mice is associated with a deregulation of JNK and Janus kinase/STAT pathways. J Biol Chem. 2004;279:8608–8616. doi: 10.1074/jbc.M308613200. [DOI] [PubMed] [Google Scholar]
- Gildenhuys S, Wallace LA, Burke JP, Balchin D, Sayed Y, Dirr HW. Class pi glutathione transferase unfolds via a dimeric and not monomeric intermediate: functional implications for an unstable monomer. Biochemistry. 2010;49:5074–5081. doi: 10.1021/bi100552d. [DOI] [PubMed] [Google Scholar]
- Golemis EA, Tew KD, Dadke D. Protein interaction-targeted drug discovery: evaluating critical issues. Biotechniques. 2002;32:636–638. 640, 642. doi: 10.2144/02323dd01. passim. [DOI] [PubMed] [Google Scholar]
- Goplen D, Wang J, Enger PO, Tysnes BB, Terzis AJ, Laerum OD, et al. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res. 2006;66:9895–9902. doi: 10.1158/0008-5472.CAN-05-4589. [DOI] [PubMed] [Google Scholar]
- Gravina SA, Mieyal JJ. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry. 1993;32:3368–3376. doi: 10.1021/bi00064a021. [DOI] [PubMed] [Google Scholar]
- Haendeler J. Thioredoxin-1 and post-translational modifications. Antioxid Redox Signal. 2006;8:1723–1728. doi: 10.1089/ars.2006.8.1723. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Marchan R, Krance SM, Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J Biol Chem. 2007;282:14337–14347. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci U S A. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CJ, Wolf CR, Kitteringham N, Powell H, Otto D, Park BK. Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase pi. Proc Natl Acad Sci U S A. 2000;97:12741–12745. doi: 10.1073/pnas.220176997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J Biol Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- Hutchens S, Manevich Y, He L, Tew KD, Townsend DM. Cellular resistance to a nitric oxide releasing glutathione S-transferase P-activated prodrug, PABA/NO. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9407-5. [Epub Mar 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Sci. 2009;100:1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Tordova M, O'Donnell R, Parsons JF, Hayden JB, Gilliland GL, et al. Structure and function of the xenobiotic substrate-binding site and location of a potential non-substrate-binding site in a class pi glutathione S-transferase. Biochemistry. 1997;36:9690–9702. doi: 10.1021/bi970805s. [DOI] [PubMed] [Google Scholar]
- Kavanagh JJ, Gershenson DM, Choi H, Lewis L, Patel K, Brown GL, et al. Multi-institutional phase 2 study of TLK286 (TELCYTA, a glutathione S-transferase P1-1 activated glutathione analog prodrug) in patients with platinum and paclitaxel refractory or resistant ovarian cancer. Int J Gynecol Cancer. 2005;15:593–600. doi: 10.1111/j.1525-1438.2005.00114.x. [DOI] [PubMed] [Google Scholar]
- Kellen E, Hemelt M, Broberg K, Golka K, Kristensen VN, Hung RJ, et al. Pooled analysis and meta-analysis of the glutathione S-transferase P1 Ile 105Val polymorphism and bladder cancer: a HuGE-GSEC review. Am J Epidemiol. 2007;165:1221–1230. doi: 10.1093/aje/kwm003. [DOI] [PubMed] [Google Scholar]
- Kohler A, Schmithorst V, Filippi MD, Ryan MA, Daria D, Gunzer M, et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood. 2009;114:290–298. doi: 10.1182/blood-2008-12-195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraggerud SM, Oldenburg J, Alnaes GI, Berg M, Kristensen VN, Fossa SD, et al. Functional glutathione S-transferase genotypes among testicular germ cell tumor survivors: associations with primary and post-chemotherapy tumor histology. Pharmacogenet Genomics. 2009;19:751–759. doi: 10.1097/FPC.0b013e3283304253. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Wu JW, Lin CP. In vivo imaging of hematopoietic stem cells and their microenvironment. J Biophotonics. 2009;2:619–631. doi: 10.1002/jbio.200910072. [DOI] [PubMed] [Google Scholar]
- Lo HW, Ali-Osman F. Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr Opin Pharmacol. 2007;7:367–374. doi: 10.1016/j.coph.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Lyttle MH, Satyam A, Hocker MD, Bauer KE, Caldwell CG, Hui HC, et al. Glutathione-S-transferase activates novel alkylating agents. J Med Chem. 1994;37:1501–1507. doi: 10.1021/jm00036a016. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc Natl Acad Sci U S A. 2004;101:3780–3785. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B, Danielson UH. Glutathione transferases—structure and catalytic activity. CRC Crit Rev Biochem. 1988;23:283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- Naka K, Ohmura M, Hirao A. Regulation of the self-renewal ability of tissue stem cells by tumor-related genes. Cancer Biomark. 2007;3:193–201. doi: 10.3233/cbm-2007-34-504. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Matsushima M, Song H, Kikuchi M. A role of PDI in the reductive cleavage of mixed disulfides. J Biochem. 1996;120:525–530. doi: 10.1093/oxfordjournals.jbchem.a021445. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- Noguera-Mazon V, Lemoine J, Walker O, Rouhier N, Salvador A, Jacquot JP, et al. Glutathionylation induces the dissociation of 1-Cys D-peroxiredoxin non-covalent homodimer. J Biol Chem. 2006;281:31736–31742. doi: 10.1074/jbc.M602188200. [DOI] [PubMed] [Google Scholar]
- Okamura T, Singh S, Buolamwini J, Haystead T, Friedman H, Bigner D, et al. Tyrosine phosphorylation of the human glutathione S-transferase P1 by epidermal growth factor receptor. J Biol Chem. 2009;284:16979–16989. doi: 10.1074/jbc.M808153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou W, Silver J. Role of protein disulfide isomerase and other thiol-reactive proteins in HIV-1 envelope protein-mediated fusion. Virology. 2006;350:406–417. doi: 10.1016/j.virol.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Park B, Lee S, Kim E, Cho K, Riddell SR, Cho S, et al. Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. Cell. 2006;127:369–382. doi: 10.1016/j.cell.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T, Helaakoski T, Tasanen K, Myllyla R, Huhtala ML, Koivu J, et al. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987;6:643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, Paracchini V, Autrup H, Barros-Dios JM, Benhamou S, Boffetta P, et al. Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J Epidemiol. 2006;164:1027–1042. doi: 10.1093/aje/kwj321. [DOI] [PubMed] [Google Scholar]
- Reinemer P, Dirr HW, Ladenstein R, Huber R, Lo Bello M, Federici G, et al. Three-dimensional structure of class pi glutathione S-transferase from human placenta in complex with S-hexylglutathione at 2.8 A resolution. J Mol Biol. 1992;227:214–226. doi: 10.1016/0022-2836(92)90692-d. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Jeong W, Chang TS, Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int Suppl. 2007:S3–S8. doi: 10.1038/sj.ki.5002380. [DOI] [PubMed] [Google Scholar]
- Romero L, Andrews K, Ng L, O’Rourke K, Maslen A, Kirby G. Human GSTA1-1 reduces c-Jun N-terminal kinase signalling and apoptosis in Caco-2 cells. Biochem J. 2006;400:135–141. doi: 10.1042/BJ20060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario LA, O'Brien ML, Henderson CJ, Wolf CR, Tew KD. Cellular response to a glutathione S-transferase P1-1 activated prodrug. Mol Pharmacol. 2000;58:167–174. doi: 10.1124/mol.58.1.167. [DOI] [PubMed] [Google Scholar]
- Rosen LS, Brown J, Laxa B, Boulos L, Reiswig L, Henner WD, et al. Phase I study of TLK286 (glutathione S-transferase P1-1 activated glutathione analogue) in advanced refractory solid malignancies. Clin Cancer Res. 2003;9:1628–1638. [PubMed] [Google Scholar]
- Rosen LS, Laxa B, Boulos L, Wiggins L, Keck JG, Jameson AJ, et al. Phase 1 study of TLK286 (Telcyta) administered weekly in advanced malignancies. Clin Cancer Res. 2004;10:3689–3698. doi: 10.1158/1078-0432.CCR-03-0687. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rossi R, Giustarini D, Milzani A, Dalle-Donne I. Membrane skeletal protein S-glutathionylation and hemolysis in human red blood cells. Blood Cells Mol Dis. 2006;37:180–187. doi: 10.1016/j.bcmd.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Ruscoe JE, Rosario LA, Wang T, Gate L, Arifoglu P, Wolf CR, et al. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther. 2001;298:339–345. [PubMed] [Google Scholar]
- Saavedra JE, Srinivasan A, Buzard GS, Davies KM, Waterhouse DJ, Inami K, et al. PABA/NO as an anticancer lead: analogue synthesis, structure revision, solution chemistry, reactivity toward glutathione, and in vitro activity. J Med Chem. 2006;49:1157–1164. doi: 10.1021/jm050700k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gomez FJ, Diez-Dacal B, Pajares MA, Llorca O, Perez-Sala D. Cyclopentenone prostaglandins with dienone structure promote cross-linking of the chemoresistance-inducing enzyme glutathione transferase P1-1. Mol Pharmacol. 2010;78:723–733. doi: 10.1124/mol.110.065391. [DOI] [PubMed] [Google Scholar]
- Sau A, Pellizzari Tregno F, Valentino F, Federici G, Caccuri AM. Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys. 2010;500:116–122. doi: 10.1016/j.abb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Sequist LV, Fidias PM, Temel JS, Kolevska T, Rabin MS, Boccia RV, et al. Phase 1-2a multicenter dose-ranging study of canfosfamide in combination with carboplatin and paclitaxel as first-line therapy for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:1389–1396. doi: 10.1097/JTO.0b013e3181b6b84b. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- Shelton MD, Kern TS, Mieyal JJ. Glutaredoxin regulates nuclear factor kappa-B and intercellular adhesion molecule in Müller cells: model of diabetic retinopathy. J Biol Chem. 2007;282:12467–12474. doi: 10.1074/jbc.M610863200. [DOI] [PubMed] [Google Scholar]
- Singh S, Okamura T, Ali-Osman F. Serine phosphorylation of glutathione S-transferase P1 (GSTP1) by PKCalpha enhances GSTP1-dependent cisplatin metabolism and resistance in human glioma cells. Biochem Pharmacol. 2010;80:1343–1355. doi: 10.1016/j.bcp.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Sohn J, Rudolph J. Catalytic and chemical competence of regulation of cdc25 phosphatase by oxidation/reduction. Biochemistry. 2003;42:10060–10070. doi: 10.1021/bi0345081. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Swiatkowska M, Szymanski J, Padula G, Cierniewski CS. Interaction and functional association of protein disulfide isomerase with alphaVbeta3 integrin on endothelial cells. FEBS J. 2008;275:1813–1823. doi: 10.1111/j.1742-4658.2008.06339.x. [DOI] [PubMed] [Google Scholar]
- Tager M, Kroning H, Thiel U, Ansorge S. Membrane-bound protein disulfide isomerase (PDI) is involved in regulation of surface expression of thiols and drug sensitivity of B-CLL cells. Exp Hematol. 1997;25:601–607. [PubMed] [Google Scholar]
- Tanaka K, Pracyk JB, Takeda K, Yu ZX, Ferrans VJ, Deshpande SS, et al. Expression of Id1 results in apoptosis of cardiac myocytes through a redox-dependent mechanism. J Biol Chem. 1998;273:25922–25928. doi: 10.1074/jbc.273.40.25922. [DOI] [PubMed] [Google Scholar]
- Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- Tew KD. Redox in redux: emergent roles for glutathione S-transferase P (GSTP) in regulation of cell signaling and S-glutathionylation. Biochem Pharmacol. 2007;73:1257–1269. doi: 10.1016/j.bcp.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Tian G, Xiang S, Noiva R, Lennarz WJ, Schindelin H. The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell. 2006;124:61–73. doi: 10.1016/j.cell.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Townsend D, Tew K. Cancer drugs, genetic variation, and the glutathione-S-transferase gene family. Am J Pharmacogenomics. 2003;3:157–172. doi: 10.2165/00129785-200303030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. 2007;7:313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Findlay VJ, Fazilev F, Ogle M, Fraser J, Saavedra JE, et al. A glutathione S-transferase pi-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69:501–508. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009a;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Manevich Y, He L, Xiong Y, Bowers RR, Jr., Hutchens S, et al. Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res. 2009b;69:7626–7634. doi: 10.1158/0008-5472.CAN-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, Shen H, Staros AL, Gate L, Tew KD. Efficacy of a glutathione S-transferase pi-activated prodrug in platinum-resistant ovarian cancer cells. Mol Cancer Ther. 2002;1:1089–1095. [PMC free article] [PubMed] [Google Scholar]
- Vergote I, Finkler N, del Campo J, Lohr A, Hunter J, Matei D, et al. Phase 3 randomised study of canfosfamide (Telcyta, TLK286) versus pegylated liposomal doxorubicin or topotecan as third-line therapy in patients with platinum-refractory or -resistant ovarian cancer. Eur J Cancer. 2009;45:2324–2332. doi: 10.1016/j.ejca.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Voso MT, Hohaus S, Guidi F, Fabiani E, D'Alo F, Groner S, et al. Prognostic role of glutathione S-transferase polymorphisms in acute myeloid leukemia. Leukemia. 2008;22:1685–1691. doi: 10.1038/leu.2008.169. [DOI] [PubMed] [Google Scholar]
- Wang AL, Tew KD. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985;69:677–682. [PubMed] [Google Scholar]
- Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, et al. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001a;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- Wang T, Arifoglu P, Ronai Z, Tew KD. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem. 2001b;276:20999–21003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- Wetzelberger K, Baba SP, Thirunavukkarasu M, Ho YS, Maulik N, Barski OA, et al. Postischemic deactivation of cardiac aldose reductase: role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids. J Biol Chem. 2010;285:26135–26148. doi: 10.1074/jbc.M110.146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Wink DA, Cook JA, Christodoulou D, Krishna MC, Pacelli R, Kim S, et al. Nitric oxide and some nitric oxide donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide. 1997;1:88–94. doi: 10.1006/niox.1996.0108. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem. 2010;285:38641–38648. doi: 10.1074/jbc.M110.162578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Ivanov VN, Habelhah H, Tew K, Ronai Z. Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res. 2000;60:4053–4057. [PubMed] [Google Scholar]
- Yu CX, Li S, Whorton AR. Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol. 2005;68:847–854. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]