Abstract
Porphyromonas gingivalis is one of the keystone pathogens associated with chronic periodontitis. All P. gingivalis strains examined thus far produce outer membrane vesicles. Recent studies have found that vesicles possess some well-known virulence factors of P. gingivalis such as adhesins, toxins and proteolytic enzymes. Carrying most of the characteristic features of their parent P. gingivalis cells, vesicles communicate with host cells and other members of microbial biofilms, resulting in the transmission of virulence factors into these host cells and the formation of pathogenic bacteria-dominated microbial communities. An in-depth understanding of both the nature and role of vesicles in the pathogenicity of P. gingivalis is both important and timely, particularly when speaking of periodontitis and its related systemic effects.
Keywords: Gram-negative bacteria, interspecies interactions outer membrane vesicles, regulation of OMV biogenesis virulence factors
Most Gram-negative bacteria (both pathogenic and nonpathogenic) secrete outer membrane vesicles (OMV) that can be surface-bound or cell-free spherical structures. Gram-positive bacteria also produce membrane-derived vesicles, such as the oral bacterium Streptococcus mutans [1]. OMVs have recently gained special recognition for their role in bacterial virulence and are known to play similar roles as their parent cells with regard to mediation of adherence, biofilm formation, invasion, host cell damage and modulation of host immune responses [2]. More importantly, OMVs appear to confer distinct functional advantages over whole bacterial cells. For example, vesicles that are enriched for bacterial virulence factors are protected from dilution and proteolytic degradation, and are able to travel to distant targets [2]. Besides the important role of vesicles in bacterial pathogenicity, there is also an interest in bacterial vesicles for their potential as a natural adjuvant, one that contains a mixture of antigens [3]. Several recent reviews have documented the biogenesis and function of bacterial OMV [4–6].
P. gingivalis is a Gram-negative bacterium associated with chronic periodontitis. Vesiculation in P. gingivalis was first reported in the 1980s [7–9], although its central function in the physiology and pathogenicity of the organism was not immediately understood. Consistent with vesicles of other Gram-negative bacteria, P. gingivalis vesicles are between 50 and 250 nm in diameter but are predominantly around 50 nm (Figure 1). Recent studies using genetic, proteomic and morphologic tools have demonstrated that P. gingivalis vesicles may act as intermediaries that carry a wide variety of virulence factors provided by their parent cells. This particular review will focus on the characteristics of vesiculation and the virulence functions of P. gingivalis vesicles. Hopefully, this discussion will uncover opportunities to utilize the characteristics of P. gingivalis vesicles for at least two distinct purposes, namely to reduce virulence and to engineer vaccines.
Figure 1. Porphyromonas gingivalis cells and vesicles shown by negative-stain transmission electron microscopy.
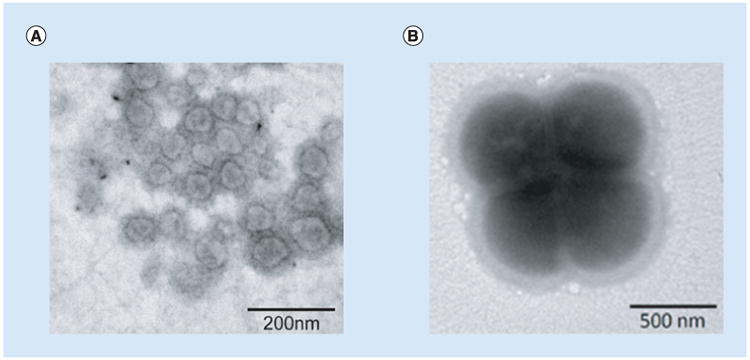
(A) The purified vesicles from P. gingivalis 33277 growth medium, showing the single membrane surrounding spherical structures. (B) Outer membrane blebbing on the surface of P. gingivalis 33277 cells.
Vesiculation of P. gingivalis & its regulation
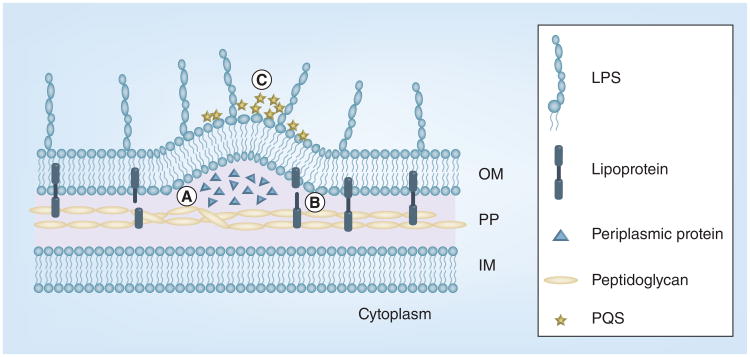
Biogenesis of bacterial OMV can be understood through several models (Figure 2). First, the bacterial outer membrane is pushed out through a physical force induced by accumulation of misfiled or overexpressed envelope proteins. This model is supported by a study using an Escherichia coli strain carrying an inducible DegP protein [10]. DegP is a member of the high-temperature requirement A (HtrA) family and functions as a chaperone at low temperatures in the periplasm of Gram-negative bacteria. McBroom and Kuehn demonstrated that the increased level of vesiculation in wildtype E. coli corresponded to the level of DegP overexpression [10]. Furthermore, these authors found that periplasmic protein overexpression-induced vesiculation was not specific to DegP, since there was also overexpression of periplasmic maltose-binding protein. Second, the linkage between the outer membrane and the underneath peptidoglycan layer was disrupted, which led to OMV shedding. This assumption was based on the observation that deficiencies in several proteins involved in the interconnection of the outer membrane and peptidoglycan or in lipopolysacchride formation had a dramatic impact on vesiculation [11,12]. An example is a 6.95 kDa lipoprotein (OprI) of Pseudomonas aeruginosa [13]. OprI is an abundant outer membrane protein and appears to covalently interact with the peptidoglycan layer [14,15]. Deletion of the oprI gene significantly enhances vesiculation in P. aeruginosa, which presumably results from loss of tethering outer membrane to peptidoglycan [14]. Finally, vesiculation may result in an increased local curvature of bacterial outer membrane. This model is supported by recent studies describing a bilayer-couple model involving a signaling molecule 2-heptyl-3-hydroxy-4-quinolone (P. aeruginosa quinolone signals [PQS]) of P. aeruginosa [16,17]. PQS appears necessary and sufficient for OMV formation through direct interaction with the bacterial membrane and expanding the outer membrane, which increases membrane curvature and leads to formation of vesicles.
Figure 2. Models of bacterial outer membrane vesicles biogenesis.
Three models are presented. (A) A physical force induced by accumulation of misfiled or overexpressed envelope proteins pushes out outer membrane vesicles. (B) The linkage between the outer membrane and the underneath peptidoglycan layer is disrupted. (C) Local curvature of bacterial outer membrane is enhanced by extracellular signals including PQS.
IM: Inner membrane; LPS: Lipopolysaccharide; OM: Outer membrane; PG: Peptidoglycan; PQS: P. aeruginosa quinolone signal.
P. gingivalis has been shown to express OMVs on its cell surface and release them into the environment [7–9]. Since the size of P. gingivalis vesicles ranges from 50 to 250 nm in diameter (predominately 50 nm), it was suggested that similar structures, previously reported in dental plaque samples [18], were likely vesicles derived from P. gingivalis or other Gram-negative oral bacteria [8]. Although all P. gingivalis strains tested (fimbriated or afimbriated) were observed to display small membranous vesicles budding from the outer membrane [19], the level of vesiculation varied among the strains [20].
Recent publications revealed a fimbrial type or expression-dependent vesiculation in P. gingivalis. P. gingivalis strains are classified into six types (type I and Ib–V) based on the strain-specific nucleotide sequences of the fimA gene that encodes a subunit of major (long) fimbriae [21,22]. When comparing vesicles expressed on the surface of P. gingivalis strains with a fimbrial allele type I (33277), type III (49417) and type IV (W83) using transmission electron microscopy, Kerr et al. found much more OMV on the surfaces of 33,277 and 49,417 than those on W83 cells [23]. Interestingly, when type IV FimA was introduced into the isogenic background of 33277, vesiculation was similar between the fimA parent strain and the recipient strain, suggesting that the fimbrial subtype was involved in vesicle biogenesis. Our recent studies also showed a fimbrial expression-mediated vesiculation in P. gingivalis. A higher number of vesicles were isolated in the growth media of P. gingivalis 33277 when compared with vesicles found in the media of its fimA mutant [20]. In addition, a reduced level of vesicle release was found in the media of W83, an afimbriated strain. The results also revealed that there was a positive correlation between the expression level of FimA and the level of vesicle production in a given P. gingivalis strain. Interestingly, a requirement for the synthesis of flagellar proteins for vesicle production was recently found in E. coli W3110 [24]. In fact, the deletion or overexpression of several membrane-associated proteins was known to affect vesiculation in many Gram-negative bacteria, likely due to an alteration in the envelope structure and/or a decrease in membrane stability [25].
Besides the expression of FimA, vesiculation of P. gingivalis has been linked to the expressions of an OmpA-like protein (Pgm6/7) and galactose 4-epimerase (GalE) [26,27]. GalE is known to catalyze the interconversion between uridine diphosphate (UDP)-glucose and UDP-galactose. It was reported that vesicles could not be found on either the surface of a galE mutant or in the growth media when monitored at different time points over 3 days in culture [27]. The authors felt that this could be due to the pleiotropic effects of the galE mutation on truncation of LPS O-antigen and nonglycosylated form of the outer membrane Omp85 homolog, which were also observed in the galE mutant. By contrast, vesicles were overproduced on the surface and in the media surrounding an ompA mutant of P. gingivalis [26]. In fact, hypervesiculation was reported in the ompA mutants of Salmonella, Vibrio cholerae and E. coli [28]. Although the mechanisms were not completely elucidated, the proteins appeared to play an important role in the maintenance of bacterial outer membrane integrity and cell wall turnover. E. coli OmpA, which was found to interact with peptidoglycan [29], presumably crosslinked the outer membrane with peptidoglycan to provide cell wall stability. One of the mechanisms of vesicle biogenesis proposed is the loss of a link between the outer membrane and peptidoglycan [5]. P. gingivalis Pgm6/7 proteins share a high degree of similarity to E. coli OmpA, and these P. gingivalis proteins may also modulate vesiculation of P. gingivalis through a similar mechanism as that seen in E. coli.
Vesiculation was also modulated in P. gingivalis, grown under different conditions. When observed under a transmission electron microscope, P. gingivalis W50 grown under hemin limited conditions displayed significantly more vesicles on its surface and released more cell-free vesicles into the surrounding environment than the organism grown under hemin excess conditions [30]. A similar observation was reported from an independent laboratory, in that, a P. gingivalis mutant with a deficiency in the utilization and transport of hemin produced twice as many vesicles as its parent strain did [31]. The mutant was also found to be more infectious and invasive than the parent strain when tested in a mouse model. These data suggest that there is an inverse correlation between the extent of vesiculation of P. gingivalis and hemin growth concentrations.
Two questions often asked are: why does P. gingivalis produce vesicles, and what functional advantage does vesiculation confer upon the bacteria against harmful environmental factors? In an earlier study of P. gingivalis vesicles, Grenier et al. demonstrated that vesicles interacted with chlorhexidine and, as a result, this interaction protected the organism against antibacterial treatment by acting as a decoy [32]. These authors further identified vesicle LPS as the major component involved in the binding to chlorhexidine. It was also reported that human β-defensin-3 bound specifically to hemagglutinin B of P. gingivalis [33]. Human β-defensins produced by gingival epithelia are known for their activity against oral bacteria including P. gingivalis [34]. Since hemagglutinin B is a major protein detected in P. gingivalis vesicles [20], it is reasonable to speculate that secretion of vesicles may reduce sensitivity of the bacteria to human β-defensin and thus facilitate survival of P. gingivalis in the oral environment. In addition, P. gingivalis vesicles may also act as decoys to absorb specific antibodies toward the bacteria. Thus, preincubation of vesicles with serum samples from periodontitis patients was able to reduce the immune reactivity of sera against P. gingivalis cells [35]. Another likely advantage of vesiculation is that cell associated vesicles serve as an expanded outer membrane, which optimizes membrane functionality. Outer membrane proteins including fimbrial proteins and gingipains are necessary for controlling autoaggregation, microcolony morphology and biovolume, or those processes required for maturation of P. gingivalis biofilms [36–38]. Moreover, the lack of cell-associated vesicles was recently linked with a decrease in the autoaggregation of P. gingivalis, which provided evidence of an enhanced function of the outer membrane mediated by vesicles [23].
P. gingivalis OMV contents
Bacterial vesicles originate from outer membrane blebbing and contain mostly outer membrane lipids including LPS, outer membrane proteins and some periplasmic and inner membrane components [39]. DNA and RNA are also packed within some bacterial vesicles. Biller et al. showed earlier that Prochlorococcus vesicles contain DNA fragments covering 50% of the entire chromosomal sequence and RNA from 95% of all open reading frames [40]. Recently, we observed presence of ribonucleic acids in P. gingivalis vesicles [41]. DNA fragments of genes encoding the major subunit of fimbriae (fimA and mfa1), superoxide dismutase (sod) and gingipains were detected, using PCR at relatively higher levels. Sizes of some DNA fragments in vesicles appear large enough to encode a virulence factor, such as the fimA gene. RNAs were also isolated and identified from purified vesicles of P. gingivalis. Furthermore, vesicle-mediated horizontal gene transfer was detected between P. gingivalis strains with efficiency at 1.9 × 10−7 [41], suggesting that packing DNA and RNA within vesicles could be one of the driving forces for the evolution of bacteria in the oral cavity ecosystem.
Assay of LPS staining patterns demonstrated that LPS profiles in the envelope fraction were almost identical in both P. gingivalis parent cells and their corresponding vesicles [26]. This was not the case when protein profiles of P. gingivalis cells and the vesicles were compared. Thus, accumulating evidence indicates that a select protein cargo exists within bacterial vesicles. In general, some outer membrane-associated virulence factors are enriched in vesicles [2]. Gingipains, a group of proteinases produced by P. gingivalis, were found preferentially packed into vesicles [42,43]. Therefore, a three- to fivefold enrichment of gingipains was observed in vesicles derived from P. gingivalis 33277 and W83, respectively, as compared with levels in their parent bacterial strains [20]. The mechanism(s) responsible for this selective assembly of protein cargos in P. gingivalis strains has not been identified. A study by Haurat et al. reported that the major outer membrane proteins RagA/B were excluded from vesicles of P. gingivalis W50, however, these proteins were detected in the vesicles of a porS- mutant with a deficiency in LPS biosynthesis [43]. RagA/B was also undetectable in the vesicles of the porS+ complement strain. Therefore, the authors suggested that P. gingivalis' LPS plays an important role in the selective exclusion of RagA/B from vesicles. It is proposed that LPS may be responsible for compartmentalization of the bacterial surface based on polysaccharide composition or length [43]. Some microdomains that are considered ‘hot spots’ for vesicle budding selectively contain or have an enriched subset of proteins. A selective protein cargo was also recently identified in P. gingivalis vesicles. When comparing protein ratios of vesicles/cell outer membrane using proteomic analyses, Veith et al. demonstrated that all proteins with a C-terminal secretion signal (CTD), including gingipains, exhibited mid-to-high ratios in vesicles, indicating an enrichment of these proteins in P. gingivalis vesicles, whereas some outer membrane proteins with a peptidoglycan-binding domain such as Omp40 and 41 and RagA/B showed lower ratios and had reduced levels in vesicles [42]. It was proposed that enriched CTD proteins promote formation of an electron dense surface layer via their connection with anionic LPS (A-LPS), and that this electron dense surface layer provides vesicles with a smooth surface layer [44].
Strain variations were found when the protein contents of vesicles derived from P. gingivalis 33277, W50 and W83 were analyzed. Not surprisingly, fimbrial proteins (FimA, C, D, E and Mfa1) were exclusively detected in 33277 vesicles, which is consistent with expression of these proteins in P. gingivalis 33277 cells [20,35,42]. A most striking finding was that tetratricopeptide repeat (TPR) domain proteins were not detectable in 33277 vesicles (Supplementary Table 1), while four or five TPR domain proteins were found in the vesicles from W50 and W83, even though there are eight genes encoding TPR proteins in the P. gingivais genome. Proteins with a TPR domain are found in both eukaryotic and prokaryotic cells and are involved in diverse cellular processes including transcriptional regulation, mRNA processing and protein folding and translocation [45]. Previous studies, using a mouse subcutaneous infection model, showed that expression of TprA protein (PG1385 identified in W50 and W83 vesicles) was upregulated in P. gingivalis W83 grown in a mouse subcutaneous chamber, and that the survival rates of mice infected with the tprA mutant were significantly higher than those of mice infected with the parent strain W83, suggesting a role of TprA in P. gingivalis' virulence [46]. However, the virulence mechanism of TprA remains to be clarified.
A recent study, using LC–MS/MS, identified 151 proteins from P. gingivalis W50, and all but one likely originated from the bacterial outer membrane or the periplasm [42]. This study further classified the proteins based on their known localization; 79/151 proteins were membrane proteins and the rest were assigned either as extracellular, luminal or undetermined. The importance of these findings is that the identification and the understanding of the distinct features of P. gingivalis vesicles may provide a molecular basis for the development of a safer vaccine using vesicles, rather than their parental bacterial cells. One of the most successful OMV-based vaccines is the one against Neisseria meningitides, which has been clinically used in several countries outside of the USA [47]. There is concern, however, that the complex contents of OMVs could contain risk factors leading to a clinical complication. For example, LPS, especially lipid A (also known as endotoxin), can induce a strong innate immune response in humans and cause a severe complication called endotoxic or septic shock. In the initial development of OMV-based vaccines, efforts have been focused on a reduction in LPS content by detergent extractions, and more recently, OMV vaccines have been developed from bacterial mutants producing an attenuated nontoxic LPS [47]. It is worthy to note that P. gingivalis LPS elicits a much weaker immune response compared with E. coli LPS and can be agonistic or antagonistic with respect to Toll-like receptor 4 activation based on the lipid A structure in the bacterial subpopulations [48]. Therefore, it is promising to know that investigators may take advantage of some unique features of P. gingivalis' LPS to identify and engineer safer liposome-based vaccine.
Virulence of P. gingivalis vesicles
In addition to carrying the major outer membrane contents of P. gingivalis, vesicles have shown the primary characteristic features of this organism, such as induction of inflammatory responses, impairment of host cells and transmission of virulence factors into host cells [27,35]. A recent study showed that after treatment with P. gingivalis vesicles (5–10 μg ml-1) for 1 h, oral epithelial cells in a confluent monolayer were detached from the wells of culture plates [35]. In agreement with these results, we found detachment of human epithelial cells or gingival fibroblasts from wells that were exposed to vesicles (>3 μg ml-1) [20]. However, the detachment of the cells was not observed with a lower concentration of vesicles (0.5 μg ml-1). The detachment effect was abolished when the vesicles were treated at 100°C for 30 min or in the presence of antigingipain antibodies suggesting a gingipain-dependent host cell impairment [35].
One of important features of P. gingivalis is its ability to invade and survive within host cells including fibroblasts, epithelial and endothelial cells [49]. Several well-known membrane proteins involved in P. gingivalis attachment to, and invasion of, host cells have been identified within vesicles, including FimA, hemagglutinin A and heat-stress protein (HtrA) [50 –53]. Not surprisingly, therefore, vesicles derived from P. gingivalis have been found to invade human primary oral epithelial cells, gingival fibroblasts and human umbilical vein endothelial cells [20,54–56]. Consistent with the invasive activity of P. gingivalis cells, 33277 vesicles, a strain with relatively high invasive activity was found to enter gingival epithelial cells and fibroblasts more efficiently than W83 vesicles. Notably, the effect of vesicle invasion on host cells has not as yet been fully established. However, a depletion of the intracellular transferrin was observed in epithelial cells invaded by P. gingivalis vesicles, which resulted from degradation of the cellular transferrin receptor within 1 h after vesicle invasion [54]. Another important question is, if internalization of both P. gingivalis cells and their vesicles into human epithelial cells are established using a similar mechanism. It can be said that at least both of them depend upon endocytic pathways for entry [55,57]. One key step of P. gingivalis host cell entry appears to involve secretion of the serine phosphatase SerB, which dephosphorylates the Ser 3 residue of the actin-depolymerizing molecule cofilin [58]. However, SerB was not found in P. gingivalis vesicles [42]. Thus, it is likely that P. gingivalis vesicles utilize a different mechanism for epithelial cell internalization. Another important feature of P. gingivalis invasion is the ability of the internalized cells to disseminate from one cell to another through actin-mediated membrane protrusions or an endocytic recycling pathway [57,59]. It will be interesting to determine, if some of the intracellular vesicles can also exit and re-enter other host cells. In opposition to this suggestion, Furuta et al. showed that P. gingivalis vesicles were sorted to lysosomes after being internalized via an endocytic pathway [55].
Chronic periodontitis is known to be the result of a breakdown of periodontal tissue-microbe homeostasis, which then leads to uncontrolled inflammation. A study using a mouse model demonstrated that after using an intranasal immunization method, vesicles effectively elicited P. gingivalis-specific serum IgG and IgA as well as salivary IgA 5 weeks after the initial immunization, whereas whole cells did not [27]. It was suggested that the increased antigenicity found in the vesicles might result from the more concentrated immune-dominant determinants on the vesicles compared with P. gingivalis cell surfaces. P. gingivalis vesicles are also known to play a role in the induction of inflammatory responses. Kou et al. found that stimulation of human gingival epithelial cells with vesicles led to an increased expression of cyclooxygenase-2, IL-6, IL-8 and matrix metalloproteinases (MMP-1 and MMP-3) [60]. Production of IL-8 is well known for its role in innate immune responses in periodontal tissues since this chemokine is involved in the recruitment of neutrophils from the vascularized gingival tissue to the gingival crevice [61]. By contrast, P. gingivalis vesicles appeared to repress immune responses induced by IFN-γ [62]. Expression of several genes involved in IFN-γ signal transduction, including genes encoding class II transactivator, Janus kinases (Jak1 and Jak2), were downregulated in vascular endothelial cells in the presence of P. gingivalis vesicles [62]. Since major histocompatibility complex class II molecules are essential for antigen presentation, it is likely that inhibition of their expression facilitates P. gingivalis escape from immune surveillance. Overall, these studies suggest that the effect of P. gingivalis vesicles on the human immune response system is a complicated matter, which may depend on both their strain-specific origins and the growth conditions of the parent cells.
Besides the role of P. gingivalis in periodontitis, an association between P. gingivalis and atherosclerosis has been extensively investigated in vitro, ex vivo and in animal models [63–67], which has made this bacterium a model of atherosclerosis pathogenesis initiated by microorganisms [68]. Previous studies have focused on intact P. gingivalis cells, based on the discovery of proteins and DNA of this bacterium in ex vivo samples. It was speculated that P. gingivalis may enter microvasculature following tooth brush or other dental procedures, which may lead to a transient bacteremia [69,70]. However, it has not been confirmed if live cells of P. gingivalis cause low-grade inflammation in the walls of arterial vessels. With recent findings, including the efficient invasive activity of vesicles and the presence of vesicle-associated major outer membrane proteins, DNA and RNA in P. gingivalis vesicles [20,41,42], it is likely that vesicles serve a significant role in atherosclerosis and represent a ‘Trojan horse’ to induce infections at secondary sites, such as in the walls of vessels than intact P. gingivalis cells. This concept is supported by in vitro studies that P. gingivalis vesicles, like their originating cells, were able to induce macrophages to form foam cells [71] and to serve as an activator of platelet aggregation [72].
Interspecies interaction mediated by Porphyromonas gingivalis vesicles
Dental plaque is a multispecies microbial biofilm. Interactions between/among different species are established by the specific recognition between each adhesin and its receptor. P. gingivalis expresses multiple adhesins that mediate coaggregation with many other oral bacteria [73–75]. All of these well-known adhesive molecules including fimbrial proteins, gingipains and hemagglutinin are found in P. gingivalis vesicles. Therefore, once released, vesicles can act as representatives of P. gingivalis to communicate with other oral bacteria. Studies of interspecies bacterial interactions revealed that P. gingivalis vesicles play a central role in introducing some bacteria to dental plaque (biofilm) and in promoting the microbial diversity of multibacterial structures. One example is that Staphylococcus aureus, frequently found in the nasal cavity and pharynx, was able to coaggregate with predominant oral microbes such as Streptococcus, Actinomyces and the mycelium-type Candida albicans, only after they were treated with P. gingivalis vesicles [76]. In another instance, P. gingivalis vesicles mediated coaggregation of Treponema denticola and Lachnoanaerobaculum saburreum [77], a process that did not occur in the absence of P. gingivalis vesicles or in the presence of heat-treated vesicles. Thus, it is conceivable that vesicles serve as a tether between these two bacteria. Binding of P. gingivalis to T. denticola is known to involve P. gingivalis fimbriae and T. denticola dentilisin [78], however, it is not clear what specific molecular mechanism is involved in the interaction between P. gingivalis and L. saburreum. Moreover, P. gingivalis vesicles were also able to enhance the attachment and invasion of Tannerella forsythia to epithelial cells, and showed a greater ability to do this than the originating cells [79]. Although this effect is not fully understood, bacterial molecules enriched in these vesicles are likely involved.
Conclusion & future perspective
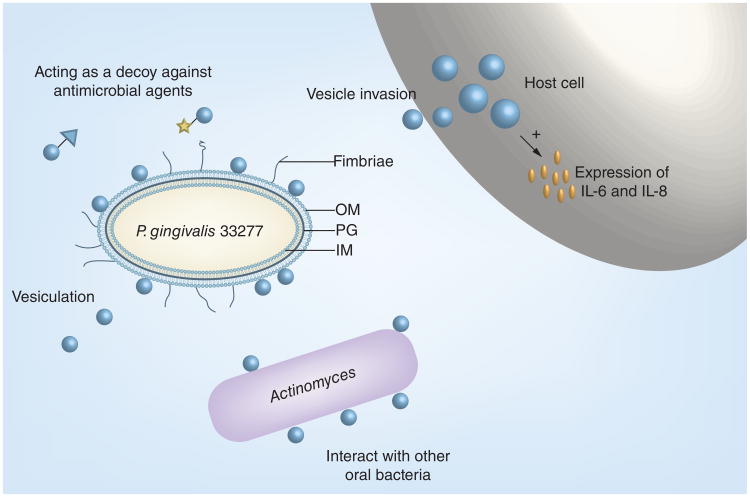
Vesicles carrying most of the bacterial virulence factors are probably the best weapon of P. gingivalis for its survival in the oral cavity (Figure 3). As evidenced above, they have diverse abilities, including the mediation of biofilm formation, organism–host interactions and immune responses. Characteristic features of vesicles, such as concentrated proteinases, virulence factors and the ability to travel to distant sites, may make them more important not only for the initiation and progression of periodontitis but also for the secondary pathophysiological effect of periodontitis-associated systemic disorders. Therefore, manipulating and/or blocking vesiculation of P. gingivalis and other periodontitis-associated oral pathogens may be one of therapeutic targets for virulence reduction. Future studies of vesicles may also focus on taking advantage of this acellular organelle to develop vaccines against P. gingivalis infection, since virulence mechanisms among different P. gingivalis strains vary and are strain specific. A comparison of functionally different vesicles derived from different P. gingivalis strains may provide an opportunity to select distinct vesicles, which can be modulated and utilized as a safer vaccine than their parental cells.
Figure 3. Porphyromonas gingivalis vesicle and its functions.
The formation of outer membrane vesicless is through the blebbing and pinching-off of the OM. Arrows show OM, PG and IM, respectively. Bacterial cell-free vesicles may function as decoys neutralizing antimicrobial agents, interacting with other oral bacteria, host cells and inducing host immune responses. This is a schematic presentation and is not to scale.
IM: Inner membrane; OM: Outer membrane; PG: Peptidoglycan.
Supplementary Material
Executive Summary.
Porphyromonas gingivalis
Porphyromonas gingivalis, a Gram-negative bacterium, is one of the keystone pathogens associated with chronic periodontitis and several systemic diseases.
All P. gingivalis strains examined thus far produce outer membrane vesicles with size ranging from 50–250 nm.
Porphyromonas gingivalis outer membrane vesicles contents
LPS profiles in the envelope fraction were almost identical in both Porphyromonas gingivalis parent cells and their corresponding vesicles.
DNA and RNA are packed within P. gingivalis vesicles, including DNA fragments of genes encoding fimA, mfa1, sod, rgp and kgp. Sizes of some DNA fragments in vesicles are large enough to encode a virulence factor.
Major outer membrane-associated virulence factors of P. gingivalis are exported through vesicles. Gingipains, a group of proteinases, are found preferentially packed into vesicles.
Virulence of Porphyromonas gingivalis vesicles
Vesicles derived from Porphyromonas gingivalis are able to invade human primary oral epithelial cells, gingival fibroblasts and human umbilical vein endothelial cells.
The increased antigenicity found in the vesicles might result from the more concentrated immune-dominant determinants on the vesicles compared with P. gingivalis cell surfaces.
Vesicles serve a significant role in atherosclerosis and represent a ‘Trojan horse’ to induce infections at secondary sites, such as in the walls of vessels than intact P. gingivalis cells.
P. gingivalis vesicles were also able to enhance the attachment and invasion of Tannerella forsythia to epithelial cells, and showed a greater ability to do this than the originating cells.
Acknowledgments
We thank D Marver for a critical reading of the manuscript.
This work was supported by Public Health Service grants DE020915 and DE022428 (H Xie) from NIDCR, and by U54MD007593 from NIMHD.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Supplementary data: To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/full/10.2217/fmb.15.63
Disclaimer: The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Liao S, Klein MI, Heim KP, et al. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol. 2014;196(13):2355–2366. doi: 10.1128/JB.01493-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Bonnington KE, Kuehn MJ. Protein selection and export via outer membrane vesicles. Biochim Biophys Acta. 2014;1843(8):1612–1619. doi: 10.1016/j.bbamcr.2013.12.011. Reviews protein trafficking and secretion in bacteria via outer membrane vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acevedo R, Fernandez S, Zayas C, et al. Bacterial outer membrane vesicles and vaccine applications. Front Immunol. 2014;5:121. doi: 10.3389/fimmu.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Ann Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Berleman J, Auer M. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ Microbiol. 2013;15(2):347–354. doi: 10.1111/1462-2920.12048. Summarizes the role of outer membrane vesicles (OMVs) in cell-to-cell exchange of DNA, toxins, protein and small signalling molecules in microbial communities and pathogenic processes. [DOI] [PubMed] [Google Scholar]
- 6.Manning AJ, Kuehn MJ. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J Mol Microbiol Biotechnol. 2013;23(1–2):131–141. doi: 10.1159/000346548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smalley JW, Birss AJ. Trypsin-like enzyme activity of the extracellular membrane vesicles of Bacteroides gingivalis W50. J Gen Microb. 1987;133(10):2883–2894. doi: 10.1099/00221287-133-10-2883. [DOI] [PubMed] [Google Scholar]
- 8.Grenier D, Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams GD, Holt SC. Characteristics of the outer membrane of selected oral Bacteroides species. Can J Microb. 1985;31(3):238–250. doi: 10.1139/m85-046. [DOI] [PubMed] [Google Scholar]
- 10.Mcbroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63(2):545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deatherage BL, Lara JC, Bergsbaken T, Rassoulian Barrett SL, Lara S, Cookson BT. Biogenesis of bacterial membrane vesicles. Mol Microbiol. 2009;72(6):1395–1407. doi: 10.1111/j.1365-2958.2009.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TT, Saxena A, Beveridge TJ. Effect of surface lipopolysaccharide on the nature of membrane vesicles liberated from the Gram-negative bacterium Pseudomonas aeruginosa. J Electron Microsc. 2003;52(5):465–469. doi: 10.1093/jmicro/52.5.465. [DOI] [PubMed] [Google Scholar]
- 13.Duchene M, Barron C, Schweizer A, Von Specht BU, Domdey H. Pseudomonas aeruginosa outer membrane lipoprotein I gene: molecular cloning, sequence, and expression in Escherichia coli. J Bacteriol. 1989;171(8):4130–4137. doi: 10.1128/jb.171.8.4130-4137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wessel AK, Liew J, Kwon T, Marcotte EM, Whiteley M. Role of Pseudomonas aeruginosa peptidoglycan-associated outer membrane proteins in vesicle formation. J Bacteriol. 2013;195(2):213–219. doi: 10.1128/JB.01253-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock RE, Irvin RT, Costerton JW, Carey AM. Pseudomonas aeruginosa outer membrane: peptidoglycan-associated proteins. J Bacteriol. 1981;145(1):628–631. doi: 10.1128/jb.145.1.628-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Schertzer JW, Whiteley M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. mBio. 2012;3(2) doi: 10.1128/mBio.00297-11. (Epub ahead of print). Presents a ‘bilayer couple’ model that may account for OMV formation under all conditions and raise the possibility of a universal paradigm for vesicle production in prokaryotes with features strikingly different from what is known in eukaryotes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437(7057):422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 18.Halhoul N, Colvin JR. The ultrastructure of bacterial plaque attached to the gingiva of man. Arch Oral Biol. 1975;20(2):115–118. doi: 10.1016/0003-9969(75)90164-8. [DOI] [PubMed] [Google Scholar]
- 19.Handley PS, Tipler LS. An electron microscope survey of the surface structures and hydrophobicity of oral and non-oral species of the bacterial genus Bacteroides. Arch Oral Biol. 1986;31(5):325–335. doi: 10.1016/0003-9969(86)90047-6. [DOI] [PubMed] [Google Scholar]
- 20.Mantri CK, Chen CH, Dong X, et al. Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis. Microbiology Open. 2015;4(1):53–65. doi: 10.1002/mbo3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara T, Morishima S, Takahashi I, Hamada S. Molecular cloning and sequencing of the fimbrilin gene of Porphyromonas gingivalis strains and characterization of recombinant proteins. Biochem Biophys Res Comm. 1993;197(1):241–247. doi: 10.1006/bbrc.1993.2467. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa I, Amano A, Ohara-Nemoto Y, et al. Identification of a new variant of fimA gene of Porphyromonas gingivalis and its distribution in adults and disabled populations with periodontitis. J Peridontol Res. 2002;37(6):425–432. doi: 10.1034/j.1600-0765.2002.01637.x. [DOI] [PubMed] [Google Scholar]
- 23.Kerr JE, Abramian JR, Dao DH, et al. Genetic exchange of fimbrial alleles exemplifies the adaptive virulence strategy of Porphyromonas gingivalis. PLoS ONE. 2014;9(3):e91696. doi: 10.1371/journal.pone.0091696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manabe T, Kato M, Ueno T, Kawasaki K. Flagella proteins contribute to the production of outer membrane vesicles from Escherichia coli W3110. Biochem Biophys Res Comm. 2013;441(1):151–156. doi: 10.1016/j.bbrc.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Baker JL, Chen L, Rosenthal JA, Putnam D, Delisa MP. Microbial biosynthesis of designer outer membrane vesicles. Curr Opin Biotechnol. 2014;29C:76–84. doi: 10.1016/j.copbio.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwami J, Murakami Y, Nagano K, Nakamura H, Yoshimura F. Further evidence that major outer membrane proteins homologous to OmpA in Porp hyromonas gingivalis stabilize bacterial cells. Oral Micriobiol Immunol. 2007;22(5):356–360. doi: 10.1111/j.1399-302X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 27••.Nakao R, Hasegawa H, Ochiai K, et al. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS ONE. 2011;6(10):e26163. doi: 10.1371/journal.pone.0026163. Discovers differential immune responses induced by Porphyromonas gingivalis vesicles and their parent cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwechheimer C, Sullivan CJ, Kuehn MJ. Envelope control of outer membrane vesicle production in Gram-negative bacteria. Biochemistry. 2013;52(18):3031–3040. doi: 10.1021/bi400164t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Mot R, Vanderleyden J. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both Gram-positive and Gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol Microbiol. 1994;12(2):333–334. doi: 10.1111/j.1365-2958.1994.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 30.Mckee AS, Mcdermid AS, Baskerville A, Dowsett AB, Ellwood DC, Marsh PD. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986;52(2):349–355. doi: 10.1128/iai.52.2.349-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genco CA, Simpson W, Forng RY, Egal M, Odusanya BM. Characterization of a Tn4351-generated hemin uptake mutant of Porphyromonas gingivalis: evidence for the coordinate regulation of virulence factors by hemin. Infect Immun. 1995;63(7):2459–2466. doi: 10.1128/iai.63.7.2459-2466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Grenier D, Bertrand J, Mayrand D. Porphyromonas gingivalis outer membrane vesicles promote bacterial resistance to chlorhexidine. Oral Micriobiol Immunol. 1995;10(5):319–320. doi: 10.1111/j.1399-302x.1995.tb00161.x. Provides solid evidence to support the idea that vesicles may act as decoy protect bacterial cells from antibacterial agents. [DOI] [PubMed] [Google Scholar]
- 33.Pingel LC, Kohlgraf KG, Hansen CJ, et al. Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immuniol Cell Biol. 2008;86(8):643–649. doi: 10.1038/icb.2008.56. [DOI] [PubMed] [Google Scholar]
- 34.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42(3):1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakao R, Takashiba S, Kosono S, et al. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. 2014;16(1):6–16. doi: 10.1016/j.micinf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura F, Murakami Y, Nishikawa K, Hasegawa Y, Kawaminami S. Surface components of Porphyromonas gingivalis. J Peridontol Res. 2009;44(1):1–12. doi: 10.1111/j.1600-0765.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 37.Lin X, Wu J, Xie H. Porphyromonas gingivalis minor fimbriae are required for cell-cell interactions. Infect Immun. 2006;74(10):6011–6015. doi: 10.1128/IAI.00797-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuboniwa M, Amano A, Hashino E, et al. Distinct roles of long/short fimbriae and gingipains in homotypic biofilm development by Porphyromonas gingivalis. BMC Microbiol. 2009;9:105. doi: 10.1186/1471-2180-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74(1):81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW. Bacterial vesicles in marine ecosystems. Science. 2014;343(6167):183–186. doi: 10.1126/science.1243457. Identifies a striking feature of Prochlorococcus vesicles that may play a role in carbon cycling, gene transfer and viral defense. [DOI] [PubMed] [Google Scholar]
- 41.Ho MH, Chen CH, Goodwin JS, Wang BY, Xie H. Functional advantages of Porphyromonas gingivalis vesicles. PLoS ONE. 2015;10(4):e0123448. doi: 10.1371/journal.pone.0123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veith PD, Chen YY, Gorasia DG, et al. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res. 2014;13(5):2420–2432. doi: 10.1021/pr401227e. [DOI] [PubMed] [Google Scholar]
- 43••.Haurat MF, Aduse-Opoku J, Rangarajan M, et al. Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem. 2011;286(2):1269–1276. doi: 10.1074/jbc.M110.185744. Establishes that the P. gingivalis has a mechanism to selectively sort proteins into OMV, resulting in the preferential packaging of virulence factors into OMV and the exclusion of abundant outer membrane proteins from the protein cargo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen YY, Peng B, Yang Q, et al. The outer membrane protein LptO is essential for the O-deacylation of LPS and the co-ordinated secretion and attachment of A-LPS and CTD proteins in Porphyromonas gingivalis. Mol Microbiol. 2011;79(5):1380–1401. doi: 10.1111/j.1365-2958.2010.07530.x. [DOI] [PubMed] [Google Scholar]
- 45.Allan RK, Ratajczak T. Versatile TPR domains accommodate different modes of target protein recognition and function. Cell Stress Chaperones. 2011;16(4):353–367. doi: 10.1007/s12192-010-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimura M, Ohara N, Kondo Y, et al. Proteome analysis of Porphyromonas gingivalis cells placed in a subcutaneous chamber of mice. Oral Micriobiol Immunol. 2008;23(5):413–418. doi: 10.1111/j.1399-302X.2008.00444.x. [DOI] [PubMed] [Google Scholar]
- 47.Van Der Ley P, Van Den Dobbelsteen G. Next-generation outer membrane vesicle vaccines against Neisseria meningitidis based on nontoxic LPS mutants. Human Vaccines. 2011;7(8):886–890. doi: 10.4161/hv.7.8.16086. [DOI] [PubMed] [Google Scholar]
- 48.Jain S, Darveau RP. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontology 2000. 2010;54(1):53–70. doi: 10.1111/j.1600-0757.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontology 2000. 2010;52(1):68–83. doi: 10.1111/j.1600-0757.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belanger M, Kozarov E, Song H, Whitlock J, Progulske-Fox A. Both the unique and repeat regions of the Porphyromonas gingivalis hemagglutin A are involved in adhesion and invasion of host cells. Anaerobe. 2012;18(1):128–134. doi: 10.1016/j.anaerobe.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, Ju J, Rigney T, Tribble GD. Fimbriae of Porphyromonas gingivalis are important for initial invasion of osteoblasts, but not for inhibition of their differentiation and mineralization. J Peridontol. 2011;82(6):909–916. doi: 10.1902/jop.2010.100501. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz O, Watanabe K, Lamont RJ. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol. 2002;4(5):305–314. doi: 10.1046/j.1462-5822.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 53.Yuan L, Rodrigues PH, Belanger M, Dunn WA, Jr, Progulske-Fox A. Porphyromonas gingivalis htrA is involved in cellular invasion and in vivo survival. Microbiology. 2008;154(Pt 4):1161–1169. doi: 10.1099/mic.0.2007/015131-0. [DOI] [PubMed] [Google Scholar]
- 54.Furuta N, Takeuchi H, Amano A. Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infect Immun. 2009;77(11):4761–4770. doi: 10.1128/IAI.00841-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Furuta N, Tsuda K, Omori H, Yoshimori T, Yoshimura F, Amano A. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect Immun. 2009;77(10):4187–4196. doi: 10.1128/IAI.00009-09. Illustrates mechanism and pathway of P. gingivalis vesicles invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho MCC, Goodwin JS, Wang BY, Xie H. Functional advantages of Porphyromonas gingivalis vesicles. PLoS ONE. 2015;10(4):e0123448. doi: 10.1371/journal.pone.0123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeuchi H, Furuta N, Morisaki I, Amano A. Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway. Cell Microbiol. 2011;13(5):677–691. doi: 10.1111/j.1462-5822.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- 58.Hasegawa Y, Tribble GD, Baker HV, Mans JJ, Handfield M, Lamont RJ. Role of Porphyromonas gingivalis SerB in gingival epithelial cell cytoskeletal remodeling and cytokine production. Infect Immun. 2008;76(6):2420–2427. doi: 10.1128/IAI.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect Immun. 2006;74(1):703–710. doi: 10.1128/IAI.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kou Y, Inaba H, Kato T, et al. Inflammatory responses of gingival epithelial cells stimulated with Porphyromonas gingivalis vesicles are inhibited by hop-associated polyphenols. J Peridontol. 2008;79(1):174–180. doi: 10.1902/jop.2008.070364. [DOI] [PubMed] [Google Scholar]
- 61.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 62.Srisatjaluk R, Kotwal GJ, Hunt LA, Justus DE. Modulation of gamma interferon-induced major histocompatibility complex class II gene expression by Porphyromonas gingivalis membrane vesicles. Infect Immun. 2002;70(3):1185–1192. doi: 10.1128/IAI.70.3.1185-1192.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Peridontol. 1996;67(10 Suppl.):1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 64.Kozarov E, Sweier D, Shelburne C, Progulske-Fox A, Lopatin D. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect. 2006;8(3):687–693. doi: 10.1016/j.micinf.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Peridontol. 2000;71(10):1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 66.Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108(Suppl. 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elkaim R, Dahan M, Kocgozlu L, et al. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: a preliminary study. J Peridontol Res. 2008;43(2):224–231. doi: 10.1111/j.1600-0765.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 68.Reyes L, Herrera D, Kozarov E, Roldan S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Peridontol. 2013;40(Suppl. 14):S30–S50. doi: 10.1111/jcpe.12079. [DOI] [PubMed] [Google Scholar]
- 69.Iwai T. Periodontal bacteremia and various vascular diseases. J Peridontol Res. 2009;44(6):689–694. doi: 10.1111/j.1600-0765.2008.01165.x. [DOI] [PubMed] [Google Scholar]
- 70.Kinane DF, Riggio MP, Walker KF, Mackenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Peridontol. 2005;32(7):708–713. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 71.Qi M, Miyakawa H, Kuramitsu HK. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microbial Pathogen. 2003;35(6):259–267. doi: 10.1016/j.micpath.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Sharma A, Novak EK, Sojar HT, Swank RT, Kuramitsu HK, Genco RJ. Porphyromonas gingivalis platelet aggregation activity: outer membrane vesicles are potent activators of murine platelets. Oral Micriobiol Immunol. 2000;15(6):393–396. doi: 10.1034/j.1399-302x.2000.150610.x. [DOI] [PubMed] [Google Scholar]
- 73.Lamont RJ, Jenkinson HF. Subgingival colonization by Porphyromonas gingivalis. Oral Micriobiol Immunol. 2000;15(6):341–349. doi: 10.1034/j.1399-302x.2000.150601.x. [DOI] [PubMed] [Google Scholar]
- 74.Imai M, Murakami Y, Nagano K, Nakamura H, Yoshimura F. Major outer membrane proteins from Porphyromonas gingivalis: strain variation, distribution, and clinical significance in periradicular lesions. Eur J Oral Sci. 2005;113(5):391–399. doi: 10.1111/j.1600-0722.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 75.Veith PD, Talbo GH, Slakeski N, et al. Major outer membrane proteins and proteolytic processing of RgpA and Kgp of Porphyromonas gingivalis W50. Biochem J. 2002;363(Pt 1):105–115. doi: 10.1042/0264-6021:3630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamaguchi A, Nakayama K, Ichiyama S, et al. Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr Microbiol. 2003;47(6):485–491. doi: 10.1007/s00284-003-4069-6. [DOI] [PubMed] [Google Scholar]
- 77.Grenier D. Porphyromonas gingivalis outer membrane vesicles mediate coaggregation and piggybacking of Treponema denticola and Lachnoanaerobaculum saburreum. Int J Dentistry. 2013;2013:305476. doi: 10.1155/2013/305476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashimoto M, Ogawa S, Asai Y, Takai Y, Ogawa T. Binding of Porphyromonas gingivalis fimbriae to Treponema denticola dentilisin. FEMS Microbiol Lett. 2003;226(2):267–271. doi: 10.1016/S0378-1097(03)00615-3. [DOI] [PubMed] [Google Scholar]
- 79.Inagaki S, Onishi S, Kuramitsu HK, Sharma A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by ‘Tannerella forsythia’. Infect Immun. 2006;74(9):5023–5028. doi: 10.1128/IAI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.