Abstract
OBJECTIVE
To examine the outcome of simultaneous resection for rectal cancer with synchronous liver metastases.
BACKGROUND
One quarter of colorectal cancer patients will present with liver metastasis at the time of diagnosis. Recent studies have shown that simultaneous resections are safe and feasible for stage IV colon cancer. Limited data are available for simultaneous surgery in stage IV rectal cancer patients.
METHODS
One hundred ninety-eight patients underwent surgical treatment for stage IV rectal cancer. In 145 (73%) patients, a simultaneous procedure was performed. Fifty-three (27%) patients underwent staged liver resection. A subpopulation of 69 (35%) patients underwent major liver resection (3 segments or more) and 30 (44%) patients with simultaneous surgery.
RESULTS
The demographics of the 2 groups were similar. Complication rates were comparable for simultaneous or staged resections, even in the group subjected to major liver resection. Total hospital stay was significantly shorter for the simultaneously resected patients (P < .01).
CONCLUSIONS
Simultaneous resection of rectal primaries and liver metastases is a safe procedure in carefully selected patients at high-volume institutions, even if major liver resections are required.
Keywords: Stage IV rectal cancer, Simultaneous resection, Complications
Each year about 40,000 patients are newly diagnosed with rectal cancer and about 20,000 rectal cancer–related deaths are documented in the United States.1 Fifteen to 25% of patients present with synchronous liver metastasis at the time of diagnosis.2,3 Surgical resection of the primary tumor and the liver metastasis remains the only potential treatment for cure, with 5-year survival rates between 25% and 40%.4–7
The traditional surgical strategy for colorectal cancer presenting with synchronous liver metastases has been to resect the primary cancer, followed by resection of the hepatic tumors after chemotherapy.8–11 Because of improved safety of hepatic surgery in recent years, the surgical management of synchronous disease has begun a paradigm change. Several experienced centers have reported safety of combined procedures for resection of colon cancer and synchronous colorectal cancer.8,9,12–14 It is understandable that concerns remain regarding the perceived risks of combining pelvic surgery with hepatectomy. Few studies, however, have reported the actual clinical outcome of such combined rectal and liver resections. In this study, we present data from a tertiary referral center showing the safety and feasibility of simultaneous rectal and liver resections for stage IV rectal cancer.
Methods
After obtaining Investigational Review Board approval, a review of the prospective hepatic resection database of the Memorial Sloan Kettering Cancer Center (MSKCC) identified 198 patients who underwent rectal and liver surgery for stage IV rectal cancer. Synchronous metastasis was defined as patients presenting with rectal cancer and liver metastasis at the time of diagnosis. Preoperative tumor staging followed the guidelines of the American Joint Committee on Cancer.15
Hepatic metastases were detected by combinations of computed tomography, magnetic resonance imaging), and intraoperative ultrasound. Preoperative comorbidities were classified as previously described.14 Chemotherapy before rectal and/or hepatic resection included any systemic or regional chemotherapy with or without concomitant external beam radiation. Level of rectal primary was defined as the distance measured from the anal verge to the tumor at the time of presurgical evaluation. The rectal resections were classified according to the ASCRS textbook of Colon and Rectal Surgery.16 The type of liver resection was defined by the Couinaud classification.17 Resections of 3 or more contiguous liver segments were considered as a major liver procedure.8 The technique for anesthetic management has been reported previously.18 All patients were classified using the Clinical Risk Score defined by Fong.7
All postoperative complications were captured for the entire hospitalization and for at least 30 days following rectal and/or hepatic resection. Complications were graded according to the criteria described by Dindo et al.19 Postoperative mortality included any death during postoperative hospitalization or within 30 days after rectal and/or hepatic procedure. Clinical data evaluated included total operation time, estimated blood loss, and length of hospitalization. For patients who underwent a staged treatment approach, complications and length of hospitalization were generated as the sum from rectal and liver procedure.
Statistical analysis
Univariate tests for differences between the simultaneous resection cohort and the staged resection cohort were conducted using Fisher's exact test for categorical covariates and 2-sample t tests for continuous covariates. Analysis of variance models were used to estimate correlations between several disease treatment variables such as length of hospitalization and size of metastases. Logistic regression was used to estimate the probability and odds ratios for several variables relating to complication severity. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Between 1984 and 2008,198 patients underwent rectal and liver surgery for synchronous metastasized rectal cancer at MSKCC; 145 patients (73.2%) underwent simultaneous rectal and hepatic resection. Fifty-three patients (26.8%) received staged resection with a mean interval between the rectal and the liver procedure of 5.3 ± 5.9 months (median: 2.9 months).
Patient demographics
Mean age of patients was 56 ± 14 years (median: 56 years) for simultaneous resections and 58 ± 11 years (median: 59 years) for staged treatment (P = .4). In both study groups, the minority of patients were female (simultaneous n = 57, 39.3%; staged n = 21, 39.6%; P = nonsignificant [NS]). In the simultaneously resected study population, 79 (55%) patients suffered from at least one comorbidity. Six (4.1%) patients were preoperatively diagnosed with a cardiovascular comorbidity, 6 (4.1%) with pulmonary comorbidity, and 3 (2.1%) patients with combined cardiovascular and pulmonary comorbidity. In the staged group, 30 (56.6%) patients were documented with at least one comorbidity. Three (5.7%) patients were suffering from cardiovascular disease, 7 (13.2%) patients from pulmonary disease, and 3 (5.7%) from combined disease. Severity of comorbidities was not statistically significant.
A previous abdominal surgical procedure was documented in 20% of patients for both study groups (19.3% in simultaneous, 20.8% in staged group; P = NS).
Tumor characteristics (Table 1)
Table 1.
Demographics and cancer characteristics
| Simultaneous | Staged | P value | |
|---|---|---|---|
| Patients | 145 | 53 | |
| Age (years) | 56 ± 14 | 58 ± 11 | .4 |
| Female | 57 (40%) | 21 (40%) | .8 |
| Comorbidities | 79 (55%) | 30 (57%) | NS |
| Rectal primary | |||
| Level (cm) | 9.9 ± 5.3 | 9.0 ± 5.2 | .3 |
| pT 3/4 | 118 (82%) | 44 (82%) | .7 |
| pN 1/2 | 85 (59%) | 33 (62%) | .7 |
| LNN density (%) | 15.0 ± 22.2 | 11.7 ± 14.3 | .3 |
| Hepatic metastasis | |||
| Largest size (cm) | 2.7 ± 2.3 | 5.6 ± 3.6 | <.01 |
| Number of lesions | 2.1 ± 1.3 | 2.2 ± 1.2 | .8 |
| >5 lesions | 19 (13%) | 6 (11%) | .8 |
LNN = lymph node; NS = nonsignificant; pN = pathological regional lymph node involvement; pT = pathological tumor size.
Primary lesions
The level of the primary rectal cancer was similar in the 2 groups (median 9.0 vs 9.9 cm, P = .32). The majority of rectal lesions in patients undergoing simultaneous resection were histopathologically classified as pT3 or pT4 (118 patients, 81.4%); comparable T-staging was found for patients with staged surgical approach (pT3/ 4 = 44 patients, 83.0%; P = .7). The distribution regarding pN1/2 did not show statistically significant differences (59% vs 62%; P = .7). The lymph node density was 15.0 ± 22.3% for the simultaneous versus 11.7 ± 14.3% for the staged group (P = .25) Details are listed in Table 1.
Hepatic lesions
With 5.6 ± 3.6 cm (median: 4.2 cm), the mean size of the largest hepatic lesion was significantly larger (P < .01) in the staged-resected study population compared with 2.7 ± 2.3 cm (median: 2.0 cm) in the simultaneously resected group. The median number of metastasis was 2 in both groups (P = NS). In the simultaneous resection group, 13% of patients (19 patients) with more than 5 metastases were identified, compared with 11% (6 patients) in staged resection patients (P = .81) Details are listed in Table 1.
Neoadjuvant treatment
In the simultaneously resected study population, 58 (40%) patients received chemotherapy only, 42 (29%) patients received chemoradiation, and 4 (3%) patients received radiation only. The rest (41 patients; 28%) of this study population did not receive neoadjuvant treatment.
In the staged-resected study population for the rectal primary, 3 (6%) patients received chemotherapy only, 14 (26%) patients received chemoradiation, and 9 (17%) patients received radiation only. The rest (27 patients; 51%) of this study population did not receive neoadjuvant treatment. Chemotherapy before liver resection was provided in 25 (47%) patients in the staged study population.
Surgical aspects (Table 2)
Table 2.
Surgical details classified for simultaneous and staged procedure
| Simultaneous | Staged | P value | |
|---|---|---|---|
| OR colon | |||
| LAR | 130 (90%) | 40 (76%) | |
| APR | 15 (10%) | 13 (25%) | <.01 |
| OR liver | |||
| Wedge | 59 (41%) | 3 (6%) | |
| Segment | 56 (39%) | 11 (21%) | |
| Major (R3) | 30 (21%) | 39 (74%) | <.01 |
| Liver first | 100 (69%) | 0 | |
| Pump placement | 29 (20%) | 10 (19%) | NS |
| R status | |||
| R1 | 18 (12%) | 6 (6%) | .3 |
| R2 | 11 (8%) | ||
| OR time | |||
| Total (hours) | 5.9 ± 1.6 | 9.2 ± 2.2 | <.01 |
| Estimated blood loss (cc) | 630 ± 530 | 1,200 ± 760 | <.01 |
| Transfusion | |||
| PRBC | 45 (31.0%) | 15 (28.3%) | NS |
APR = abdominoperineal resection; LAR = low anterior resection; Major = resection of 3 or more liver segments; NS = nonsignificant; OR = ; PRBC = packed red blood cells.
Simultaneous treatments were performed by a midline laparotomy. Surgical treatment of primary rectal lesions mainly consisted of low anterior resection in both groups (90% for simultaneous versus 76% for staged; P < .01). Pouch construction was performed in 38 (26%) patients of the simultaneous group and in 4 (8%) patients of the staged group (P < .01). In the simultaneous group, 60 (41%) patients received a temporary stoma compared with 9 (17%) patients in the staged group (P < .01) (Table 2).
Surgical procedures addressing the liver lesions are listed in detail in Table 3. In patients with simultaneous re-sections, 69% of the liver procedures were performed before the rectal resection. Pump placement was performed in 20.0% of simultaneously resected patients and in 18.9% of staged-resected patients (P = .57). Thirty simultaneous (20.7%) and 39 (73.6%) staged hepatic resections were defined as major liver procedures (P < .01).
Table 3.
Perioperative course
| Simultaneous | Staged | P value | |
|---|---|---|---|
| Complications | |||
| 0 | 86 (59%) | 28 (53%) | |
| 1 | 20 (14%) | 7 (13%) | |
| 2 | 18 (12%) | 8 (15%) | |
| 3 | 17 (12%) | 8 (15%) | |
| 4 | 4 (3%) | 2 (4%) | |
| Mortality | 0 (0%) | 0 (0%) | .30 |
| Hospitalization time (days) | 10 ± 5 | 18 ± 7 | <.01 |
The combined rates of positive resection margins for both procedures were 21% in the simultaneously resected group compared with 13% in the staged-resected group (P = .31). The treatment approach did not result in a significant difference in the rate of R1 liver resections (simultaneous 11% vs staged 6%; P = .28). In 7.5% of patients in the staged group, a positive resection margin at the rectal side was detected compared with 2.1% in the simultaneous group (P = .08).
In neither of the groups was a patient found to have positive resection margins in both the liver and the rectum. In the simultaneously resected group, 7.6% of patients underwent an R2 resection with pump placement for adjuvant chemotherapy for 2-staged liver resection.
Total operation time was one and a half time longer for staged resection compared with the simultaneous approach (5.9 ± 1.6 vs 9.2 ± 2.2 hours; P < .01). Estimated mean blood loss was significantly less in the simultaneously resected population (630 ± 530 vs 1,200 ± 760 cc; P < .01). In both groups, around 30% of patients required at least one transfusion of packed red blood cells (P = NS).
Outcome (Table 3)
Complications
No perioperative mortalities were noted for either the simultaneous or the staged resection approach. Overall, no significant differences were found between the 2 surgical approaches regarding the distribution of complication grading (P = .30). Severe complications (stage III and IV according to Dindo et al19) occurred only in 15% of simultaneous- and 19% of staged-resected patients (P = .51). Details on perioperative complications are provided in Tables 3 and 4. In addition, neither OR time (P = .17), the number of liver lesions (P = .14), the level of rectal cancer (P = .80), nor the size of liver lesions (P = .14) predicted the occurrence of severe complications.
Table 4.
Details on perioperative complications (total study population)
| Simultaneous | Staged | ||
|---|---|---|---|
| Patients | 145 | 53 | |
| Rectal | Liver | ||
| GI bleeding | 0 (.0%) | 0 (.0%) | 2 (3.8%) |
| GI leakage | 3 (2.1%) | 0 (.0%) | 0 (.0%) |
| Bile leak | 4 (2.8%) | 0 (.0%) | 0 (.0%) |
| Pneumothorax | 2 (1.4%) | 1 (1.9%) | 4 (7.5%) |
| Effusion | 2 (1.4%) | 0 (.0%) | 3 (5.7%) |
| Fistula/abscess | 13 (9.0%) | 1 (1.9%) | 1 (1.9%) |
| Ventral hernia | 0 (.0%) | 1 (1.9%) | 1 (1.9%) |
| Dehydration | 0 (.0%) | 1 (1.9%) | 1 (1.9%) |
| Wound infection | 17 (11.7%) | 6 (11.3%) | 3 (5.7%) |
| Ileus | 17 (11.7%) | 4 (7.5%) | 3 (5.7%) |
| Urinary retention | 3 (2.1%) | 4 (7.5%) | 1 (1.9%) |
| Urinary tract infection | 9 (6.2%) | 2 (3.8%) | 1 (1.9%) |
| Cardiovascular | 6 (4.1%) | 0 (.0%) | 5 (9.4%) |
| Colitis | 2 (1.4%) | 0 (.0%) | 1 (1.9%) |
| Pump associated | 2 (1.4%) | 0 (.0%) | 1 (1.9%) |
GI = gastrointestinal.
Neither the type of rectal or liver procedure had an impact on the severity of complications (P = .40 and .19).
Hospitalization
The mean hospitalization for patients receiving simultaneous resections was significantly shorter when compared with patients treated with a staged approach (10 ± 5 vs 18 ± 7 days; P < .01). Patients who underwent an APR had a longer mean hospitalization period of 3.8 ± 1.4 days compared with patients who underwent a low anterior resection (P = .01). In addition, patients who were treated with a major liver resection had to stay significantly longer (4.9 ± .9 days, P < .01) in the hospital.
Adjuvant therapy
In the simultaneous group, 137 (95%) patients received adjuvant chemotherapy. In the staged study population, 46 (87%) patients received chemotherapy after staged liver resection (P = .12).
Subgroup major liver resections
Sixty-nine patients (34.8%) underwent major liver resection, which was defined by the removal of 3 or more consecutive liver segments; 30 of these (43.5%) received a simultaneous primary tumor resection. Details are provided in Tables 5 and 6.
Table 5.
Details on perioperative complications in patients requiring major liver resections
| Simultaneous | Staged | ||
|---|---|---|---|
| Patients | 30 | 39 | |
| Rectal | Liver | ||
| GI bleeding | 0 (.0%) | 0 (.0%) | 1 (2.6%) |
| GI leakage | 1 (3.3%) | 0 (.0%) | 0 (.0%) |
| Bile leak | 2 (6.7%) | 0 (.0%) | 0 (.0%) |
| Pneumothorax | 0 (.0%) | 1 (2.6%) | 4 (10.3%) |
| Effusion | 0 (.0%) | 0 (.0%) | 1 (2.6%) |
| Fistula/abscess | 4 (13.3%) | 1 (2.6%) | 1 (2.6%) |
| Ventral hernia | 0 (.0%) | 0 (.0%) | 1 (2.6%) |
| Wound infection | 6 (20.0%) | 4 (10.3%) | 3 (7.7%) |
| Ileus | 4 (13.3%) | 3 (7.7%) | 3 (7.7%) |
| Urinary retention | 0 (.0%) | 3 (7.7%) | 0 (.0%) |
| Urinary tract infection | 3 (10.0%) | 2 (5.1%) | 1 (2.6%) |
| Cardiovascular | 1 (3.3%) | 0 (.0%) | 3 (7.7%) |
| Pump associated | 0 (.0%) | 0 (.0%) | 1 (2.6%) |
GI = gastrointestinal.
Table 6.
Details for patients requiring major liver resections (R3 segments)
| Simultaneous | Staged | P value | |
|---|---|---|---|
| Patients | 30 | 39 | |
| Tumor characteristics | |||
| Level primary (cm) | 10.1 ± 5.0 | 8.8 ± 5.2 | .3 |
| Size (cm) | 4.7 ± 3.3 | 6.4 ± 3.7 | .01 |
| >5 liver lesions | 10 (33.3%) | 3 (7.7%) | .01 |
| Surgical characteristics | |||
| APR | 2 (6.7%) | 8 (20.5%) | .16 |
| OR time (hours) | 6.9 ± 1.3 | 9.2 ± 2.3 | <.01 |
| Complications | |||
| 0 | 11 (36.6%) | 17 (43.6%) | |
| 1 | 8 (26.7%) | 3 (7.7%) | |
| 2 | 4 (13.4%) | 12 (30.8%) | |
| 3 | 6 (20.0%) | 5 (12.8%) | |
| 4 | 1 (3.3%) | 2 (5.1%) | |
| Mortality | 0 (.0%) | 0 (.0%) | .7 |
| Hospitalization time (days) | 12 ± 5 | 18 ± 7 | <.01 |
APR = abdominoperineal resection;OR = operation.
Tumor characteristic/surgical aspects
The level of the rectal lesions in this subgroup analysis did not show significant differences between staged and simultaneous-resected patients (10.1 ± 5.0 vs 8.8 ± 5.2 cm; P = .29). The size of the largest liver lesion was slightly bigger in the staged resection group (4.7 ± 3.3 vs 6.4 ± 3.7 cm; P = .01), but 33% of patients in the simultaneous group were treated for more than 5 liver lesions, compared with only 8% in the staged study group (P = .01). Significantly more APR procedures were performed in the staged patient group (P < .01).
Complications/outcome
No significant differences regarding postoperative complications were observed between the 2 treatment strategies (P = .70). No perioperative mortalities were reported in either group. The mean hospitalization period was significantly longer in staged resected patients (P < .01).
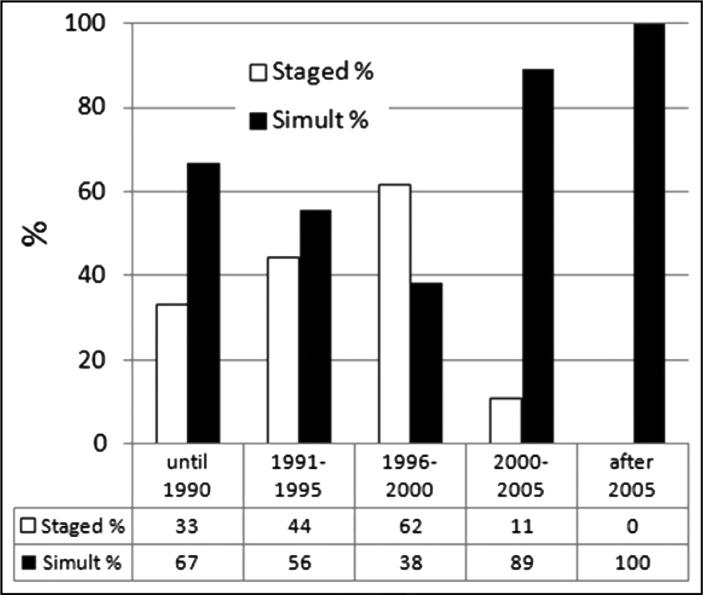
Changes in practice over time
The use of staged versus simultaneous resections for patients presenting with primary cancers in places as it related to time period is shown in Fig. 1. Before 2000, 51% of patients had a staged procedure. Since 2000, only 6% of patients have been treated with a staged procedure.
Figure 1.
Changes in practice over time. The use of staged or simultaneous (Simult) resections for patients is shown.
Comments
As almost a quarter of rectal cancer patients present with stage IV disease, the optimal timing of surgery for the primary and the liver disease has significant clinical implications.20 Most centers still recommend a staged surgical approach with removal of the primary cancer first, followed by liver resection after adjuvant chemotherapy.21,22 This traditional standard posits that a simultaneous liver resection to be too challenging for these patients undergoing pelvic surgery. Combined procedures were considered to have prohibitive rates of morbidity and mortality. In particular, for patients requiring major liver resection, some results showing increased rates of morbidity and mortality have been reported.8,23
Recent improvements in the surgical and anesthetic techniques have greatly enhanced the safety of major operative procedures including pelvis procedures and hepatectomies. This has allowed a reconsideration of combined pelvic and hepatic procedures. Early series reporting simultaneous resections for stage IV colorectal cancer patients showed acceptable morbidity and mortality rates.24–26 Nevertheless, most authors of prior studies recommended a simultaneous treatment approach only in patients with small liver lesions or with colon cancer.21 In addition, the use of adjuvant chemotherapy after resection of the primary tumor before the liver resection has been advocated as providing oncologic benefit although without definitive supporting data.27–29 Most of these prior recommendations are based on series with very small numbers of patients with rectal tumors.10,13,22
We have previously reported the safety of a simultaneous primary and liver resection approach for stage IV colon cancer patients.14 This study is an extension of our previous work and documents the safety and feasibility of a combined treatment approach even for rectal cancer. In this retrospective study, only patients receiving the rectal as well as the liver procedure at MSKCC were included to provide uniform data documentation and surgical standards. This study design also demonstrates the patient selection criteria at a tertiary center for simultaneous resection. Thus, rectal tumors requiring APR were more likely to be chosen for a staged approach. Larger liver tumors requiring major liver resection were more likely to be chosen for a staged approach. This article therefore shows not only the safety of a simultaneous approach, but also that such safety relies on patient selection by experienced surgeons.
The tendency to have sphincter preservation in the simultaneously resected patients may also reflect the increasing numbers of sphincter preserving procedures after 2000. The option to preserve the sphincter has been optimized in recent years by better neoadjuvant treatment options, as well as by improvements in stapling devices and improvements in surgical techniques.30–32 Similarly, a propensity for simultaneously resected patients to have limited liver resections may reflect the trend for more parenchyma-sparing procedures during the last decade.33,34 In our study population, around 70% of simultaneously resected patients received neoadjuvant chemotherapy compared with around 50% of patients in the staged group. Smaller sizes of liver lesions might be caused by improved response of liver metastases to currently established neoadjuvant chemotherapy agents.35,36
In these simultaneous resection procedures, we prefer to perform the liver resection before the rectal procedure.37 This order allows the “clean” liver procedure to be performed before the “contaminated” intestinal procedure.38 During the hepatectomy, low fluid administration is generally used to prevent venous bleeding from the resection surface of the liver.39,40 Thus, performing the liver resection first minimizes the relative hypotension during this “low central venous pressure anesthesia” and allows fluid resuscitation during the rectal portion of the procedure. Furthermore, the venous congestion from the Pringle maneuver might endanger a newly created bowel anastomosis, if the rectal has been performed before the liver resection.
The main message of this study is that combined procedures for stage IV rectal cancer patients are safe and feasible in carefully selected and evaluated patients at experienced centers. The rates of complications between staged- and simultaneously resected patients did not show statistical difference. Complication rates of this rectal study population were comparable with recently published studies combining stage IV colon cancer patients.8,12,13 Mortality in this current series is also consistent with the mortality rate of 2% generally reported for rectal procedures alone41 or for major liver resection alone.42 In patients undergoing major liver resection, the rate of severe complications was acceptable with 23% in the simultaneous and 18% in the staged group, which is comparable with other studies.43
This combined treatment approach resulted in lower total blood loss, shorter total operative time, and a shorter hospital stay. The economic benefits of reduction in hospitalization cost and more efficient OR utilization are clear. This simultaneous approach also allows faster recovery and initiation of chemotherapy. Furthermore, one cannot underestimate the psychological benefits of allowing a single procedure for eradication of all disease instead of employing 2 procedures scheduled over a period of months. The possibility that some patients will never be candidates for staged liver resection because of tumor progression under adjuvant chemotherapy will be eliminated by a synchronous resection approach. For example, in a Dutch study, only 10% of patients presenting with stage IV colorectal cancer finally underwent liver resection.44
In summary, our data show simultaneous resection of primary rectal cancer, and liver metastases is safe for the majority of patients and is an efficient way to render surgical care. At experienced centers, low morbidity and mortality rates can be achieved even in patients requiring major liver resection. A synchronous treatment strategy could be considered when liver and colorectal surgeons agree on safety of this approach.
Acknowledgments
This study was funded in part by NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
The authors declare no conflicts of interest.
Uncited table
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–9. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leporrier J, Maurel J, Chiche L, et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–74. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 4.Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–80. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 5.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–66. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farid SG, Aldouri A, Morris-Stiff G, et al. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91–100. doi: 10.1097/SLA.0b013e3181bfda3c. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–91. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins LT, Millikan KW, Bines SD, et al. Hepatic resection for metastatic colorectal cancer. Am Surg. 1997;63:605–10. [PubMed] [Google Scholar]
- 10.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 11.Verhoef C, van der Pool AE, Nuyttens JJ, et al. The “liver-first approach” for patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2009;52:23–30. doi: 10.1007/DCR.0b013e318197939a. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Wang L, Chen C, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastases. J Gastrointest Surg. 2010;14:1974–80. doi: 10.1007/s11605-010-1284-x. [DOI] [PubMed] [Google Scholar]
- 13.Martin RC, 2nd, Augenstein V, Reuter NP, et al. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg. 2009;208:842–50. doi: 10.1016/j.jamcollsurg.2009.01.031. discussion, 850–2. [DOI] [PubMed] [Google Scholar]
- 14.Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal re-sections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233–41. doi: 10.1016/S1072-7515(03)00390-9. discussion, 241–2. [DOI] [PubMed] [Google Scholar]
- 15.Bertino JR. Chemotherapy of colorectal cancer: history and new themes. Semin Oncol. 1997;24(5 Suppl 18):S18–S18. [PubMed] [Google Scholar]
- 16.Wolf B. The ASCRS Textbook of Colon and Rectal Surgery [Google Scholar]
- 17.Couinaud C. Le Foie: Etudes Anatomiques et Chirurgicales. Masson & Cie; Paris: 1957. [Google Scholar]
- 18.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–5. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 21.Kemeny N. The management of resectable and unresectable liver metastases from colorectal cancer. Curr Opin Oncol. 2010;22:364–73. doi: 10.1097/CCO.0b013e32833a6c8a. [DOI] [PubMed] [Google Scholar]
- 22.Hillingso JG, Wille-Jorgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancerda systematic review. Colorectal Dis. 2009;11:3–10. doi: 10.1111/j.1463-1318.2008.01625.x. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Shimada H, Matsuo K, et al. Outcome after simultaneous colorectal and hepatic resection for colorectal cancer with synchronous metastases. Surgery. 2004;136:650–9. doi: 10.1016/j.surg.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Vogt P, Raab R, Ringe B, et al. Resection of synchronous liver metastases from colorectal cancer. World J Surg. 1991;15:62–7. doi: 10.1007/BF01658964. [DOI] [PubMed] [Google Scholar]
- 25.Elias D, Detroz B, Lasser P, et al. Is simultaneous hepatectomy and intestinal anastomosis safe? Am J Surg. 1995;169:254–60. doi: 10.1016/S0002-9610(99)80146-9. [DOI] [PubMed] [Google Scholar]
- 26.Scheele J, Stangl R, Altendorf-Hofmann A, et al. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 27.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–61. doi: 10.1097/01.sla.0000145964.08365.01. discussion, 1061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemo-therapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy SK, Barbas AS, Clary BM. Synchronous colorectal liver metastases: is it time to reconsider traditional paradigms of management? Ann Surg Oncol. 2009;16:2395–410. doi: 10.1245/s10434-009-0372-1. [DOI] [PubMed] [Google Scholar]
- 30.Denost Q, Laurent C, Capdepont M, et al. Risk factors for fecal incontinence after intersphincteric resection for rectal cancer. Dis Colon Rectum. 2011;54:963–8. doi: 10.1097/DCR.0b013e31821d3677. [DOI] [PubMed] [Google Scholar]
- 31.Rullier E, Goffre B, Bonnel C, et al. Preoperative radiochemotherapy and sphincter-saving resection for T3 carcinomas of the lower third of the rectum. Ann Surg. 2001;234:633–40. doi: 10.1097/00000658-200111000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxter NN, Garcia-Aguilar J. Organ preservation for rectal cancer. J Clin Oncol. 2007;25:1014–20. doi: 10.1200/JCO.2006.09.7840. [DOI] [PubMed] [Google Scholar]
- 33.Pamecha V, Gurusamy KS, Sharma D, et al. Techniques for liver parenchymal transection: a meta-analysis of randomized controlled trials. HPB (Oxford) 2009;11:275–81. doi: 10.1111/j.1477-2574.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schemmer P, Friess H, Dervenis C, et al. The use of endo-GIA vascular staplers in liver surgery and their potential benefit: a review. Dig Surg. 2007;24:300–5. doi: 10.1159/000103662. [DOI] [PubMed] [Google Scholar]
- 35.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to pre-operative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–51. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 36.Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–82. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 37.Van Dessel E, Fierens K, Pattyn P, et al. Defining the optimal therapy sequence in synchronous resectable liver metastases from colorectal cancer: a decision analysis approach. Acta Chir Belg. 2009;109:317–20. doi: 10.1080/00015458.2009.11680432. [DOI] [PubMed] [Google Scholar]
- 38.Kemeny N, Jarnagin W, Paty P, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23:4888–96. doi: 10.1200/JCO.2005.07.100. [DOI] [PubMed] [Google Scholar]
- 39.Jarnagin WR, Gonen M, Maithel SK, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intra-operative management in patients undergoing major hepatic resection. Ann Surg. 2008;248:360–9. doi: 10.1097/SLA.0b013e318184db08. [DOI] [PubMed] [Google Scholar]
- 40.Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–60. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 41.Paun BC, Cassie S, MacLean AR, et al. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251:807–18. doi: 10.1097/SLA.0b013e3181dae4ed. [DOI] [PubMed] [Google Scholar]
- 42.Virani S, Michaelson JS, Hutter MM, et al. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1284–92. doi: 10.1016/j.jamcollsurg.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 43.Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–41. doi: 10.1016/j.jamcollsurg.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 44.van der Pool AE, Damhuis RA, Ijzermans JN, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis. 2012;14:56–61. doi: 10.1111/j.1463-1318.2010.02539.x. [DOI] [PubMed] [Google Scholar]