Abstract
Carbon dioxide (CO2) is a colorless, odorless gas which occurs naturally in the atmosphere and human body. With the advent of digital subtraction angiography, the gas has been used as a safe and useful alternative contrast agent in both arteriography and venography. Because of its lack of renal toxicity and allergic potential, CO2 is a preferred contrast agent in patients with renal failure or contrast allergy, and particularly in patients who require large volumes of contrast medium for complex endovascular procedures. Understanding of the unique physical properties of CO2 (high solubility, low viscosity, buoyancy, and compressibility) is essential in obtaining a successful CO2 angiogram and in guiding endovascular intervention. Unlike iodinated contrast material, CO2 displaces the blood and produces a negative contrast for digital subtraction imaging. Indications for use of CO2 as a contrast agent include: aortography and runoff, detection of bleeding, renal transplant arteriography, portal vein visualization with wedged hepatic venous injection, venography, arterial and venous interventions, and endovascular aneurysm repair. CO2 should not be used in the thoracic aorta, the coronary artery, and cerebral circulation. Exploitation of CO2 properties, avoidance of air contamination and facile catheterization technique are important to the safe and effective performance of CO2 angiography and CO2-guided endovascular intervention.
Keywords: Contrast medium, Carbon dioxide, Angiography, Renal failure, Contrast-induced nephropathy
INTRODUCTION
The use of carbon dioxide (CO2) as a contrast agent goes back to 1920s when the gas was used to visualize retroperitoneal structures. In the 1950s and early 1960s, CO2 was injected intravenously to delineate the right atrium for the detection of pericardial effusion [1,2]. This imaging technique developed from animal and clinical studies which demonstrated that CO2 was safe and well tolerated with venous injections [3]. With the advent of digital subtraction technique (DSA) in 1980s, CO2 has evolved into a safe and useful contrast agent for vascular imaging [4].
CO2 is the only proven safe contrast agent in patients with renal failure and hypersensitivity to iodinated contrast medium. Now CO2 is used widely as an intravascular contrast agent in both the arterial and venous circulations for various indications. Because of the potential neurotoxicity and cardiac arrhythmia, CO2 should not be used in the thoracic aorta, the coronary artery and the cerebral circulation.
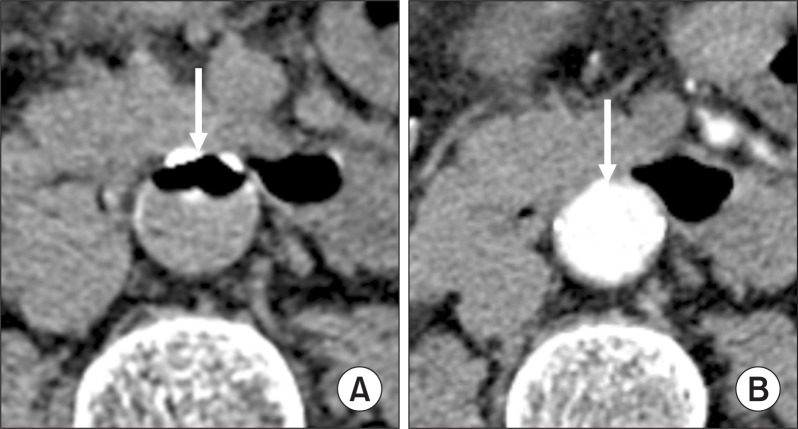
When injected into an artery or vein, CO2 displaces blood whereas contrast medium mixes with blood (Fig. 1). Despite the difference in the physical property between the gaseous CO2 and liquid contrast medium, CO2 arteriograms are quite comparable to contrast arteriograms, providing much of the vascular information that can be derived from contrast medium angiography with less risk and at lower cost. With the availability of high-resolution DSA and a reliable gas delivery system, CO2 can be easy to inject and image for diagnostic angiography and endovascular intervention [5]. For years the development of each of these vascular diagnostic and endovascular applications has demonstrated the unique benefits of CO2 as a contrast agent [6].
Fig. 1.
Axial computed tomography (CT) scans during intra-aortic infusion of CO2 or contrast medium. (A) CT section through the infra-renal aorta demonstrates the large, coalesced CO2 bubble displacing blood in the anterior part of the aorta (arrow). (B) Contrast medium has mixed with blood, enhancing the entire aortic lumen (arrow).
This review will discuss the scientific principles, techniques and practice of CO2 angiography.
PROPERTIES OF CO2
Performance, imaging and interpretation of CO2 angiograms and CO2-guided vascular intervention are dependent upon the knowledge of CO2 properties. CO2 constitutes 0.03% of air and is treated as if its partial pressure were zero. The density and atomic weight of the substance determine whether it will be more or less dense than the body tissues, and accordingly is classified as a positive or a negative contrast medium. Because of the low atomic number and density, CO2 is a negative contrast agent, and absorbs therefore x-ray to a lesser extent than the surrounding blood and vessel wall. Therefore, CO2 imaging requires the DSA technique with good contrast resolution.
When injected into a vein, CO2 is carried by the blood to the lungs, where the gas is eliminated in a single pass. There is no evidence of passage of CO2 through the pulmonary capillaries into the left atrium. Likewise, CO2 bubbles do not enter the portal vein when an injection is made into the proximal superior mesenteric or splenic artery. If CO2 is injected into a catheter wedged in a peripheral splenic artery branch, CO2 then flows through the splenic pulp and splenic vein to the portal vein. When arteriovenous shunting is present as in vascular tumors, arteriovenous fistula and malformation, and traumatic arteriovenous fistula, the draining vein is filled with CO2 following an arterial injection.
1. Solubility
CO2 is 28 times more soluble than oxygen and 54 times more soluble than nitrogen. This high solubility of CO2 allows its injection into the arteries below the diaphragm and veins without clinically significant gas embolism. The solubility of CO2 and air can be assessed by DSA or fluoroscopy of the gas trapped in the right atrium in the left lateral decubitus position (right-side up position). Five mL of CO2 trapped in the right atrium will dissolve within 45 seconds. Larger volumes of CO2 will take a longer time to dissolve [7]. With the patient in the supine position the CO2 bubble in the pulmonary outflow tract following an intravenous injection will disappear in 15 seconds. The same amount of air trapped in the right atrium or pulmonary outflow tract will take much longer time to dissolve. When multiple CO2 injections are to be made, the time interval between the injections should be increased to 3 to 5 minutes, especially in patients with emphysema and pulmonary hypertension.
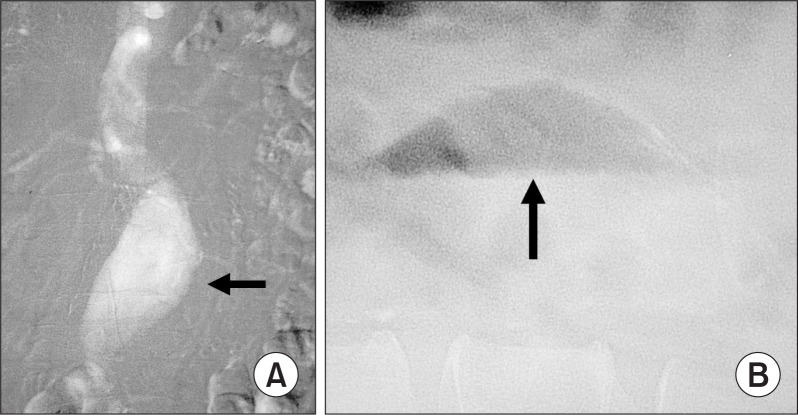
When performing CO2 aortography in the patient with abdominal aortic aneurysm (AAA), lateral fluoroscopy of the aneurysm should be done following the injection. If trapped CO2 is seen in the ventral portion of the AAA (Fig. 2), the body position should be changed from the side to the side to wash out the gas.
Fig. 2.
CO2 trapped in the abdominal aortic aneurysm (AAA). (A) CO2 digital subtraction angiography demonstrates an AAA (arrow). The aortic branches fill poorly because much of the gas injected is trapped in the anterior part of the aneurysm. (B) Cross-table lateral image demonstrates CO2 trapped in the aneurysm (arrow).
2. Viscosity
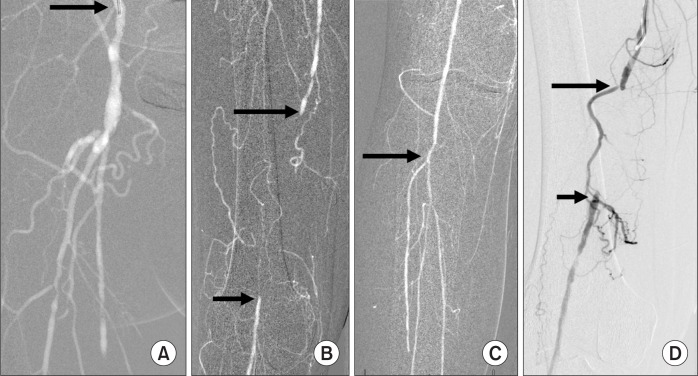
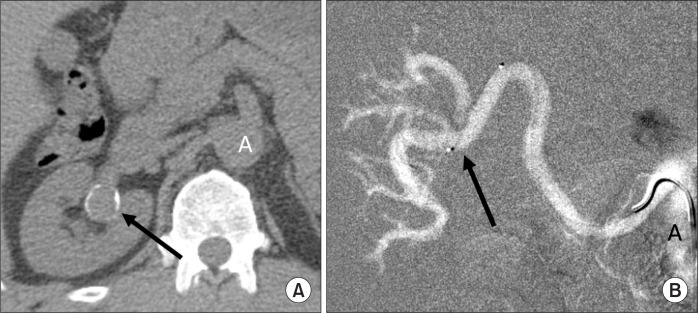
The viscosity of a fluid or a gas is a measure of its resistance to flow. CO2 is 400 times less viscous than contrast medium. Therefore, CO2 in diagnostic quantities (15–30 mL) can be injected through a 3-Fr catheter (Fig. 3), small bore needles (22–25 gauge), an end-hole catheter, between the guide wire and the catheter using a Touhy-Borst fitting (y-connector), and through the side ports of the introducer sheath and stent delivery system. The low viscosity of CO2 allows for detection of bleeding (traumatic and gastrointestinal bleeding), type 11 endoleak, portal vein visualization with a wedged hepatic vein injection, central vein visualization with a peripheral vein injection, demonstration of tumor vessels, and visualization of collateral vessels in both arterial and venous occlusive disease. Because CO2 does not mix with blood, the gas bubbles remain undiluted, better visualizing peripheral vessels through collaterals (Fig. 4).
Fig. 3.
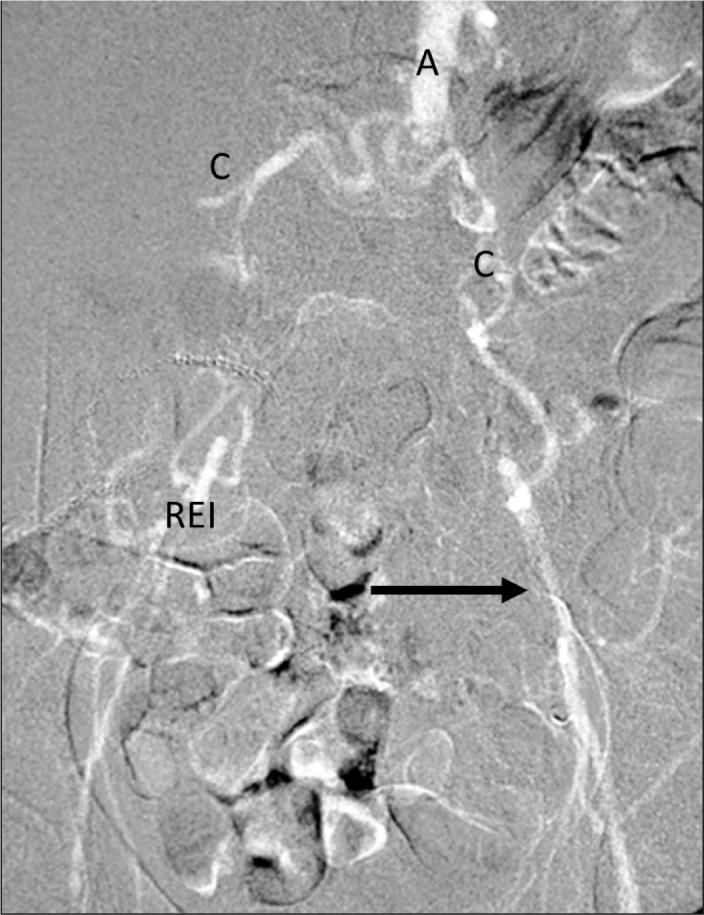
Aortoiliac occlusive disease. After unsuccessful passage of a guide wire into the aorta from either the right or left femoral artery, a 3-Fr dilator was introduced into the left external iliac artery where 30 mL of CO2 was injected (arrow). The CO2 flowed countercurrent through collateral (C) and refluxed into the aorta (A). Antegrade collateral blood flow from the aorta filled the right external iliac artery (REI).
Fig. 4.
Superficial femoral artery (SFA) occlusion. (A) Injection of CO2 into the external iliac artery (arrow) showing occlusion of the distal SFA and patent deep femoral artery. (B) CO2 shows occluded SFA (longer arrow) and collaterals reconstituting the popliteal artery (shorter arrow). (C) The popliteal and infra-popliteal arteries (arrow) are well filled with CO2. (D) Injection of iodinated contrast medium into the SFA proximal to the occlusion (longer arrow) demonstrates fewer collateral vessels and dilution of the contrast medium distally in the reconstituted popliteal artery (shorter arrow). The patient underwent successful recanalization of the occlusion and angioplasty.
3. Buoyancy
When CO2 is submerged in a fluid, the gas bubble rises very quickly because the upward force exerted by the fluid is much stronger than the weight of CO2. This property is called buoyancy that means floating on blood. The buoyancy of CO2 may not be apparent on the anteroposterior (AP) projection but can be seen on the cross-table lateral projection of large diameter vessels, such as the aorta, the inferior vena cava and the AAA (Fig. 2). With selective injection and reflux technique, CO2 can visualize visceral arteries, such as the celiac, splenic, hepatic and superior mesenteric arteries.
Using a circulatory system model, Song et al. [8] evaluated the gas dispersion patterns from different angiographic catheters, gas flow dynamics, and the effect of the vessel size and inclination on luminal gas filling. Regardless of the vessel size, the CO2 bubble shows a parabolic flow profile along the anterior (nondependent) part of the vessel with incomplete fluid displacement along its posterior (dependent) portion. The thickness of the fluid in the dependent part depends upon vessel diameter, increasing in width with an increase in the vessel size. In the 15.9 mm-diameter vessel, its luminal filling with CO2 was 65% which increased to 85% at the 15-degree incline. The buoyancy of CO2 results in better imaging of the vessels coursing anterior to the injection site, such as the celiac and superior mesenteric arteries (Fig. 5). Therefore, CO2 is preferable to iodinated contrast medium when performing stent placement of the celiac and superior mesenteric arteries. Cross-table lateral fluoroscopy and DSA demonstrate their origins in profile that can help stent positioning. The buoyancy of CO2 is disadvantageous for renal angiography since the renal artery courses in the dependent position in relation to the aortic injection site (Fig. 6).
Fig. 5.
Lateral CO2 aortograms in a patient with median arcuate ligament compression syndrome. (A) Full inspiration. The origin of the celiac artery is smooth (arrow) and there is no stenosis. The superior mesenteric artery is patent. (B) Full expiration. There is narrowing at the origin of the celiac artery (arrow) due to median arcuate ligament compression.
Fig. 6.
Renal artery aneurysm in a patient with renal failure. (A) Computed tomography section through the right renal hilus demonstrates a calcified renal artery aneurysm (arrow). (B) CO2 renal arteriogram. Injection of 20 mL of CO2 into the segmental renal artery branch (arrow) via a microcatheter resulted in reflux of CO2 and filling of the proximal main renal artery which was not seen on the CO2 aortogram (not shown). A, aorta.
Because of the buoyancy and low viscosity, CO2 is preferable to contrast medium in upper extremity venography. The central veins, such as the axillary, subclavian and innominate veins fill well with peripheral CO2 injection. The buoyancy is not a problem with CO2 arteriography of the lower extremity (Fig. 4). Elevation of the feet, intra-arterial injection of nitroglycerin, and distal injection with gas reflux can improve the image quality of CO2 DSA of lower extremity arteries.
4. Compressibility
Another property of CO2 that plays a significant role in CO2 angiography is compressibility and explosive delivery of CO2. The volume of a given amount of gas at constant temperature is inversely proportional to the pressure. The density of CO2 increases as the injection exerts a force on the gas, resulting in decrease in its volume but increase its pressure. When the compressed gas exits from the catheter, there will be expansion of the gas, known as “explosive delivery” that may cause discomfort to the patient and poor image quality. Clearing the fluid or blood of the catheter with an injection of 3 to 5 mL of CO2 decreases gas compression and explosion.
ADVANTANGES
The main benefit of CO2 as a contrast agent is the lack of renal toxicity and anaphylactic response. Therefore, it is the preferred alternative contrast agent in patients with renal failure and contrast allergy. Since CO2 is eliminated by the lungs in a single pass, unlimited volumes of CO2 can be used but injections should be separated by 2 to 3 minutes.
The other advantage of CO2 stems from its low viscosity. CO2 can be injected through a microcatheter with the inner diameter of 0.021-inch (0.533 mm), skinny needles (21 g to 25 g), between the catheter and guide wire, and side port of the sheath and stent delivery system. No significant bleeding has occurred following CO2 injections into the splenic or hepatic parenchyma for portal vein visualization. CO2 has been effective in visualizing lower extremity arteries, tumor vessels, arteriovenous fistulas, and visceral arteries. CO2 is sensitive in detecting the gastrointestinal and traumatic bleeding. CO2 is also used to visualize the portal vein by wedged hepatic venography and gas injection in the spleen and liver.
CO2 reflux can fill the proximal vessels that cannot be seen with the contrast medium. This reflux technique is very useful in a variety of diagnostic and interventional procedures.
The other important advantage of using CO2 is that it is a safe and effective flushing medium for the catheter and sheath. Because CO2 is immiscible with blood, it can prevent clots developing in the catheter. During the angiographic and endovascular procedures 5 mL of CO2 is injected into the catheter every 2–3 minutes. As a cost benefit, CO2 is inexpensive in comparison with nonionic iodinated contrast medium
DISADVANTAGES
CO2 requires a special delivery system to prevent air contamination and gas compression [9,10]. CO2 should not be used as a contrast agent for imaging the thoracic aorta, the coronary artery, and the cerebral circulation because of its potential neurotoxicity and coronary artery gas embolism causing myocardial ischemia [11–13]. The intracoronary CO2 injection has a profound effect on the left ventricular function in swine [14]. Potential image degradation presents a problem with CO2 use. Since CO2 is a negative contrast agent imaged using the DSA technique, bowel gas and peristalsis, and other motion degrade CO2 imaging. The buoyancy of CO2 causes incomplete filling of the vessels dependent upon vessel size and the location of area of interest. The renal artery coursing posterior to the aorta may not fill with CO2 injection in the aorta. Elevation of the side of the target vessel and the CO2 reflux technique should be used to image the renal artery (Fig. 6).
INDICATIONS
CO2 is used as a contrast agent for diagnostic angiography and endovascular intervention. The diagnostic indications include: 1) aortogram and runoff; 2) renal arteriography; 3) mesenteric angiography; 4) detection of traumatic arterial bleeding (spleen, kidney, liver and pelvis); 5) renal transplant arteriography; 6) central venography; 7) wedged hepatic venography; 8) evaluation of failing dialysis access; and 9) splenoportography. CO2 is used in the following endovascular procedures: 1) angioplasty and stenting (aorta, iliac, mesenteric, and superficial femoral, popliteal, and tibial); 2) tumor embolization (kidney, liver and bone); 3) embolization of arteriovenous fistula and malformations; 4) roadmapping for foreign body retrieval; 5) catheter-directed thrombolysis; 6) transjugular intrahepatic portosystemic shunt (TIPS); 7) transjugular liver biopsy; and 9) endovascular aneurysm repair (EVAR).
CONTRAINDICATIONS
Absolute contraindications to the use of CO2 as a contrast agent include thoracic aortography, coronary arteriography and cerebral arteriography. A rat study with the carotid injection of CO2 has shown that the gas can be neurotoxic with multifocal ischemic infarctions and disruption of the blood-brain barrier [12,13]. Clinically, seizures and loss of consciousness have been encountered when CO2 refluxed into the thoracic aorta and cerebral circulation following an injection of CO2 into the brachial artery [15]. When imaging dialysis fistula and graft, CO2 should be injected into the venous limb of the graft or fistula and at the arterial anastomosis to prevent reflux of the gas into the brachial and subclavian arteries.
CO2 should not be injected into the abdominal aorta in the prone position since the buoyant gas may fill the spinal and lumbar arteries, and cause spinal cord ischemia. Similarly CO2 should not be injected into the abdominal aorta with the patient’s head in an elevated position since the gas can flow in the opposite direction of blood flow, especially in a hypoplastic aorta in children.
In the patient undergoing nitrous oxide anesthesia, concurrent use of CO2 should be avoided since the nitrous oxide can diffuse into the CO2 bubble, increasing the CO2 volume significantly. In the venous system, this rapid expansion of CO2 bubble may result in pulmonary artery vapor lock.
Relative contraindications to the intravenous use of CO2 include pulmonary hypertension and chronic obstructive pulmonary disease. Since the intravenous injection of CO2 in diagnostic quantities can increase pulmonary arterial pressure, CO2 injections should be separated by 3 to 5 minutes to prevent accumulation of CO2 and subsequent pulmonary artery vapor lock. CO2 should be used cautiously in patients with patent foramen ovale (PFO) or atrial septal defect.
INJECTION OF CO2
CO2 can be delivered through any pre-shaped end-hole catheter and microcatheters. A pigtail catheter is not necessary for CO2 aortography or vena cavography. CO2 should be injected at a rate that slightly exceeds the rate of blood flow in the artery being studied. Injecting too slowly results in incomplete filling of the artery injected. Too rapid an injection, on the other hand, results in reflux of CO2 into the aorta and filling of extraneous aortic branches. However, reflux of CO2 is used to better visualize the undulating arteries, proximal vessels, and arteries in the dependent areas. For example, an injection into the common femoral artery results in reflux of CO2 centrally and filling of the iliac arteries, the aorta and even contralateral iliac arteries. An injection into the deep femoral artery results in reflux of CO2 and filling of the common femoral artery. An injection of CO2 in the renal artery results in gas reflux into the aorta and filling of the renal artery orifice. Amounts of CO2 for the aorta are 15 to 30 mL/sec for 1.5 seconds. Amounts for mesenteric, renal, iliac and femoral arteries are 10 to 20 mL/sec. Regardless of the volume injected, CO2 is delivered within 2 sec. In small vessels such as tibial arteries, CO2 is injected over two to three seconds.
A disposable aluminum cylinder containing CO2 United States Pharmacopeia (USP) 99.9% pure CO2 with gas fitting and regulators is used to fill the hand-held syringe and the plastic bag for CO2 delivery. There is no possibility for inadvertent injection of large volumes and air contamination if the system is used properly. Most CO2 cylinders contain over 3 million cc of CO2 under a high pressure, and should not be connected directly to the catheter.
The plastic bag system [9] has been used for more than 15 years in more than 10,000 patients at the University of Florida, Gainesville, FL, and the University of Michigan, Ann Arbor, MI, without any major complication. If the operator uses the bag system properly, air contamination can be prevented with delivery of CO2 in the non-explosive manner. A 0.2 μm filters (Acrodisc; Gelman Sciences, Ann Arbor, MI, USA) is used to remove any particulate or microbial contaminates from the cylinder, regulator and connecting tubing. Unfortunately the plastic bag delivery system (AngioDynamics, Queensbury, NY, USA) has been discontinued due to a serious complication in which the bag was inadvertently filled with oxygen rather than CO2 [10]. A similar plastic bag system is available from Merit Medical Systems Inc. (South Jordan, Utah).
The CO2mmander (PMDA, Ft. Myers, FL, USA) is a Food and Drug Administration-approved portable medical CO2 delivery system. It allows for the delivery of CO2 at a low pressure to any reservoir (the plastic bag or a large syringe) with the standard Lure-lock fitting. The AngiAssist system (AngioAdvancements, Ft. Myers, FL, USA) that is used with the CO2mmander, is a unique stopcock system with valves that control direction of gas flow and allows gas delivery in a non-explosive fashion. A 60-mL reservoir syringe connected to the AngiAssist system is filled with CO2 from the CO2mmander, which is used to fill a 30-mL injection syringe.
The hand-held syringe method has been used for several decades and found to be safe if the ‘operator uses it properly. One-way or three-way stopcock is connected to a 30 mL or 60 mL Luer-lock tip syringe before filling with CO2. The syringe is filled and emptied with CO2 from the CO2 cylinder to remove residual air from the syringe. Once the syringe has been filled with CO2, the stopcock is quickly opened and closed to decrease the gas pressure within the syringe to the atmospheric pressure. Prior to CO2 injection for vascular imaging, the catheter should be purged with 5 mL of CO2 via the three-way stopcock or by exerting slow, low pressure on the syringe plunger to fill the catheter with a small amount of CO2. Air contamination is a potential risk for the use of a hand-held syringe for CO2 delivery. If the stopcock of the CO2-filled syringe is opened, air contamination occurs because of the CO2 partial pressure differential between the CO2-filled syringe and air [16].
The purity of CO2 or air contamination can be assessed by fluoroscopy of the main pulmonary artery after an injection of 10 to 15 mL of CO2 into a central vein. CO2 bubbles are usually seen in the main pulmonary artery following an injection, which should disappear within 15–20 seconds. If the gas remains visible for more than 30 seconds, air contamination should be suspected, and the delivery system should be checked for air-tight connection. Cross-table lateral DSA can be used to determine the speed of absorption of CO2 trapped in the right atrium in the left lateral decubitus position. The CO2 bubble will dissolve within 45 seconds [7]. If the gas bubble remains visible for more than one minute, there is a possibility of air contamination.
IMAGING of CO2
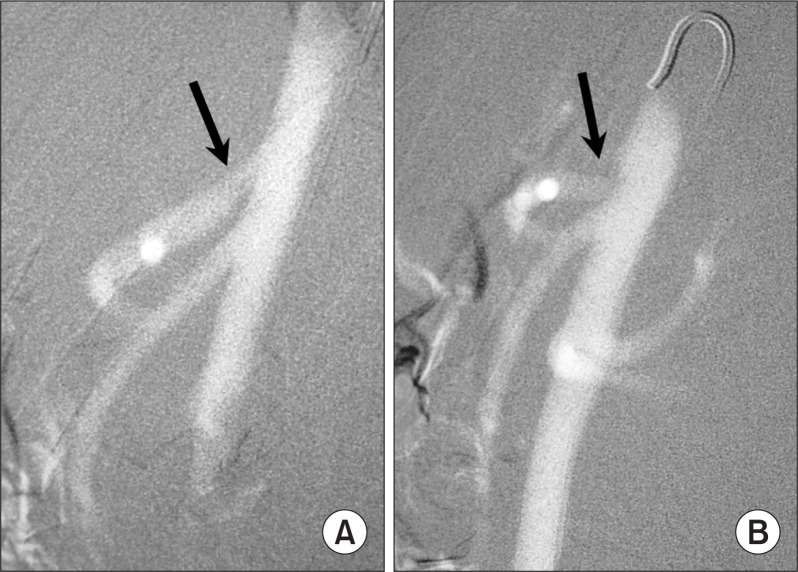
CO2 must be imaged using the digital subtraction equipment with the 1024×1024 system and the CO2 software program. The modern DSA equipment has the stacking software program that allows integration of multiple images into a single composite image. The image stacking helps improve the image quality when the vessel is incompletely filled secondary to CO2 bubble breakup (Fig. 7). Image stacking is also useful in CO2 venography. Since motion degrades CO2 images, obtaining additional mask images helps eliminate misregistration artifacts by using new mask image subtraction. The exposure rate should range from 3 to 6 frames per second and the exposure covers only the arterial and capillary phases since CO2 will not pass through the capillary bed into the vein.
Fig. 7.
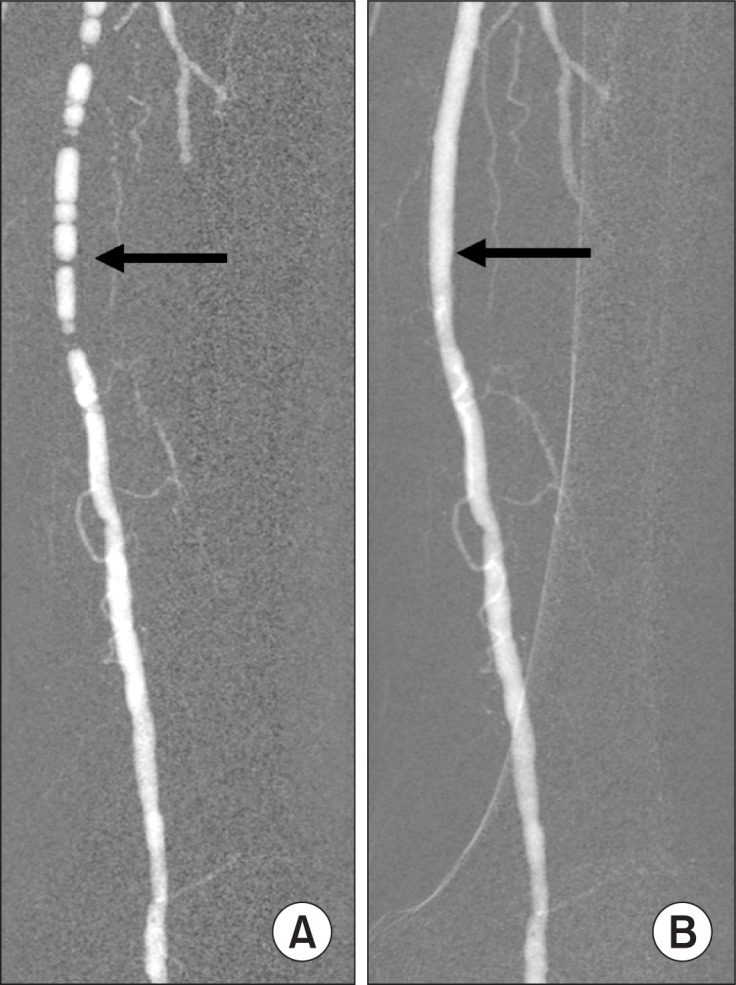
CO2 femoral arteriogram. (A) Breakup of CO2 bubbles into smaller bubbles resulted in incomplete luminal filling that mimics stenosis (arrow). (B) After image stacking, the superficial femoral artery is completely filled (arrow) and no stenosis is seen.
1. Technique
① Use any shape of distal catheter bend and any size of catheter, such as a 3-Fr microcatheter for CO2 injection. An end-hole catheter can be used for both CO2 aortography and vena cavography, and thus eliminates the need for flush catheters.
② Use the plastic bag system, CO2mmander with Angi-Assist, or the hand-held syringe method for CO2 delivery to prevent explosive gas delivery and air contamination
③ Never connect the catheter directly to the CO2 cylinder to avoid inadvertent large volume CO2 injection
④ Close the stopcock of the CO2-filled hand-held syringe until injection to prevent air contamination.
⑤ Purge the catheter with 5 mL of CO2 immediately prior to a CO2 angiogram to prevent explosive gas delivery.
⑥ Increase CO2 volumes to improve CO2 imaging quality.
⑦ Separate CO2 injections by 2–3 minutes.
⑧ Elevate the area of interest (15 degrees for the lower extremity imaging and 45 degree for renal artery imaging).
⑨ Inject 100 μg of nitroglycerin intra-arterially prior to a lower extremity CO2 arteriogram.
⑩ Perform selective injection when imaging a dilated, tortuous artery
⑪ Use the CO2 reflux technique for better imaging the iliac and femoral arteries with CO2 injection into the superficial femoral artery (SFA).
⑫ If motion is a problem for CO2 imaging, use rapid exposure (4–6 frames/sec) with additional mask images for better subtraction, pixel shifting, and image stacking.
PATIENT PREPARATION
The standard evaluation and preparation used for catheter-based angiography with contrast medium are used for CO2 angiography. Since CO2-guided endovascular procedures may require the use of small amounts of iodinated contrast medium, hydration is recommended. Patients with a history of hypersensitivity to iodinated contrast medium require steroid preparation. The majority of simple procedures do not require any preparation. These include abdominal aortography and runoff, transplant renal angiography, upper extremity venography, hepatic venography, and vena cavography.
MONITORING THE PATIENT
The following monitors should be placed on the patient before administration of sedative drugs and injection of CO2: pulse oximeter, electrocardiogram (ECG), blood pressure cuff, and capnometer (if available). Mild sedation should be used to allow the patient hold his or her breath during CO2 imaging. Sedation is not required for CO2 venography.
The highly soluble CO2 is well tolerated when injected into the arterial or venous circulation with the exception of the coronary and cerebral circulations. A rapid injection of CO2 in the artery or vein in quantities sufficient for vascular imaging will not cause any significant changes in vital signs. A significant vital sign change will occur if there was air contamination or if accidental delivery of excessive volumes of CO2 has occurred. The patient should be carefully monitored for the level of sedation as deep sedation can mimic air contamination of the CO2 delivery.
All patients receiving sedation should be carefully monitored for the level of sedation. Delay in the treatment of air embolism from incorrect diagnosis can result in serious clinical outcomes. Pulse oximetry is used to monitor arterial hemoglobin saturation reflecting the status of lung perfusion and oxygen delivery to the tissues. However, it is not an early warning monitor for air embolism. Oxygen saturation may remain above 90% despite severe hypotension from air embolism or accidental delivery of excessive volumes of CO2. Blood pressure is monitored either continuously or at one minute after CO2 delivery. Blood pressure will start to drop within 20 seconds after CO2 injection if there was air contamination in the delivery system. If the blood pressure drops by more than 10 mmHg after CO2 injection, the delivery system and the cylinder must be checked for any source of air contamination.
Capnography is the most reliable monitor for air contamination since it provides the status of hemodynamic and ventilatory functions of the lungs in a real time fashion [17]. It is the graphical and numerical representation of exhaled CO2 concentration during the respiratory cycle and can give an early warning about respiratory depression and pulmonary vapor lock. In addition, ECG, heart rate, and respiratory rate are monitored.
ARTERIAL APPLICATIONS
1. Aortogram and Runoff
CO2 angiography is safe and effective in evaluating the aorta and its branches, and runoff vessels. It can provide enough information to institute appropriate therapy and to guide endovascular intervention [18]. Because of the unique physical properties of CO2 the technical approach to CO2 aortography and runoff is different from contrast medium angiography. After percutaneous catheterization of the femoral artery using the standard Seldinger technique, a 4-or 5-Fr introducer sheath is placed in the femoral artery. A 4- or 5-Fr end-hole catheter is then inserted through the sheath into the upper abdominal aorta, just cephalad to the origin of the celiac artery at the T-12 level. Aortography is performed with the injection of 30–40 mL of CO2 for 1.5 seconds at the frame rate of 4 frames/second. Lateral aortography is performed for visualization of the origins of the celiac and superior mesenteric arteries using the same technique of the AP aortography.
When an aortic aneurysm is present, much of the injected CO2 will be trapped in the most anterior portion of the aneurysm (Fig. 2). The buoyancy effect of CO2 may mislead to incorrect diagnosis due to inadequate filling of the aorta. The aneurysm may appear to be smaller due to trapping of the gas in the aneurysm. On the lateral projection, only the ventral portion of the aneurysm is filled (Fig. 2). The trapped gas can be flowed away by turning the patient from the side to the side. Opacification of the renal arteries may require an injection of CO2 at the level of the neck of the aneurysm, imaging with the DSA technique at the frame rate of 6 frames per second. When imaging AAA and mesenteric arterial occlusive disease, only small volumes of CO2 (less than 20 mL) should be injected every 5 minutes to decrease the amounts of gas trapped and prevent mesenteric gas embolism.
Pelvic arteriogram is performed with CO2 injection into the aortic bifurcation and imaged in the AP and oblique projections. When CO2 fails to fill the entire iliac arteries, the stacking technique is used. The internal iliac arteries will not fill well because of their dependent course. The catheter is then advanced into the contralateral femoral artery and CO2 is injected to visualize the contralateral iliac arteries. Additional injections of CO2 are made into the SFA to visualize the outflow vessels. Elevation of the foot and intra-arterial vasodilator (100 μg of nitroglycerin) can improve filling of the distal vessels. Since CO2 does not mix with blood, it maintains a negative density throughout the outflow vessels (Fig. 4). It can also visualize the distal vessels bilaterally in the presence of aortoiliac occlusive disease (Fig. 3).
Occasionally, with the severe aortic atherosclerosis, the catheter cannot be advanced into the desired level of the contralateral lower extremity. If this occurs, a Cobra or Shepherd hook catheter is placed in the contralateral common iliac artery and a 3-Fr microcatheter is advanced coaxially into the superficial femoral or popliteal artery, and injections are made with 15 to 20 mL of CO2 to fill the distal vessels. A distal CO2 injection usually results in reflux of the gas into the proximal vessels.
Conversion of retrograde into antegrade femoral artery catheterization may be required for diagnostic arteriography and infrainguinal arterial intervention. After retrograde femoral artery catheterization, aortography, pelvic arteriography and arteriography of the contralateral lower extremity are performed. A reverse curve catheter, such as sidewinder or Simmons configuration, is then converted to antegrade catheterization, and a microcatheter is advanced coaxially to the ipsilateral SFA for better filling of the distal vessels. An injection of CO2 into the distal superficial femoral or popliteal artery usually results in reflux of CO2, and fill the proximal femoral and iliac arteries. Unlike contrast medium, the low-viscosity CO2 will flow more easily through collateral vessels, filling the distal arteries without dilution (Fig. 4). In patients with popliteal artery aneurysms, CO2 injection into the aneurysm through a microcatheter will better fill the aneurysm and distal vessels.
- ① A & O Procedure
- Retrograde femoral artery puncture on the less symptomatic side
- Aortogram (may be performed after the runoff) in AP and lateral projections with 30–40 mL of CO2
- Pelvic arteriography with 30 mL of CO2 in AP, right anterior oblique and left anterior oblique projections
- Advance a catheter or a sheath into the contralateral external iliac artery for runoff. Advance a microcatheter coaxially to the popliteal artery for better filling of the distal vessels.
- Ipsilateral runoff with multiple CO2 injections into the external iliac artery. Inject 100 μg of nitroglycerin and elevate the foot to 15 degrees. If peripheral arterial filling is poor, convert the retrograde to antegrade femoral catheterization and advance a 3-Fr microcatheter coaxially to the popliteal artery and inject CO2. Inject dilute contrast medium (140 mg I/mL) into the popliteal artery to better visualize the infrapopliteal arteries and plantar arch.
- Antegrade common femoral artery access for infrainguinal arterial intervention. Enter a 21 g needle into the common femoral artery under ultrasound guidance, and advance a 0.018-inch (0.457 mm) wire. The guide wire occasionally passes down the deep femoral artery instead of continuing into the SFA. When this occurs, a 3-Fr dilator is inserted into the deep femoral artery, and digital subtraction angiography with the injection of 10–15 mL of CO2 is performed to confirm the puncture of the common femoral artery by refluxing CO2. A 5-Fr sheath is then introduced into the deep femoral artery and the wire is reintroduced into the deep femoral artery. While the wire is left in place as a safety wire, the sheath is retracted back to the common femoral artery, and a 0.035-inch (0.889 mm) Terumo wire is advanced medially into the SFA and the 0.018-inch (0.457 mm) wire is withdrawn. Over the Terumo wire, the sheath is advanced into the SFA. If CO2 is injected into the SFA, the gas will reflux and visualize the proximal vessels including the iliac arteries and aorta.
2. Renal artery angioplasty and stenting
When the left renal artery arises from the posterolateral aspect of the aorta, the buoyancy of CO2 may result in incomplete filling of the artery. Turning the patient into the left-side-up position usually results in filling of the artery. Alternatively, the left renal artery is catheterized, and CO2 is injected to fill the proximal renal artery by reflux of CO2. On occasion it is difficult to catheterize the renal artery originating from the atherosclerotic aorta. If this difficulty occurs, one should catheterize the superior mesenteric artery, and injection of CO2 may result in reflux of CO2 into the aorta and the renal artery. If the renal artery is still not seen after any of these injections, the catheter tip is placed in the aorta at the L1–2 vertebral body level, inferior to the origin of the superior mesenteric artery and CO2 is injected into the aorta with DSA to identify the origin of the renal artery. Once the renal artery has been located, an attempt is made to catheterize the renal artery using a Shepherd hook or a cobra catheter. If the renal artery is occluded, delayed images are obtained to demonstrate filling of the distal renal artery through the collaterals.
The lack of nephrotoxicity and allergic responses together with the low viscosity make CO2 a useful contrast agent for renal stent placement [19,20]. The procedure can be done with CO2 alone or with injections of small test doses of contrast medium when the catheter is in the renal artery. Once the stent is positioned across the renal artery stenosis, CO2 is injected into the aorta and imaged in the AP, right and left oblique projections to confirm the correct position of the stent before deployment. After stent deployment, renal artery pressure gradient is measured. If there is no significant gradient across the stent, a completion arteriogram is performed with an injection of CO2 into the renal artery distal to the stent.
3. Visceral angiography and intervention
Celiac, superior and interior mesenteric arteriograms should begin with the injection of CO2 into the aorta in the AP and lateral projections. Lateral aortography is performed in full inspiration to visualize the origin of the celiac and superior mesenteric arteries. The median arcuate ligament of the diaphragm can compress the celiac artery. Such compression is usually associated with an epigastric bruit and pain in young women. The lateral aortogram shows a typical concave impression on the cranial aspect of the celiac artery just distal to its origin [21]. If CO2 aortography is performed in both full inspiration and expiration, the degree of the stenosis usually changes, increasing with expiration and decreasing or disappearing with inspiration (Fig. 5). CO2 is a useful contrast agent for the evaluation of visceral vascular anatomy, visceral arterial occlusive disease, aneurysms and pseudoaneurysms, traumatic bleeding of the liver and spleen, and endovascular intervention.
The buoyancy of CO2 makes the gas an effective contrast agent for stenting celiac or superior mesenteric artery stenosis. Using the cross-table lateral imaging and fluoroscopy, CO2 is injected into the aorta superior to the celiac artery to determine stent positioning. After deploying the stent, an arterial pressure gradient is obtained and then CO2 is injected into the superior mesenteric artery for a completion arteriogram.
4. Transplant renal artery DSA
Transplant renal artery stenosis is a curable cause of post-transplant arterial hypertension, allograft dysfunction and graft loss. Doppler ultrasonography and magnetic resonance angiography are used as the initial diagnostic modality. However, the definitive diagnosis of a hemodynamically significant renal artery stenosis requires catheter angiography and measurement of the arterial pressure gradient across the lesion. If a significant stenosis is present, percutaneous transluminal angioplasty and stenting is performed to restore kidney perfusion. Because of the buoyancy, CO2 is a useful contrast agent for imaging the renal transplant artery located anterior to the iliac artery injection site [22].
5. CO2 for detection of bleeding
CO2 is a safe and useful contrast agent for the diagnosis of bleeding in the gastrointestinal tract, spleen, liver, kidney and pelvis [23,24]. It is sensitive in detecting the bleeding since CO2 is non-viscous, compressible and immiscible with blood. In gastrointestinal bleeding, angiography is performed only when endoscopy fails to reveal the bleeding site. When the bleeding site has been demonstrated by endoscopy or computed tomography (CT) angiography, the patient is referred for angiographic localization of the bleeding site and embolization. The procedure should begin with injection of CO2 into the aorta. If CO2 extravasation is seen in the lower gastrointestinal tract or colon, superior and inferior mesenteric arteriograms are performed with contrast medium for a roadmap for selective catheterization and embolization. Once a bleeding site is seen, a 3-Fr microcatheter is advanced coaxially into the bleeding artery, and CO2 is injected to demonstrate gas extravasation. Peristalsis makes CO2 very difficult to image in superior mesenteric angiography. The administration of glucagon, a high frame rate and the use of new mask can improve image quality. After arterial embolization with appropriate embolic materials (usually microcoils), CO2 or contrast medium is injected for a completion arteriogram. In the patient with recurrent bleeding but no active extravasation, provocative angiography may be performed with intra-arterial injections of heparin, vasodilators and a thrombolytic agent. When the bleeding has been induced and the bleeding site is identified, repeat arteriogram is done with the injection of contrast medium for vascular roadmap for selective embolization.
In traumatic bleeding, contrast-enhanced CT plays an important role in the evaluation of active bleeding from the spleen, liver, kidney and pelvis. It also provides enough information to institute angiographic therapy and direct the angiographer to the precise area of interest. Because of the low viscosity, CO2 is sensitive in detecting extravasation. If extravasation is seen on the aortogram, the bleeding artery is catheterized and the arteriogram is performed with CO2 and contrast medium for a roadmap before embolization.
6. CO2-guided EVAR
Renal failure is a potentially serious complication among patients undergoing EVAR with the use of iodinated contrast material. CO2 DSA can provide much of the necessary vascular information for safe deployment of the aortic stent graft with the use of small amounts of iodinated contrast medium if necessary [25,26]. The author’s experience with CO2-guided EVAR has involved the deployment of Zenith Flex aortic endograft (Cook Medical Inc., Bloomington, IN, USA). CO2 angiography is used to localize the orifice of the renal and hypogastric arteries before deploying the stent graft. CO2 angiography is useful in the diagnosis of endoleaks following an EVAR procedure [27].
The plastic bag system has been used for delivery of CO2 through the side port of the main body and the iliac limb delivery sheath. CO2 can also be injected using a diagnostic catheter. Immediately prior to introduction of the main body endograft, the side port of the delivery sheath is purged with 20-mL of CO2. After placing the main body endograft, 20-mL of CO2 is injected through the side port to identify the orifice of the renal arteries with the side of interest elevated to 8–10 degrees. After the four radiopaque markers of the proximal graft are positioned caudal to the renal artery, repeat CO2 DSA is performed to re-demonstrate the endograft position. The hypogastric arteries are identified with injections of 20-mL CO2 through the side ports of the femoral sheaths. A completion angiogram is performed with injection of CO2 through a 5-Fr end-hole catheter at the level of the renal arteries and in the graft.
VENOUS APPLICATIONS
1. CO2 upper extremity venography
CO2 is a useful contrast agent for upper extremity venography and allows visualization of the basilic, cephalic and subclavian veins. Because of the low viscosity of CO2 it can be injected through a small needle placed in a peripheral vein. Indications for CO2 venography include: evaluation of arm swelling and prior to creation of dialysis fistulas, replacement of an implantable cardioverter-defibrillator or a pacemaker system, and peripherally inserted central catheter placement in patients with difficult venous access. When performing CO2 venography for planning fistula creation for hemodialysis, an intravenous line is placed in a vein on the dorsum of the hand on the radial side for CO2 injection to visualize the cephalic vein, which is used for creation of the radial artery-cephalic fistula. Digital subtraction venography is done with the injection of 10 mL of CO2 per second for three seconds at the frame rate of 3 frames per second. Purging the intravenous line with 3–5 mL of CO2 before CO2 injection for venography will decrease pain from gas explosion at the injection site. Intravenous injections of Lidocaine and 100 μg of nitroglycerin may help alleviate pain with CO2 injection.
2. CO2 for hemodialysis access
CO2 can be used as a contrast agent for fistulography and access declotting procedure [15]. The patient is placed supine on the fluoroscopy table and the arm being examined is placed in the anatomical position on an arm board. The technique used for CO2 imaging of hemodialysis graft and fistula is similar to that used with contrast medium. Fifteen to twenty mililiters of CO2 is injected into the venous outflow to visualize the venous anastomosis and central veins. The injection rate should be adjusted to avoid reflux of CO2 into the brachial and subclavian arteries. Declotting procedure, angioplasty and stenting of venous stenosis can be performed using CO2 as a contrast agent.
3. CO2 vena cavography prior to filter placement
CO2 is a safe and effective contrast agent for vena cavography prior to filter placement. It can provide the necessary information for filter placement including caval patency, the level of left renal vein, and venous anomalies such as caval duplication, retroaortic and circumaortic renal vein. The transverse diameter of the inferior vena cava (IVC) can be accurately measured on a CO2 cavogram obtained in the AP projection [28].
The CO2 cavography technique prior to filter placement is as follows: after percutaneous catheterization of the right femoral vein under ultrasound guidance, a 5-Fr end-hole catheter with Cobra configuration (C-3) is introduced into the external iliac vein over a guide wire through a 6-Fr sheath. Digital subtraction cavography is performed with injection of 30 mL of CO2 into the external iliac vein at the frame rate of 4 frames per second. The catheter is then advanced into the contralateral iliac vein, and left iliac venography is performed with an injection of 30 mL of CO2. This will visualize the left iliac vein, the inferior vena cava, and caval duplication if present. When left femoral vein has been punctured, CO2 is injected into the left external iliac vein using a 5-Fr Cobra catheter to demonstrate the iliac vein and the inferior vena cava. If the left renal vein has not been filled by any of the iliac vein and IVC injection, the Cobra catheter is advanced into the left renal vein at the L1–2 vertebral level and digital subtraction left renal venography is performed with an injection of 20 mL of CO2. The cobra catheter is used to search for retroaortic left renal vein. If the catheter has been placed in the left renal vein, the catheter is retracted to the IVC, and the guide wire advanced to the right atrium. The filter sheath/dilator is advanced over the wire to the level of the left renal vein. After removal of the dilator, the filter is deployed just below the lowest renal vein. Thirty mililiters of CO2 is injected into the IVC through the sheath to demonstrate filter placement.
4. CO2 wedged hepatic venography
Wedged hepatic venography is indicated for the evaluation of portal hypertension, portal vein patency, and targeting the portal vein in TIPS [29]. Once the catheter tip is wedged in a peripheral hepatic vein from the transjugular or transfemoral route, wedged hepatic venous pressure is obtained to calculate a pressure gradient between the portal vein and right atrium. Wedged hepatic venography is performed with 30 mL of CO2 injected at a rate of 15 mL per second and at a rate of 4 frames per second. If the portal vein fails to fill with CO2, the catheter should be wedged in another hepatic vein for repeat CO2 injection. If this still fails to fill the portal vein, balloon occlusion hepatic venogram or CO2 parenchymal injection is done. The success rate for portal vein visualization with CO2 is over 90 percent.
The potential complications of CO2 wedged hepatic venography is hepatic capsular perforation and bleeding as a result of explosive delivery of a compressed gas. Purging the catheter with 3 to 5 mL of CO2 prior to CO2 injection for wedged hepatic venography can prevent the explosive delivery of the gas, thus reducing the risk of capsular perforation.
5. CO2 splenoportography
For years splenoportography was the important angiographic method for evaluating patients with cirrhosis and portal hypertension. It can demonstrate the portal venous system and portosystemic collateral veins. In 1970s, because of the risk of bleeding associated with splenoportography using a larger needle and contrast medium, the procedure has been largely replaced by noninvasive imaging method and arterial portography (indirect portography). CO2 transsplenic portography using a 22 to 25 gauge needle is a safe and effective technique for visualizing the portal venous system and portosystemic collaterals [30,31]. CO2 splenoportography is useful, especially in pediatric patients when an imaging study of portal vein is inconclusive. It eliminates the need for femoral artery catheterization for arterial portography.
RISKS OF CO2 ANGIOGRAPHY
Adverse reactions associated with CO2 angiography are uncommon. Operator error has resulted in serious complications, including air contamination of the CO2 delivery system, injection of gases other than CO2, delivery of excessive volume of CO2, and neurotoxicity related to an inadvertent injection of CO2 into the cerebral circulation [32,33].
Injection site complications are uncommon since CO2 is less viscous than contrast material. CO2 extravasation rarely occurs with an intra-arterial injection but has been seen with peripheral venous injections. CO2 extravasation into the soft tissue has not caused any sequel since the gas is rapidly absorbed. Pain and discomfort may occur when CO2 is injected into a small peripheral vein due to an explosive delivery of the compressed gas. This adverse reaction lasts less than one to two minutes, but the pain-induced motion degrades image quality. Purging the catheter or the delivery tubing with 3–5 mL of CO2 must be done prior to a diagnostic CO2 injection. Injection of CO2 into the abdominal aorta or the celiac axis may cause epigastric pain, nausea and even vomiting that usually last a few minutes. Turning the patient from the side to the side usually helps relieve the symptoms.
Air contamination of the delivery system can result in intrapulmonary vapor lock with a cardiac event and even death. An inadvertent injection of air in the visceral arteries can cause bowel ischemia and infarction. The source of CO2 before filling a syringe or the plastic bag must be confirmed for a CO2 cylinder labeled with CO2 USP. When using the hand-held syringe for CO2 delivery, its stopcock must be closed until injection. With the plastic bag system, air tight connection must be made between the plastic bag and the delivery system.
In order to prevent inadvertent delivery of excessive volume, the catheter should not be connected directly to the CO2 cylinder. If a 60-mL syringe is filled with pressurized gas, the volume of the gas far exceeds the volume of the syringe when delivered into a vessel. Therefore, once the syringe is filled with a pressurized gas, the stopcock should be opened and closed to eliminate the gas pressure from the syringe.
Vapor lock is caused by trapped CO2 bubbles in a vessel and interrupt the blood flow. Inadvertent intravenous injection of excessive volume of CO2 can potentially obstruct the normal flow of blood in the pulmonary artery, resulting in hypoxia and hypotension. Injection of 40 mL of CO2 into a vein will not cause pulmonary artery vapor lock and any change in vital signs.
Another area of CO2 vapor lock involves the inferior mesenteric artery in the patient with AAA. When CO2 is trapped in the ventral portion of the AAA, the gas is replaced by less soluble nitrogen from the blood, which can obstruct blood flow in the inferior mesenteric artery by blocking collateral circulation from the superior mesenteric artery. This may cause diarrhea and even colonic ischemia. Another potential vapor lock may develop when CO2 angiography is performed during nitrous oxide anesthesia in which less soluble nitrogen can diffuse into the trapped CO2 bubble resulting in expansion of the bubble containing less soluble nitrogen [30].
Because of its potential neurotoxicity of CO2, the gas should not be used as an arterial contrast agent above the diaphragm. A rat study of the carotid injection of CO2 has shown that CO2 can be neurotoxic with multifocal ischemic infarctions and disruption of the blood-brain barrier [11]. However, in a rabbit study by Dimakakos et al. [12], CO2 infusion into the thoracic aorta and carotid artery at the rate 3 mL/kg, revealed no evidence of ischemic brain infarct, hemorrhage, thrombosis or foci of necrosis on MR imaging and histologic examination. In a pig study by Kozlov et al. [13] single CO2 injection into the internal carotid artery did not produce persistent clinical symptomatology. However when two sequential injections of CO2 were made, all animal had adverse effects after the second injection, including involuntary tonic-clonic muscular movements, cardiopulmonary arrest and recurrent intractable seizure activity.
Paradoxical gas embolism following venous CO2 injection in the presence of PFO is a rare event. If pulmonary artery pressure increases with CO2 injection, a potentially PFO may open and allow CO2 to flow through the left atrium into the aortic arch. If any changes in vital signs occur following a venous CO2 injection, air contamination or paradoxical gas embolism should be suspected.
Hawkins et al. [34] examined the nephrotoxicity of CO2. CO2 was injected into the canine renal arteries at a dose of 7 mL/kg to 54 mL/kg. The CO2 injection decreased renal blood flow by 11.86% but the renal blood flow returned to the baseline level 24 hours later. However, renal function remained unchanged. Mild histologic changes and one case of moderate acute tubular necrosis were found when the kidneys were placed vertically. It was concluded that CO2 was a safe contrast agent and less nephrotoxic than iodinated contrast materials.
CONCLUSION
CO2 angiography can be used as a contrast agent in all arterial and venous circulations except the thoracic aorta, the coronary artery, and the cerebral circulation. It can provide vascular information necessary for diagnosis and intervention. CO2 is the only known safe contrast agent in patients with contrast allergy and renal insufficiency, and can prevent contrast-induced nephropathy. CO2 is preferable in many diagnostic arteriography and endovascular interventions that often require large amounts of contrast medium. An understanding of the properties of CO2 and development of a facile catheterization and imaging techniques are essential in the practice of CO2 angiography.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Paul RE, Durant TM, Oppenheimer MJ, Stauffer HM. Intravenous carbon dioxide for in tracardiac gas contrast in the roentgen diagnosis of pericardial effusion and thickening. Am J Roentgenol Radium Ther Nucl Med. 1957;78:224–225. [PubMed] [Google Scholar]
- 2.Scatliff JH, Kummer AJ, Janzen AH. The diagnosis of pericardial effusion with intracardiac carbon dioxide. Radiology. 1959;73:871–883. doi: 10.1148/73.6.871. [DOI] [PubMed] [Google Scholar]
- 3.Barrera F, Durant TM, Lynch PR, Oppenheimer MJ, Stauffer HM, Stewart GH., 3rd In vivo visualization of intracardiac structures with gaseous carbon dioxide; cardiovascular-respiratory effects and associated changes in blood chemistry. Am J Physiol. 1956;186:325–334. doi: 10.1152/ajplegacy.1956.186.2.325. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins IF. Carbon dioxide digital subtraction arteriography. AJR Am J Roentgenol. 1982;139:19–24. doi: 10.2214/ajr.139.1.19. [DOI] [PubMed] [Google Scholar]
- 5.Cho KJ, Hawkins IF. Carbon. In: Cho KJ, Hawkins IF, editors. Carbon dioxide angiography: principles, techniques, and practices. New York: Informa Healthcare; 2007. [Google Scholar]
- 6.Hawkins IF, Cho KJ, Caridi JG. Carbon dioxide in angiography to reduce the risk of contrast-induced nephropathy. Radiol Clin North Am. 2009;47:813–825. doi: 10.1016/j.rcl.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Cho KJ, Cho DR, Hawkins IF., Jr A simple DSA method to detect air contamination during CO2 venous studies. Cardiovasc Intervent Radiol. 2006;29:642–645. doi: 10.1007/s00270-005-0009-0. [DOI] [PubMed] [Google Scholar]
- 8.Song K, Cho D, Shinn K, Charlton E, Cho K. Gas dynamics in CO2 angiography: in vitro evaluation in a circulatory system model. Invest Radiol. 1999;34:151–155. doi: 10.1097/00004424-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins IF, Jr, Caridi JG, Klioze SD, Mladinich CR. Modified plastic bag system with O-ring fitting connection for carbon dioxide angiography. AJR Am J Roentgenol. 2001;176:229–232. doi: 10.2214/ajr.176.1.1760229. [DOI] [PubMed] [Google Scholar]
- 10.Cho KJ, Hawkins IF., Jr Discontinuation of the plastic bag delivery system for carbon dioxide angiography will increase radiocontrast nephropathy and life-threatening complications. AJR Am J Roentgenol. 2011;197:W940–W941. doi: 10.2214/AJR.11.6639. [DOI] [PubMed] [Google Scholar]
- 11.Coffey R, Quisling RG, Mickle JP, Hawkins IF, Jr, Ballinger WB. The cerebrovascular effects of intraarterial CO2 in quantities required for diagnostic imaging. Radiology. 1984;151:405–410. doi: 10.1148/radiology.151.2.6424174. [DOI] [PubMed] [Google Scholar]
- 12.Dimakakos PB, Stefanopoulos T, Doufas AG, Papasava M, Gouliamos A, Mourikis D, et al. The cerebral effects of carbon dioxide during digital subtraction angiography in the aortic arch and its branches in rabbits. AJNR Am J Neuroradiol. 1998;19:261–266. [PMC free article] [PubMed] [Google Scholar]
- 13.Kozlov DB, Lang EV, Barnhart W, Gossler A, De Girolami U. Adverse cerebrovascular effects of intraarterial CO2 injections: development of an in vitro/in vivo model for assessment of gas-based toxicity. J Vasc Interv Radiol. 2005;16:713–726. doi: 10.1097/01.RVI.0000153114.05700.61. [DOI] [PubMed] [Google Scholar]
- 14.Lambert CR, de Marchena EJ, Bikkina M, Arcement BK. Effects of intracoronary carbon dioxide on left ventricular function in swine. Clin Cardiol. 1996;19:461–465. doi: 10.1002/clc.4960190604. [DOI] [PubMed] [Google Scholar]
- 15.Kariya S, Tanigawa N, Kojima H, Komemushi A, Shiraishi T, Kawanaka T, et al. Efficacy of carbon dioxide for diagnosis and intervention in patients with failing hemodialysis access. Acta Radiol. 2010;51:994–1001. doi: 10.3109/02841851.2010.518159. [DOI] [PubMed] [Google Scholar]
- 16.Cho DR, Cho KJ, Hawkins IF., Jr Potential air contamination during CO2 angiography using a hand-held syringe: theoretical considerations and gas chromatography. Cardiovasc Intervent Radiol. 2006;29:637–641. doi: 10.1007/s00270-005-0019-y. [DOI] [PubMed] [Google Scholar]
- 17.Sandlin D. Capnography for nonintubated patients: the wave of the future for routine monitoring of procedural sedation patients. J Perianesth Nurs. 2002;17:277–281. doi: 10.1053/jpan.2002.34336. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins IF, Caridi JG. Carbon dioxide (CO2) digital subtraction angiography: 26-year experience at the University of Florida. Eur Radiol. 1998;8:391–402. doi: 10.1007/s003300050400. [DOI] [PubMed] [Google Scholar]
- 19.Liss P, Eklöf H, Hellberg O, Hägg A, Boström-Ardin A, Löfberg AM, et al. Renal effects of CO2 and iodinated contrast media in patients undergoing renovascular intervention: a prospective, randomized study. J Vasc Interv Radiol. 2005;16:57–65. doi: 10.1097/01.RVI.0000144807.81633.79. [DOI] [PubMed] [Google Scholar]
- 20.Caridi JG, Stavropoulos SW, Hawkins IF., Jr CO2 digital subtraction angiography for renal artery angioplasty in high-risk patients. AJR Am J Roentgenol. 1999;173:1551–1556. doi: 10.2214/ajr.173.6.10584800. [DOI] [PubMed] [Google Scholar]
- 21.Reuter SR. Accentuation of celiac compression by the median arcuate ligament of the diaphragm during deep expiration. Radiology. 1971;98:561–564. doi: 10.1148/98.3.561. [DOI] [PubMed] [Google Scholar]
- 22.Moresco KP, Patel NH, Namyslowski Y, Shah H, Johnson MS, Trerotola SO. Carbon dioxide angiography of the transplanted kidney: technical considerations and imaging findings. AJR Am J Roentgenol. 1998;171:1271–1276. doi: 10.2214/ajr.171.5.9798859. [DOI] [PubMed] [Google Scholar]
- 23.Funaki B. Carbon dioxide angiography. Semin Intervent Radiol. 2008;25:65–70. doi: 10.1055/s-2008-1052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandhu C, Buckenham TM, Belli AM. Using CO2-enhanced arteriography to investigate acute gastrointestinal hemorrhage. AJR Am J Roentgenol. 1999;173:1399–1401. doi: 10.2214/ajr.173.5.10541128. [DOI] [PubMed] [Google Scholar]
- 25.Criado E, Kabbani L, Cho K. Catheterless angiography for endovascular aortic aneurysm repair: a new application of carbon dioxide as a contrast agent. J Vasc Surg. 2008;48:527–534. doi: 10.1016/j.jvs.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 26.Criado E, Upchurch GR, Jr, Young K, Rectenwald JE, Coleman DM, Eliason JL, et al. Endovascular aortic aneurysm repair with carbon dioxide-guided angiography in patients with renal insufficiency. J Vasc Surg. 2012;55:1570–1575. doi: 10.1016/j.jvs.2011.11.142. [DOI] [PubMed] [Google Scholar]
- 27.Sueyoshi E, Nagayama H, Sakamoto I, Uetani M. Carbon dioxide digital subtraction angiography as an option for detection of endoleaks in endovascular abdominal aortic aneurysm repair procedure. J Vasc Surg. 2015;61:298–303. doi: 10.1016/j.jvs.2014.07.088. [DOI] [PubMed] [Google Scholar]
- 28.Dewald CL, Jensen CC, Park YH, Hanks SE, Harrell DS, Peters GL, et al. Vena cavography with CO2 versus with iodinated contrast material for inferior vena cava filter placement: a prospective evaluation. Radiology. 2000;216:752–757. doi: 10.1148/radiology.216.3.r00au15752. [DOI] [PubMed] [Google Scholar]
- 29.Debernardi-Venon W, Bandi JC, García-Pagán JC, Moitinho E, Andreu V, Real M, et al. CO2 wedged hepatic venography in the evaluation of portal hypertension. Gut. 2000;46:856–860. doi: 10.1136/gut.46.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho KJ, Cho DR. CO2 digital subtraction splenoportography with the “skinny” needle: experimental study in a swine model. Cardiovasc Intervent Radiol. 2003;26:273–276. doi: 10.1007/s00270-003-2655-4. [DOI] [PubMed] [Google Scholar]
- 31.Caridi JG, Hawkins IF, Jr, Cho K, Sohn SY, Langham MR, Jr, Weichmann BN, et al. CO2 splenoportography: preliminary results. AJR Am J Roentgenol. 2003;180:1375–1378. doi: 10.2214/ajr.180.5.1801375. [DOI] [PubMed] [Google Scholar]
- 32.Caridi JG, Hawkins IF., Jr CO2 digital subtraction angiography: potential complications and their prevention. J Vasc Interv Radiol. 1997;8:383–391. doi: 10.1016/S1051-0443(97)70577-3. [DOI] [PubMed] [Google Scholar]
- 33.Steffey EP, Johnson BH, Eger EI., 2nd Nitrous oxide intensifies the pulmonary arterial pressure response to venous injection of carbon dioxide in the dog. Anesthesiology. 1980;52:52–55. doi: 10.1097/00000542-198001000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins IF, Jr, Mladinich CR, Storm B, Croker BP, Wilcox CS, Akins EW, et al. Short-term effects of selective renal arterial carbon dioxide administration on the dog kidney. J Vasc Interv Radiol. 1994;5:149–154. doi: 10.1016/S1051-0443(94)71474-3. [DOI] [PubMed] [Google Scholar]