Abstract
Background
Adjuvant chemotherapy after the complete resection of non-small-cell lung cancer (NSCLC) is now the standard of care. To improve survival, it is important to identify risk factors for the continuation of adjuvant chemotherapy. In this study, we analyzed chemotherapy compliance and magnitude of the prognostic impact of the prognostic nutritional index (PNI) before adjuvant chemotherapy.
Methods
We conducted a retrospective review of data from 106 patients who had received adjuvant chemotherapy. The adjuvant chemotherapy consisted of an oral tegafur agent (OT) or platinum-based chemotherapy (PB). The correlations between the PNI values and recurrence-free survival (RFS) were then evaluated.
Results
In the PB group, the percentage of patients who completed the four planned cycles of chemotherapy was not correlated with the PNI. In the OT group, however, a significant difference was observed in the percentage of patients who completed the planned chemotherapy according to the PNI before adjuvant chemotherapy. The RFS of patients with a PNI <50 before adjuvant chemotherapy was significantly poorer than that of the patients with a PNI ≥50. A multivariate analysis showed that nodal metastasis and PNI before chemotherapy were independent predictors of the RFS. However, PNI before surgery was not a predictor of the RFS. In the subgroup analysis, PNI before chemotherapy was independent predictor of the RFS in the OT group (P=0.019), but not in the PB group (P=0.095).
Conclusion
The PNI before adjuvant chemotherapy influenced the treatment compliance with the planned chemotherapy in the OT group, but not the PB group. In addition, a low PNI before adjuvant chemotherapy was associated with a poor RFS in a multivariate analysis, especially in the OT group.
Keywords: non-small-cell lung cancer, adjuvant chemotherapy, prognostic nutritional index, treatment compliance
Introduction
Lung cancer is a leading cause of cancer-related death worldwide. The most effective treatment for non-small-cell lung cancer (NSCLC) is surgical resection. In addition, adjuvant chemotherapy after the complete resection of stage II–IIIA NSCLC is now the standard of care based on three large-scale phase III trials and a meta-analysis.1–4 In Japan, moreover, tegafur–uracil (UFT) has been selected for patients with stage I disease (T1bN0M0 and T2N0M0).5,6 The aim of adjuvant chemotherapy is to eradicate micrometastatic tumor cells. Thus, it is important to continue chemotherapy for a sufficient length of time. In breast cancer, the efficacy of adjuvant chemotherapy decreased when the treatment was insufficient.7,8 To improve patient survival, it is important to identify risk factors for the continuation of adjuvant chemotherapy.
The prognostic nutritional index (PNI), which is calculated by combining the serum albumin concentration with the total peripheral blood lymphocyte count, was initially used to assess the immune-nutritional status of patients receiving gastrointestinal surgery.9 Several reports have shown that the PNI is a prognostic marker in patients with various cancers, including cancers of the esophagus, stomach, colorectum, and pancreas, and malignant pleural mesothelioma.10–14 Moreover, the PNI can predict the prognosis of patients with cancer regardless of the site of origin.15 However, few studies examining the PNI in patients with NSCLC have been performed.
On the basis of these findings, we investigated the impact of PNI among NSCLC patients who had received adjuvant chemotherapy. In this study, we analyzed chemotherapy compliance and the magnitude of the prognostic impact of the PNI. In addition, we examined the best timing for the evaluation PNI: before surgery or before adjuvant chemotherapy.
Methods
Study population
We conducted this retrospective study in a total of 552 patients with NSCLC who underwent surgery at the Kawasaki Medical School Hospital between 2005 and 2012. Of these, 157 patients received adjuvant chemotherapy. Fifty-one patients were excluded from the study. All the patients who were included in the analysis met the following criteria: 1) lobectomy with lymph node dissection; 2) neither radiotherapy nor chemotherapy administered prior to surgery; and 3) two PNI evaluations, one obtained before surgery and one obtained before adjuvant chemotherapy. The histological diagnosis of the tumors was based on the criteria of the World Health Organization, and the TNM stage was determined according to the criteria established in 2009. This study was conducted with the approval of the institutional Ethics Committee of Kawasaki Medical School (Number 1803: Approved on May 12, 2014). The requirement for informed consent from individual patients was waived for this retrospective analysis of the database.
Adjuvant chemotherapy and follow-up
The adjuvant chemotherapy consisted of an oral tegafur agent (OT) or platinum-based chemotherapy (PB). The criteria for regimen selection were based on a discussion among the hospital cancer board and on enrollment in a clinical trial (the Setouchi Lung Cancer Study Group). In practice, OT was selected for patients with stage I (T1bN0M0 and T2N0M0), and PB was selected for patients with stage II and IIIA cancer.4,6 In OT group, patients received UFT (250 mg/m2 of body surface area per day for 2 years) or S-1 (80 mg/m2 of body surface area for 4 consecutive weeks repeated every 6 weeks for 1 year) within 8 weeks after surgery. UFT is an oral agent consisting of the combination of uracil and tegafur at a molar ratio of 4:1, and S-1 is a fluorinated pyrimidine formulation that combines tegafur, 5-chloro-2,4-dihydroxypyridine, and potassium oxonate in a molar ratio of 1:0.4:1. The PB regimens mainly consisted of four cycles of carboplatin plus paclitaxel, carboplatin plus gemcitabine, carboplatin plus S-1, or cisplatin plus vinorelbine. Postoperative radiotherapy was not performed. The schedule for follow-up examinations was arranged on an individual basis; most of the patients received medical check-ups and chest X-ray or CT scans at least twice per year. The last follow-up review was performed on June 30, 2014. The median follow-up duration for the determination of the RFS was 34.6 months (range, 3–62 months).
PNI evaluation
The PNI values were calculated using data from a complete blood count that was routinely performed before surgery and before adjuvant chemotherapy. On the basis of a previous study, the PNI was calculated as 10× serum albumin (g/dL) +0.005× total lymphocyte count (per mm3).9 A PNI value of at least 50 was defined as normal, while less than 50 was regarded as mild malnutrition, less than 45 was regarded as moderate-to-severe malnutrition, and less than 40 was regarded as serious malnutrition.11 The PNI cut-off value for clinically significant malnutrition was set at below 50 in this study.
Statistical analysis
All the statistical analyses were performed using the SPSS statistical package (version 17.0; SPSS, Chicago, IL, USA). Categorical data were examined using the χ2-test. The prognostic evaluation was performed based on recurrence-free survival (RFS). RFS was defined as the time from the date of surgery until lung cancer recurrence or non-lung-cancer death. The impact of PNI was evaluated according to the type of adjuvant chemotherapy (OT or PB). The survival curves were estimated using the Kaplan–Meier method, and differences were evaluated using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Two-sided P-values of less than 0.05 were considered statistically significant.
Results
Patient characteristics
The patient characteristics are summarized in Table 1. The patients ranged in age from 44 to 81 years (mean, 66.9 years). There were 74 men and 32 women. Seventy-two patients (67.9%) had adenocarcinoma, while 20 (18.9%) had squamous cell carcinoma, 7 (6.6%) had large cell carcinoma, and 7 (6.6%) had other histological types. Pathological N0 disease was confirmed in 61 patients (57.6%), and N1 or N2 disease was confirmed in 45 patients (42.6%). Pathological stage I disease was confirmed in 48 patients (45.4%), and stage II or III disease was confirmed in 58 patients (54.6%). Fifty-three patients received PB adjuvant chemotherapy, and 53 patients received OT adjuvant chemotherapy.
Table 1.
Patient characteristics enrolled in this study (n=106)
| Number | % | |
|---|---|---|
| Sex | ||
| Men | 74 | 69.8 |
| Women | 32 | 30.2 |
| Age, mean ± SD | 66.9±8.7 | |
| Histology | ||
| Adenocarcinoma | 72 | 67.9 |
| Squamous cell carcinoma | 20 | 18.9 |
| Large-cell carcinoma | 7 | 6.6 |
| Adenosquamous carcinoma | 3 | 2.8 |
| Pleomorphic carcinoma | 4 | 3.8 |
| Tumor differentiation | ||
| Well | 26 | 24.5 |
| Moderate | 37 | 34.9 |
| Poor | 43 | 40.6 |
| Nodal status | ||
| N0 | 61 | 57.6 |
| N1 | 22 | 20.7 |
| N2 | 23 | 21.7 |
| Pathological stage | ||
| IA | 11 | 10.4 |
| IB | 37 | 35.0 |
| IIA + IIB | 29 | 27.3 |
| IIIA | 29 | 27.3 |
| Chemotherapy regimen | ||
| PB agent | 53 | 50.0 |
| CBDCA + paclitaxel | 29 | |
| CBDCA + gemcitabine | 12 | |
| CDDP + vinorelbine | 7 | |
| CBDCA + S-1 | 5 | |
| OT agent | 53 | 50.0 |
| UFT | 39 | |
| S-1 | 14 |
Abbreviations: CDDP, cisplatin; CBDCA, carboplatin; UFT, tegafur-uracil; OT, oral tegafur; PB, platinum-based.
Correlations between chemotherapy regimen and clinicopathological characteristics
The PB group had a higher proportion of tumors at a pathological lymph node status of N1 or N2 (P=0.001) and higher pathological stage (P=0.001) than the OT group, but no significant associations were observed between the chemotherapy regimen and patient sex, tumor size, histological subtype, or PNI (both before surgery and before chemotherapy) (Table 2).
Table 2.
Patient characteristics according to chemotherapy regimen
| Characteristics | PB chemotherapy | Oral-FT chemotherapy | P-value |
|---|---|---|---|
| Patients, number | 53 | 53 | |
| Age, mean ± SD | 65.1±8.4 | 68.6±8.7 | 0.036 |
| Sex | 0.526 | ||
| Male | 39 | 35 | |
| Female | 14 | 18 | |
| Histology | 0.451 | ||
| Adenocarcinoma | 32 | 40 | |
| Squamous cell carcinoma | 11 | 9 | |
| Large-cell carcinoma | 6 | 1 | |
| Adenoaquamous carcinoma | 2 | 1 | |
| Pleomorphic carcinoma | 2 | 2 | |
| Tumor size (mean), mm | 36.6 | 35.3 | 0.644 |
| Pathological nodal status | 0.001 | ||
| pN0 | 19 | 42 | |
| pN1 | 16 | 6 | |
| pN2 | 18 | 5 | |
| Pathological stage | 0.001 | ||
| IA | 0 | 11 | |
| IB | 12 | 25 | |
| IIA + IIB | 17 | 12 | |
| IIIA | 24 | 5 | |
| PNI | |||
| Before surgery, mean ± SD | 52.7±5.9 | 50.9±5.5 | 0.102 |
| Before chemotherapy, mean ± SD | 49.7±4.7 | 49.9±5.3 | 0.815 |
Abbreviations: PNI, prognostic nutritional index; PB, platinum-based; FT, tegafur.
Chemotherapy compliance according to PNI
Table 3 compares the treatment compliance of the two groups. In the PB group, no difference was observed in the percentage of patients who completed the four planned cycles of chemotherapy. In the OT group, however, a signifi-cant difference was observed in the percentage of patients who completed the planned chemotherapy when compared according to the PNI before adjuvant chemotherapy (71.4% versus 36.0%, P=0.010). The reasons for the discontinuation of chemotherapy were recurrence and adverse events.
Table 3.
Treatment compliance according to PNI
| Characteristics | PNI before chemotherapy
|
||
|---|---|---|---|
| <50 | ≥50 | P-value | |
| PB chemotherapy | |||
| Patients, number | 29 | 24 | |
| Treatment compliance | 0.999 | ||
| 4 cycle (complete) | 27 (93.1%) | 23 (95.8%) | |
| ≤3 cycle | 2 | 1 | |
| Treatment discontinuation reason | |||
| Adverse effect | 2 | 0 | |
| Recurrence | 0 | 1 | |
| Oral-FT chemotherapy | |||
| Patients, number | 25 | 28 | |
| Treatment compliance | 0.010 | ||
| Completea | 9 (36.0%) | 20 (71.4%) | |
| Incomplete | 16 | 8 | |
| Treatment discontinuation reason | |||
| Adverse effect | 8 | 4 | |
| Recurrence | 8 | 4 | |
Notes:
Complete; UFT: 2 years, S-1: 1 year.
Abbreviations: PNI, prognostic nutritional index; UFT, tegafur–uracil; PB, platinum-based; FT, tegafur.
Prognostic analysis
The RFS of patients with a PNI <50 before adjuvant chemotherapy was significantly poorer than that of the patients with a PNI ≥50 (P=0.001; Figure 1). A univariate analysis showed that nodal metastasis and the PNI were predictors of the RFS. A multivariate analysis was then performed using the Cox proportional hazards model. Using this model, we demonstrated that nodal metastasis (P<0.001) and the PNI before chemotherapy (P=0.005) were independent predictors of the RFS. However, the PNI before surgery was not a predictor of the RFS (Table 4).
Figure 1.
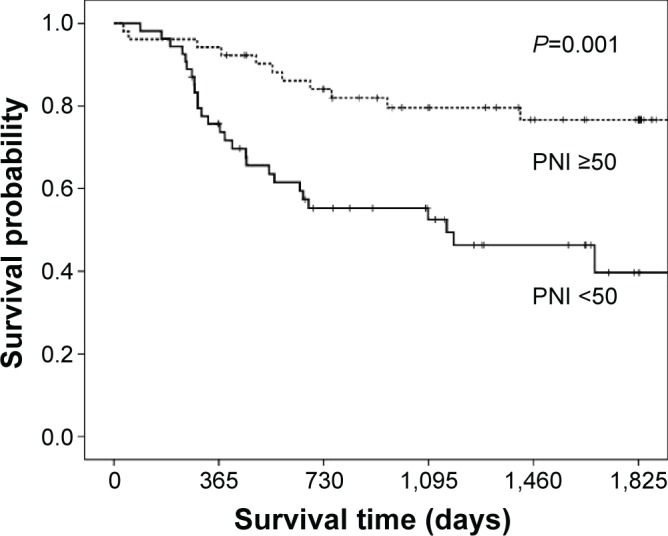
Kaplan–Meier recurrence-free survival curve according to the prognostic nutritional index before adjuvant chemotherapy: log-rank P=0.001 (number of patients; PNI <50=54, PNI ≥50=52).
Abbreviation: PNI, prognostic nutritional index.
Table 4.
Prognostic analysis of factors predicting recurrence-free survival in adjuvant chemotherapy: univariate and multivariate analysis in all cases
| Univariate
|
Multivariate
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Sex | ||||||
| Male/female | 0.89 | 0.46–1.73 | 0.731 | 1.06 | 0.50–2.24 | 0.887 |
| Age | ||||||
| >70/≤70 | 1.68 | 0.90–3.15 | 0.107 | 1.28 | 0.64–2.58 | 0.489 |
| Tumor size (mm) | ||||||
| >30/≤30 | 0.83 | 0.44–1.57 | 0.561 | 0.92 | 0.45–1.90 | 0.828 |
| Nodal metastasis | ||||||
| Positive/negative | 5.27 | 2.59–10.72 | <0.001 | 8.21 | 3.52–19.18 | <0.001 |
| Histological type | ||||||
| SQ/non-SQ | 0.57 | 0.22–1.45 | 0.235 | 0.40 | 0.15–1.06 | 0.064 |
| PNI before surgery | ||||||
| <50/≥50 | 1.19 | 0.63–2.26 | 0.590 | 1.96 | 0.87–4.44 | 0.107 |
| PNI before chemotherapy | ||||||
| <50/≥50 | 3.38 | 1.67–6.81 | 0.001 | 3.00 | 1.40–6.41 | 0.005 |
Notes: P-values in bold indicate significance (P<0.05).
Abbreviations: SQ, squamous cell carcinoma; PNI, prognostic nutritional index; HR, hazard ratio; CI, confidence interval.
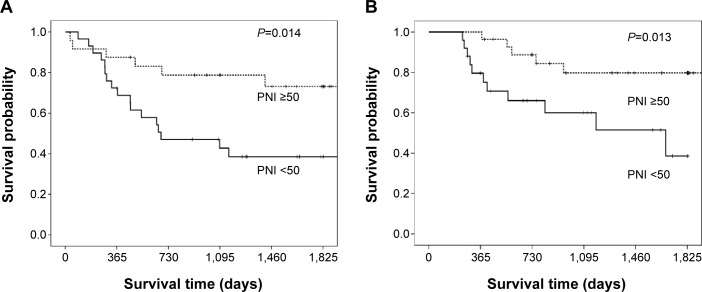
The RFS of patients with a PNI <50 before adjuvant chemotherapy was significantly poorer than that of the patients with a PNI ≥50 in both group according to chemotherapy regimen. (PB: P=0.014 and OT: P=0.013; Figure 2). A univariate analysis showed that nodal metastasis and the PNI before adjuvant chemotherapy were predictors of the RFS in the PB and the OT group. In the OT group, a multivariate analysis showed that nodal metastasis (P=0.024) and the PNI before chemotherapy (P=0.019) were independent predictors of the RFS. However in the PB group, only nodal metastasis (P=0.001) was an independent predictor of the RFS, and the PNI before chemotherapy (P=0.095) was not independent predictor (Table 5).
Figure 2.
Kaplan–Meier recurrence-free survival curve according to the prognostic nutritional index before adjuvant chemotherapy.
Notes: (A) PB chemotherapy, and (B) oral-FT chemotherapy.
Abbreviations: PNI, prognostic nutritional index; PB, platinum-based; FT, tegafur.
Table 5.
Prognostic analysis of factors predicting recurrence-free survival in adjuvant chemotherapy: multivariate analysis according to chemotherapy regimens
| PB chemotherapy
|
Oral-FT chemotherapy
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Nodal metastasis | ||||||
| Positive/negative | 35.6 | 4.00–316.4 | 0.001 | 4.93 | 1.23–19.74 | 0.024 |
| PNI before chemotherapy | ||||||
| <50/≥50 | 2.58 | 0.85–7.85 | 0.095 | 5.45 | 1.32–22.39 | 0.019 |
Notes: P-values in bold indicate significance (P<0.05).
Abbreviations: PNI, prognostic nutritional index; PB, platinum-based; HR, hazard ratio; CI, confidence interval; FT, tegafur.
Discussion
To our knowledge, this is one of the first studies to evaluate the utility of the PNI for predicting the outcomes of patients with NSCLC. In addition, this is the first study to show a correlation between the PNI and adjuvant chemotherapy among patients with resected NSCLC. Our study demonstrated that a low PNI before chemotherapy (<50 versus ≥50, P=0.005) was significantly associated with poorer chemotherapy compliance with the OT regimen and a poor RFS in a multivariate analysis.
Assessment and support of the nutritional status should be considered a valuable component of the overall oncological strategy.16 At first, the PNI was reported to predict the risk of operative morbidity and mortality after gastrointestinal surgery.17 However, their method for calculating the PNI was too difficult to use routinely. In contrast, the simplified PNI reported by Onodera et al9 was based on only two laboratory parameters, the albumin level and the lymphocyte count, which can be easily measured and are routinely used in clinical practice.9 This PNI was initially designed to assess the nutritional and immunological status of patients undergoing gastrointestinal surgery. Recently, several investigators have reported that the PNI is important for the survival of patients with various cancers, including NSCLC.18 In this study, we demonstrated that the PNI before chemotherapy was an independent predictor of the RFS as well as nodal metastasis, but the PNI before surgery was not a predictor.
The selection of chemotherapy regimens influences the prognosis. In addition, the nutritional status plays important roles in the effectiveness of chemotherapy. Ross et al19 reported the relationships between the nutrition status and weight loss, toxicity, delivery of chemotherapy, response to treatment, and prognosis in patients with lung cancer and mesothelioma. Therefore, nutrition is thought to affect the progression of disease in cancer patients. In Japan, an OT agent (UFT and S-1) is often selected for adjuvant chemotherapy in patients with various cancers, including gastric, lung, and pancreatic cancer. In lung cancer, adjuvant chemotherapy with UFT is the standard treatment for stage I patients.5,6 The rate of compliance for UFT was 74% at 1 year and 61% at 2 years. The main reasons for the discontinuation of UFT were an adverse reaction, the patient’s decision, and the doctor’s judgment.5 On the other hand, adjuvant chemotherapy with S-1 is a standard treatment for stage II or III patients with gastric cancer.20 In this study, the continuity rate of S-1 chemotherapy for 1 year was 65.8%. Aoyama et al21 reported that body weight loss after surgery is an independent risk factor for the continuation of S-1 adjuvant chemotherapy for gastric cancer. This result suggests that an early nutritional intervention might be important for gastric cancer patients undergoing S-1 adjuvant chemotherapy. Recently, in lung cancer, adjuvant chemotherapy with S-1 has been investigated in several clinical trials. In the WJOG4107L trial, the continuity rate of S-1 chemotherapy for 1 year was 52.6%.22 However, the factors that influence the continuity of S-1 chemotherapy were still unclear in NSCLC. On the other hand, adjuvant cisplatin plus vinorelbine chemotherapy is recognized as a standard regimen for patients with completely resected stage II and III NSCLC worldwide. In Japan, Sonobe et al23 reported that 83% of patients completed 3 or 4 cycles of adjuvant cisplatin plus vinorelbine chemotherapy. In this study, we mainly used the carboplatin regimen for the PB group. Chang et al24 reported that 84.1% of patients receiving carboplatin plus paclitaxel and 86.1% of patients receiving cisplatin plus vinorelbine completed 3–4 cycles of adjuvant chemotherapy. For advanced NSCLC treated with cisplatin plus paclitaxel, patients who were moderately or severely malnourished and had hypoalbuminemia developed more chemotherapy-induced toxicities compared with patients without malnutrition and normal albumin.25 However, the factors that influence the continuity of PB chemotherapy remain unclear. Our present study demonstrated that PNI before chemotherapy influenced the compliance with chemotherapy in the OT group, but not the PB group. We think that the reason for this difference might be related to the treatment period. While the treatment period for PB is about 3 months, the duration of the OT treatment is about 1–2 years. The PNI may affect the continuation of long-term treatment periods, such as that for OT.
We emphasized the significance of PNI before adjuvant chemotherapy, but not before surgery. PNI before adjuvant chemotherapy, as well as weight loss after surgery, was correlated with a decline in postoperative quality of life and is the most reliable indicator of malnutrition.21 Consequently, the need for prechemotherapy nutritional intervention in patients receiving adjuvant chemotherapy in NSCLC should be emphasized, especially in patients receiving OT chemotherapy.
This study has several limitations that should be considered when interpreting the results. The retrospective study design was a major limitation of this study. Minor limitations included insufficient evidences of the validity of the cut-off values for the PNI. Regarding the PNI, a fixed cut-off value has not yet been established, and various values have been used in previous reports.18
Conclusion
The PNI before adjuvant chemotherapy influenced the treatment compliance with planned chemotherapy in the OT group, but not the PB group. In addition, a low PNI before adjuvant chemotherapy was significantly associated with a poor RFS in a multivariate analysis, especially in the OT group.
Acknowledgments
The authors thank IMIC (http://www.imic.or.jp/) for English language review.
Footnotes
Authors’ contributions
Study concept and design: KS and MN. Data acquisition: SS, TY, AM, and YN. Data analysis and interpretation: KS and RO. Manuscript preparation: KS. Manuscript review: MN. All the authors have read and approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interests in this work.
References
- 1.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 2.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(10):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 4.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350(17):1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 6.Hamada C, Tanaka F, Ohta M, et al. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol. 2005;23(22):4999–5006. doi: 10.1200/JCO.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981;304(1):10–15. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- 8.Colleoni M, Price K, Castiglione-Gertsch M, et al. Dose-response effect of adjuvant cyclophosphamide, methotrexate, 5-fluorouracil (CMF) in node-positive breast cancer. International Breast Cancer Study Group. Eur J Cancer. 1998;34(11):1693–1700. doi: 10.1016/s0959-8049(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 9.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. in Japanese with English abstract. [PubMed] [Google Scholar]
- 10.Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396–400. doi: 10.1053/ejso.2002.1257. [DOI] [PubMed] [Google Scholar]
- 11.Migita K, Takayama T, Saeki K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20(8):2647–2654. doi: 10.1245/s10434-013-2926-5. [DOI] [PubMed] [Google Scholar]
- 12.Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532–535. doi: 10.1007/s00595-011-0061-0. [DOI] [PubMed] [Google Scholar]
- 13.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98(2):268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 14.Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117–2123. doi: 10.1007/s00432-013-1523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Andreoli A, De Lorenzo A, Cadeddu F, Iacopino L, Grande M. New trends in nutritional status assessment of cancer patients. Eur Rev Med Pharmacol Sci. 2011;15(5):469–480. [PubMed] [Google Scholar]
- 17.Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375–2381. doi: 10.1002/1097-0142(19810515)47:10<2375::aid-cncr2820471009>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Qiu C, Qu X, Shen H, et al. Evaluation of prognostic nutritional index in patients undergoing radical surgery with nonsmall cell lung cancer. Nutr Cancer. 2015;67(5):741–747. doi: 10.1080/01635581.2015.1032430. [DOI] [PubMed] [Google Scholar]
- 19.Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90(10):1905–1911. doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 21.Aoyama T, Yoshikawa T, Shirai J, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20(6):2000–2006. doi: 10.1245/s10434-012-2776-6. [DOI] [PubMed] [Google Scholar]
- 22.Iwamoto Y, Mitsudomi T, Sakai K, et al. Randomized phase II study of adjuvant chemotherapy with long-term S-1 versus cisplatin+S-1 in completely resected stage II–IIIA non-small cell lung cancer. Clin Cancer Res. 2015 Aug 7; doi: 10.1158/1078-0432.CCR-14-3160. Epub. [DOI] [PubMed] [Google Scholar]
- 23.Sonobe M, Okubo K, Teramukai S, et al. Phase II study of adju-vant vinorelbine and cisplatin in Japanese patients with completely resected stage II and III non-small cell lung cancer. Cancer Chemother Pharmacol. 2014;74(6):1199–1206. doi: 10.1007/s00280-014-2595-5. [DOI] [PubMed] [Google Scholar]
- 24.Chang WJ, Sun JM, Lee JY, Ahn JS, Ahn MJ, Park K. A retrospective comparison of adjuvant chemotherapeutic regimens for non-small cell lung cancer (NSCLC): paclitaxel plus carboplatin versus vinorelbine plus cisplatin. Lung Cancer. 2014;84(1):51–55. doi: 10.1016/j.lungcan.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Arrieta O, Michel Ortega RM, Villanueva-Rodríguez G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxelcisplatin chemotherapy: a prospective study. BMC Cancer. 2010;10:50. doi: 10.1186/1471-2407-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]