Abstract
Background
In 2012, CDC launched the first federally funded national mass media antismoking campaign. The Tips From Former Smokers (Tips) campaign resulted in a 12% relative increase in population-level quit attempts.
Purpose
Cost-effectiveness analysis was conducted in 2013 to evaluate Tips from a funding agency’s perspective.
Methods
Estimates of sustained cessations; premature deaths averted; undiscounted life years (LYs) saved; and quality-adjusted life years (QALYs) gained by Tips were estimated.
Results
Tips saved about 179,099 QALYs and prevented 17,109 premature deaths in the U.S. With the campaign cost of roughly $48 million, Tips spent approximately $480 per quitter, $2,819 per premature death averted, $393 per LY saved, and $268 per QALY gained.
Conclusions
Tips was not only successful at reducing smoking-attributable morbidity and mortality but also was a highly cost-effective mass media intervention.
Introduction
Despite declines in cigarette smoking prevalence during the past 50 years, tobacco use remains the single most preventable cause of death and disease in the U.S.1,2 Mass media campaigns can effectively reduce cigarette use by reducing smoking initiation among youth and promoting cessation among adults, particularly when combined with other evidence-based tobacco prevention and control interventions.3–8 However, with recent declines in public funding for state and local tobacco control programs,9 a critical question is whether the economic investments required for these mass media campaigns can be justified by the public health benefits.
A few studies3,8,10–16 have estimated the cost effectiveness of state and local campaigns and found them to be cost effective. National campaigns can be more economically efficient than local campaigns because of economics of scale.17 A few studies18,19 have evaluated the cost effectiveness of U.S. national mass media campaigns and also found them to be cost effective.
Tips From Former Smokers (Tips), the first federally funded national mass media antismoking campaign, launched by CDC, provides a unique opportunity to assess the cost effectiveness of a nationwide public health intervention that meets the ad exposure recommendation in CDC’s 2014 Best Practices for Comprehensive Tobacco Control Programs.20 Funded through the Prevention and Public Health Fund of the Patient Protection and Affordable Care Act (2010),21 Tips was on air from March 19 through June 10, 2012. Ads featured hardhitting, graphic, and emotional testimonials from former smokers living with the harsh consequences of smoking-related diseases. Tips ads were aimed at increasing public awareness of the immediate and longer-term health damage caused by smoking and exposure to secondhand smoke, encouraging smokers to quit, and motivating nonsmokers to communicate with family and friends about the dangers of smoking. Tips ads were delivered via TV, radio, print (magazines), outdoor (billboards and others), theater, and digital media. All ads provided free resources to help quit, including the national quitline number (1-800-QUIT-NOW) or a website (www.smokefree.gov).
The 2012 Tips campaign was effective in increasing population-level quit attempts. The campaign generated an estimated 1.64 million new quit attempts of ≥ 1 day among U.S. adult smokers and approximately 100,000 estimated sustained quits of ≥6 months.22 Although rough cost-effectiveness estimates were presented in the previous analysis,22 rigorous cost-effectiveness analyses had yet to be conducted. To address this issue, a cost-effectiveness analysis was conducted to evaluate the campaign from a funding agency’s perspective. Specifically, the cost was assessed based on the campaign-specific expenditure administered by CDC, the federal funder of the 2012 Tips. The cost effectiveness of Tips was evaluated by four measures: (1) the cost per successful quit; (2) the cost per premature death averted; (3) the cost per life year (LY) saved; and (4) the cost per quality-adjusted life year (QALY) gained.
Methods
Health Benefits
The distribution of quitters by gender was calculated over six age groups—18–24, 25–34, 35–44, 45–54, 55–64, and ≥65 years—from the 2012 GfK KnowledgePanel® survey data used to estimate the Tips campaign effect on quit attempts (shown in Appendix Table 1).22–25 Gains for former smokers who quit in the four age groups from 25 to 64 years were estimated using their respective midpoint ages (i.e., 30,40, 50, and 60 years) as the base age for benefit computations. Because survival rates for former smokers who quit before age 30 years are nearly identical to never smokers,24 survival rates for never smokers were used for former smokers who quit at age 18–24 years. Although smokers aged ≥ 65 years may still benefit from quitting,26 to be conservative, this analysis did not try to quantify benefits for this subpopulation of quitters. For each age and gender group among former smokers aged 25–64 years, LYs were calculated for former smokers and, counterfactually, for continuing smokers, using the appropriate base age as the starting year. For quits in the 18–24-year age group, LYs saved were calculated from age 30 years19. QALY gains were calculated from published estimates for current and former smokers.27 Both LYs saved and QALYs gained were then converted into a net present value using a 3% discount rate.19 Discounting used the respective base age for quitters aged 25–64 years; for former smokers aged 18–24 years, discounting was applied using a base age of 20 years. QALY gains were then calculated, by age and gender group, as total QALYs for former smokers less the total QALYs for a group of continuing smokers of the same size.
Premature deaths averted were calculated in a similar way. Working from the number of additional quits, the numbers of persons who survive to the projected former-smoker life expectancy for each age and gender group at time of quit were estimated for both former smokers and an equally sized group of continuing smokers.24,25 Taking the difference between the two groups yields the number of premature deaths averted. For age 25–64 years, this estimate includes all people alive at the respective base age. For quits among the 18–24-year age group, this estimate includes persons alive at age 30 years. Differences in the survival rates between current smokers and never smokers before age 30 years are negligible.28
Campaign Costs
Campaign costs were considered from CDC’s perspective. The CDC Office on Smoking and Health’s budget for the 2012 Tips was $54,214,962 in 2012 U.S. dollars. Of this, $6,282,153 was allocated to states for quitline support. Because similar financial support had been provided to state quitlines in the fiscal years before the campaign (e.g., $4,772,375 in fiscal year 2010), this fund was assumed to still be available if Tips were not in place, and thus was not considered part of campaign cost. However, the conclusions of this analysis remained unchanged when funding to state quitlines was taken into consideration. Consequently, the total attributable cost considered was $47,932,809, of which $6,723,386 was devoted to creative development and execution; $38,060,582 for media placement (i.e., ad buy); and $3,148,841 for evaluation. Evaluation costs are considered an essential program cost because the campaign would not be conducted in absence of rigorous evaluation research. However, the results of the analysis were not substantially different when evaluation costs were excluded. Cost per quit represents the total of the creative development, media placement, and evaluation costs, divided by the total number of additional quits.
Confidence Intervals for Premature Deaths Averted, Life Years Saved, and Quality-Adjusted Life Years Gained
To generate appropriate CIs for these cost-effectiveness measures, a Monte Carlo simulation was designed and implemented using TreeAge, version 2013. The simulation used beta distributions to generate random outcomes for each age–gender group (12 in all) simultaneously for both the as-observed campaign path and for the status-quo counterfactual of no campaign at two points for each group: (1) whether or not to attempt to quit and (2) whether or not to become a sustained quitter of ≥6 months.
In the simulation, the 6-month sustained quit rate was derived from the estimated 100,000 sustained quits reported in McAfee et al. (Figure 1).22,23 This estimate is derived by combining external estimates of long-term smoking relapse with primary survey-based estimates of post-campaign cigarette abstinence rates. Figure 2 illustrates a detailed decision tree that shows the application of the 6.1% successful quit rate to those who attempted to quit under each scenario (campaign versus no campaign).
Figure 1.
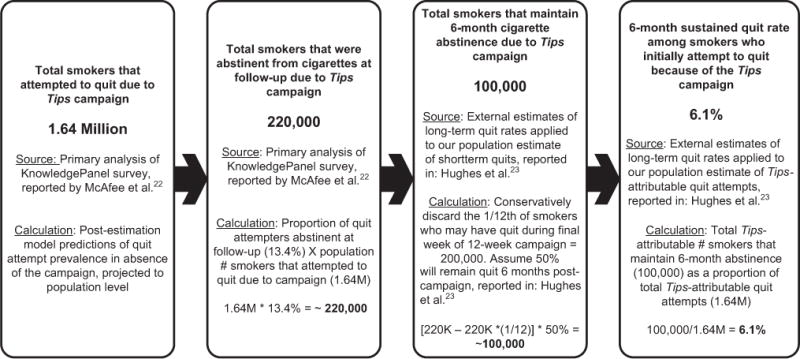
Long-term successful quits as a result of the Tips campaign.
Figure 2.
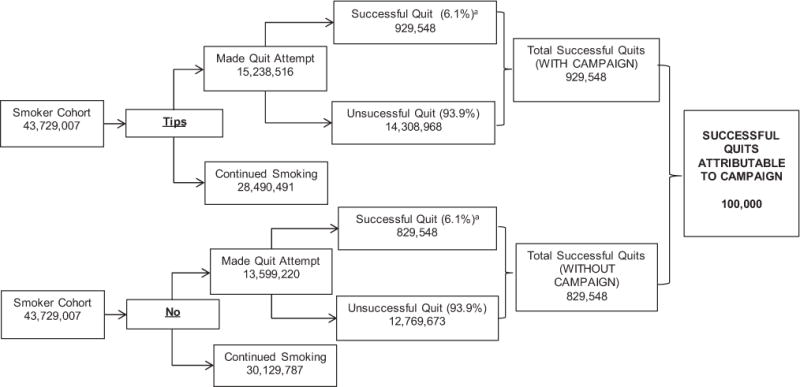
Decision tree for impact of the 2012 Tips campaign on successful quits.
aSee Figure 1 for derivation of estimated 6.1% successful quit rate as reported in McAfee et al.22
Parameters for the beta distributions were derived from the estimated size of the adult smoker cohort; its distribution by age and gender, estimated attempts to quit, and the distributions of quits are summarized in Appendix Table 2. Because the simulation was designed to vary the number of quit attempts and successful quits, benefits (including premature deaths averted, LYs saved, and QALYs gained) were input into the simulation as a per-quit value.
The primary analysis was based on a simulation with randomly selected per-quit benefits of premature death averted, LYs saved, and QALYs gained from uniform distributions based on the two recently published estimates on life expectancy described above as the extrema.24,25 Additionally, two independent analyses were conducted using the lower or upper bounds of the distributions for each age group, gender, and benefit category, respectively.24,25 The ranges of the distributions, as well as the lower and upper bound inputs of these per-quit benefits, are reported in Appendix Table 2. Each simulation was repeated 100,000 times to generate CIs. Cost-effectiveness measures were calculated at runtime of each simulation, using fixed values for total costs.
Sensitivity Analyses
Because the choice of the discount rate can have a significant impact on the net present values of LY saved and QALYs gained, sensitivity analyses were conducted based on 0%, 1%, and 5% discount rates, in addition to the 3% discount rates used in the main analysis.
As the campaign generated benefits by inducing additional quit attempts, determining how the composition of the quit attempter population affects the outcome variables of interest (i.e., quits, deaths averted, discounted QALYs, and discounted life years) is important. Therefore, these outcome variables were regressed on separate variables for quit attempts by each age group and gender and from both campaign and status quo scenarios (24 independent variables in total), using a log–log ordinary least squares specification. The results showed that the simulation output was most responsive to changes in quit attempts by women aged 18–24 and 25–34 years, and men aged 25–34 years. The full results are also presented in Appendix Table 3.
Finally, a sensitivity analysis was conducted, depicting the effect of reducing the Tips impact on successful quits by half to assess the robustness of the findings.22 These results are presented in Appendix Tables 4 and 5.
Results
Based on the 6.1% sustained quit rate derived from McAfee and colleagues,22 Table 1 presents the results and CIs estimated from three simulations on campaign-attributable sustained quitters, premature deaths averted, LYs saved, and QALYs gained among U.S. adult smokers. In the simulation with randomized benefits, stemming from 100,000 (95% CI=95,592–104,409) additional campaign-attributable sustained quitters (Figure 1), Tips contributed to approximately 17,109 (CI=15,412–18,806) additional premature deaths averted; 122,136 (CI=111,121–133,151) additional discounted LYs saved; and 179,099 (CI=165,436–192,762) additional discounted QALYs gained or 1.79 (CI=1.68–1.90) QALYs per induced quit.
Table 1.
Campaign-attributable Sustained Quits, Premature Deaths Averted, Life Years Saved, and Quality-adjusted Life Years Gained
| Outcome measures | Lower-bound simulation (95% CI) |
Randomized simulation (95% CI) |
Upper-bound simulation (95% CI) |
|---|---|---|---|
| Campaign-attributable quits | 100,000 (95,592–104,409) | 100,000 (95,592–104,409) | 100,000 (95,592–104,409) |
| Additional premature deaths averted | 13,638 (12,988–14,289) | 17,109 (15,412–18,806) | 22,099 (21,081–23,116) |
| Additional LYs saved | 98,676 (93,704–228,182) | 122,136 (111,121–133,151) | 145,578 (138,116–153,040) |
| Additional QALYs gained | 154,382 (147,015–161,750) | 179,099 (165,436–192,762) | 203,804 (193,993–213,614) |
| QALYs per induced quit | 1.54 (1.52–1.57) | 1.79 (1.68–1.90) | 2.04 (2.00–2.08) |
LY, life year; QALY, quality-adjusted life year.
In the simulation with lower-bound benefits, Tips contributed to approximately 13,638 (CI=12,988–14,289) additional premature deaths averted; 98,676 (CI=93,704–103,648) additional discounted LYs saved; and 154,382 (CI=147,015–161,750) additional discounted QALYs gained or 1.54 (CI=1.52–1.57) QALYs per induced quit. Alternatively, the simulation with upper-bound benefits suggested that around 22,099 (CI=21,081–23,116) additional premature deaths were averted; 145,578 (CI=138,116–153,040) additional discounted LYs were saved; and 203,804 (CI=193,993–213,614) additional discounted QALYs were gained or 2.04 (CI=2.00–2.08) QALYs per induced quit were obtained because of Tips.
The average campaign cost per additional sustained quitter was approximately $480 (CI=$460–$500) (Table 2). In the randomized simulation, the average campaign cost was estimated around $2,820 (CI= $2,550–$3,090) per additional premature death averted; $390 (CI=$360–$430) per attributable LY saved; and $270 (CI=$250–$290) per additional QALY gained. In the lower-bound simulation, these were estimated around $3,510 (CI=$3,340–$3,680) per additional premature death averted; $490 (CI=$460–$510) per attributable LY saved; and $310 (CI=$300–$330) per additional QALY gained. The upper-bound simulation estimated around $2,220 (CI=$2,120–$2,330) per additional premature death averted; $330 (CI=$310–$350) per attributable LY saved; and $240 (CI=$220–$250) per additional QALY gained.
Table 2.
Costs per Unit of Health Benefit Gained
| Outcome measures | Lower-bound simulation (95% CI) |
Randomized simulation (95% CI) |
Upper-bound simulation (95% CI) |
|---|---|---|---|
| Cost per quit | $480 ($460–$500) | $480 ($460–$500) | $480 ($460–$500) |
| Cost per premature death averted | $3,510 ($3,340–$3,680) | $2,820 ($2,550–$3,090) | $2,220 ($2,120–$2,330) |
| Cost per LY saved | $490 ($460–$510) | $390 ($360–$430) | $330 ($310–$350) |
| Cost per QALY gained | $310 ($300–$330) | $270 ($250–$290) | $240 ($220–$250) |
Note: The numbers shown here are rounded to the nearest $10.
LY, life year; QALY, quality-adjusted life year.
These cost-effectiveness measures were also obtained at median as well as at 2.5 and 97.5 percentiles of the distribution through the simulation to assess the robustness of the mean estimates. These estimates were very comparable to the main findings reported above.
In the randomized simulation (Table 3), the average cost per additional LY saved was $180 (CI=$160–$190) without discounting; $240 (CI=$220–$260) at 1% discounting; or $580 (CI=$500–$660) at 5% discounting. Based on the same simulation (Table 4), the average cost per additional QALY gained was $120 (CI=$110–$130) without any discount; $150 (CI=$140–$160) at 1% discounting; or $390 (CI=$360–$420) at 5% discounting. Similar estimates were also obtained based on the lower-and upper-bound simulations. Generally, the patterns of these estimates were very consistent with the findings from the randomized simulation, suggesting that the cost effectiveness of the Tips campaign might range from $100 to $460 per QALY gained.
Table 3.
Discounted Life Years Saved Estimated Under Alternative Discounting Scenarios
| Discount rate | Lower-bound simulation
|
Randomized simulation
|
Upper-bound simulation
|
|||
|---|---|---|---|---|---|---|
| Discounted LYs saved (95% CI) | Cost per discounted LY saved (95% CI) | Discounted LY saved (95% CI) | Cost per discounted LY saved (95% CI) | Discounted LY saved (95% CI) | Cost per discounted LY saved (95% CI) | |
| 0% | 240,117 (228,268–251,966) | $200 ($190–$210) | 273,867 (249,813–297,922) | $180 ($160–$ 190) | 305,046 (290,437–319,655) | $160 ($150–$160) |
|
| ||||||
| 1% | 179,084 (170,240–187,928) | $270 ($250–$280) | 202,730 (186,465–218,995) | $240 ($220–$260) | 226,312 (215,562–237,062) | $210 ($200–$220) |
|
| ||||||
| 3% | 98,676 (93,704–103,648) | $490 ($460–$510) | 122,136 (111,121–133,151) | $390 ($360–$430) | 145,578 (138,116–153,040) | $330 ($310–$350) |
|
| ||||||
| 5% | 58,603 (55,504–61,702) | $820 ($780–$860) | 83,633 (72,134–95,132) | $580 ($500–$660) | 108,665 (102,198–115,132) | $440 ($420–$470) |
Note: The costs shown here are rounded to the nearest $10.
LY, life year.
Table 4.
Discounted Quality-adjusted Life Years Gained Estimated Under Alternative Discounting Scenarios
| Discount rate | Lower-bound simulation
|
Randomized simulation
|
Upper-bound simulation
|
|||
|---|---|---|---|---|---|---|
| Discounted QALYs gained (95% CI) | Cost per discounted QALY gained (95% CI) | Discounted QALYs gained (95% CI) | Cost per discounted QALY gained (95% CI) | Discounted QALYs gained (95% CI) | Cost per discounted QALY gained (95% CI) | |
| 0% | 364,121 (346,381–381,862) | $130 ($130–$140) | 412,900 (383,043–442,756) | $120 ($110–$ 130) | 461,685 (439,740–483,629) | $100 ($100–$110) |
|
| ||||||
| 1% | 261,307 (248,624–273,989) | $180 ($170–$ 190) | 297,107 (276,192–318,023) | $150 ($140–$ 160) | 332,909 (317,058–348,761) | $140 ($140–$ 150) |
|
| ||||||
| 3% | 154,382 (147,015–161,750) | $310 ($300–$330) | 179,099 (165,436–192,762) | $270 ($250–$290) | 203,804 (193,993–213,614) | $240 ($220–$250) |
|
| ||||||
| 5% | 104,321 (99,414–109,229) | $460 ($440–$480) | 122,399 (112,524–132,274) | $390 ($360–$420) | 140,469 (133,596–147,342) | $340 ($320–$360) |
Note: The costs shown here are rounded to the nearest $10.
QALY, quality-adjusted life year.
Under the unlikely scenario assuming only half of the estimated additional sustained quits were Tips attributable, the average costs were estimated around $960 (CI=$920–$1,000) per additional quitter, $5,620 (CI=$5,060–$6,180) per additional premature death averted, $790 (CI=$720–$860) per attributable LY saved, and $540 (CI=$500–$580) per additional QALY gained in the randomized simulation (Appendix Tables 4 and 5).
Discussion
With total campaign expenditures of about $48 million, Tips spent approximately $480 per quitter, $2,820 per premature death averted, $390 per LY saved, and $270 per QALY gained. These results compare favorably with other smoking interventions, such as the EX and truth® campaigns.18,19 For example, when the EX campaign was analyzed from the program perspective using the same QALY per quitter ratio (1.79 per induced quit) as the Tips campaign, the cost per quitter is roughly $9,550 in 2012 dollars and the cost per QALY is $5,330.19 An analysis of the truth® campaign from the program perspective revealed a cost per QALY of around $1,420.18 These comparisons should be interpreted with caution owing to differences in campaign delivery (EX was delivered with less media intensity) and target audience (the truth® campaign focused on youth) as well as in analytic approach and study assumptions.
In the U.S., a commonly used but arbitrary threshold to consider an intervention to be cost effective from a societal perspective is $50,000 per LY or QALY gained.29 This study does not include information on other societal costs associated with Tips. Other campaign evaluations19 assessed that under a very generous scenario, any attempted quitters receiving help were assumed to utilize four counseling sessions for one quit attempt, a 12-week course of nicotine replacement therapy or prescriptions with brand-name medications, or both. In this case, for every dollar invested in a mass media campaign, an additional $7.30 could be spent for these evidence-based cessation treatments. This ratio would suggest that the absolute ceiling for total societal costs of Tips is $2,847 per LY saved or $1,971 per QALY gained, which is still 15 times less than the $50,000 per QALY benchmark. Alternatively, according to the WHO’s recommendation, an intervention is considered highly cost effective if it is less than the country’s per capita gross domestic product (GDP) per LY saved.30 With this threshold and U.S. per capita GDP in 2012 (approximately $51,700),31,32 Tips is also a highly cost-effective intervention.
This analysis has several limitations. First, the costs in this analysis are estimated from a funding agency’s perspective. Therefore, the additional costs that might be incurred by quitters who are aided in their quit attempts by physicians, counseling groups, or pharmacotherapy are not included. However, benefits, including averted medical costs or increased productivity of smokers who quit, are also not included. Second, the analysis does not consider possible switching behaviors between cigarettes and other combustible or non-combustible tobacco products among quitters. However, this is very rare (0.3% in the past 12 months) for smokers switching to smokeless tobacco.33 A third limitation is that this study is based on the estimated campaign-attributable successful quits that last ≥6 months. Although the original estimate of sustained quits due to the Tips campaign was conservative for several reasons,22 it is possible that the cost-effectiveness results will be overstated if additional relapses occurred after 6 months. However, the sensitivity analysis shows that Tips is cost effective, even if its effects were reduced by half.22 Finally, estimates of the population impact of the Tips campaign are based on an online sample of smokers from KnowledgePanel.22 Although this sample is based on probability sampling methods, it may differ from the U.S. smoking population as a whole. However, no substantial evidence of bias in the sample characteristics was found. For example, 77.7% of current smokers in the KnowledgePanel survey were daily smokers in 2012 compared to 78.4% of current smokers in the 2012 National Health Interview Survey. In addition, several recent studies in the survey methodology literature have shown that KnowledgePanel data are equally or more comparable to Census benchmarks as other types of probability-based samples including random-digit-dial telephone surveys.34
The estimates of program benefits are conservative in at least five ways. First, although the analysis assumes that the campaign raised the number of quit attempts, the possibility that the campaign also increased the success rates of quit attempts is not considered. In addition, estimates of the campaign-attributable increase in the number of quit attempts are based on two-tailed statistical tests, which allowed for the possibility that the campaign could have decreased the quit attempt rate. Third, the impact of the campaign on smoking initiation among adolescents and young adults is not considered and the impacts of reducing secondhand exposure related to quits are not considered. Fourth, to be conservative, the health benefits to campaign-attributable quitters aged ≥65 years are not included in the analysis. Finally, only immediate cessation effects are measured and do not capture any long-term impact of Tips on future post-campaign quit attempts.
Policymakers, public health officials, and clinicians face limited resources as they work to reduce the enormous human and financial toll of tobacco use. It is therefore important to identify interventions that have a large impact at a relatively small cost. These findings suggest that a national, federally funded mass media campaign can be highly cost effective to reduce the burden of tobacco use. These findings may also be useful to policymakers in other countries when they consider population-based mass media anti-tobacco campaigns similar to Tips.35
Supplementary Material
Acknowledgments
Special thanks to the following CDC staff members who contributed to the Tips From Former Smokers campaign: for campaign oversight, Diane Beistle, BA, and Jane Mitchko, MEd; for developing and executing the media campaign, Jeff Boal, BA, and Wendy Moniz, BA, and the PlowShare Group; for scientific, programmatic, and technical support, Rebecca Bunnell, ScD, MEd, Terry Pechacek, PhD, Robert Rodes, MS, MEd, Gabbi Promoff, MA, Karen Debrot, DrPH, and Renita Macaluso, BA; for mobilizing CDC support and providing scientific support, Ursula Bauer, PhD, MPH, and Thomas Frieden, MD, MPH; for overall support, CDC’s Office on Smoking and Health. Thanks also to Jennifer Duke, PhD, Paul Shafer, MA, and Anna MacMonegle, MA, of RTI International for data analysis and technical support.
Survey and part of the analysis was supported by CDC, USDHHS.
Appendix: Supplementary Data
Supplementary data associated with this article can be found at http://dx.doi.org/10.1016/j.amepre.2014.10.011.
Footnotes
This paper is dedicated to Terrie Hall of Lexington NC who passed away on September 16, 2013; Nathan Moose, of the Oglala Sioux tribe, who passed away on October 17, 2013; and to Bill Busse of Paw Paw, Michigan, who passed away on August 3, 2014. Terrie, Nathan, and Bill appeared in the Tips campaign and were instrumental in encouraging smokers to try to quit. Their willingness to show how smoking and secondhand smoke affected their lives in order to save others from such hardship revealed their courage, strength, and compassion. They were a source of inspiration to those who knew them and to those who viewed their ads.
None of the authors have conflicts of interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of CDC or RTI International.
References
- 1.USDHHS. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta GA: USDHHS; 2010. [PubMed] [Google Scholar]
- 2.CDC. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–8. [PubMed] [Google Scholar]
- 3.Durkin S, Brennan E, Wakefield M. Mass media campaigns to promote smoking cessation among adults: an integrative review. Tob Control. 2012;21(2):127–38. doi: 10.1136/tobaccocontrol-2011-050345. [DOI] [PubMed] [Google Scholar]
- 4.Zaza S, Briss PA, Harris KW, editors. Tobacco. Atlanta GA: Oxford University Press; 2005. Task Force on Community Preventive Services. [Google Scholar]
- 5.Hopkins DP, Briss PA, Ricard CJ, et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med. 2001;20(2S):16–66. doi: 10.1016/s0749-3797(00)00297-x. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins DP, Husten CG, Fielding JE, et al. Evidence reviews and recommendations on interventions to reduce tobacco use and exposure to environmental tobacco smoke: a summary of selected guidelines. Am J Prev Med. 2001;20(2S):67–87. doi: 10.1016/s0749-3797(00)00298-1. [DOI] [PubMed] [Google Scholar]
- 7.Davis RM, Gilpin EA, Loken B, et al. The role of the media in promoting and reducing tobacco use. NCI Tobacco Control Monograph No 19. 2008 [Report] cancercontrol.cancer.gov/tcrb/monographs/19/m19_complete.pdf.
- 8.Bala M, Strzeszynski L, Cahill K. Mass media interventions for smoking cessation in adults. Cochrane Database Syst Rev. 2008(1):CD004704. doi: 10.1002/14651858.CD004704.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Campaign for Tobacco Free Kids. A broken promise to our children: the 1998 tobacco settlement six years later. 2005 [Report] [Google Scholar]
- 10.National Cancer Institute. The role of the media in promoting and reducing tobacco use: tobacco control monograph no.19. Bethesda MD: USDHHS, NIH, National Cancer Institute; 2008. [Google Scholar]
- 11.Davis KC, Farrelly MC, Duke J, et al. Antismoking media campaign and smoking cessation outcomes, New York State, 2003–2009. Prev Chronic Dis. 2012;9:E40. [PMC free article] [PubMed] [Google Scholar]
- 12.Hyland A, Wakefield M, Higbee C, et al. Anti-tobacco television advertising and indicators of smoking cessation in adults: a cohort study. Health Educ Res. 2006;21(3):348–54. doi: 10.1093/her/cyl048. [DOI] [PubMed] [Google Scholar]
- 13.Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. Lancet. 2010;376(9748):1261–71. doi: 10.1016/S0140-6736(10)60809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biener L, McCallum-Keeler G, Nyman AL. Adults’ response to Massachusetts anti-tobacco television advertisements: impact of viewer and advertisement characteristics. Tob Control. 2000;9(4):401–7. doi: 10.1136/tc.9.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frieden TR, Bloomberg MR. How to prevent 100 million deaths from tobacco. Lancet. 2007;369(9574):1758–61. doi: 10.1016/S0140-6736(07)60782-X. [DOI] [PubMed] [Google Scholar]
- 16.Task Force on Community Preventive Health Services. The guide to community preventive services (tobacco topic) 2013 http://www.thecommunityguide.org/tobacco/index.html.
- 17.Evans P. Government action, social capital and development: reviewing the evidence on synergy. World Dev. 1996;24(6):1119–32. [Google Scholar]
- 18.Holtgrave DR, Wunderink KA, Vallone DM, Healton CG. Cost-utility analysis of the National truth campaign to prevent youth smoking. Am J Prev Med. 2009;36(5):385–8. doi: 10.1016/j.amepre.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Villanti AC, Curry LE, Richardson A, et al. Analysis of media campaign promoting smoking cessation suggests it was cost-effective in prompting quit attempts. 12. Vol. 31. Health Aff; Millwood: 2012. pp. 2708–16. [DOI] [PubMed] [Google Scholar]
- 20.CDC. Best practices for comprehensive tobacco control programs. Atlanta GA: DHHS; 2014. 2014. [Google Scholar]
- 21.Office of the Legislative Counsel. Patient Protection and Affordable Care Act (“PPACA”) Public Law. 2010:111–148. [Google Scholar]
- 22.McAfee T, Davis KC, Alexander RL, et al. Impact of the first federally funded U.S. anti-smoking national media campaign. Lancet. 2013;382(9909):2003–11. doi: 10.1016/S0140-6736(13)61686-4. [DOI] [PubMed] [Google Scholar]
- 23.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 24.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 25.Woloshin S, Schwartz LM, Welch HG. The risk of death by age, sex, and smoking status in the United States: putting health risks in context. J Natl Cancer Inst. 2008;100(12):845–53. doi: 10.1093/jnci/djn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sloan FA, Ostermann J, Picone G, et al. The price of smoking. Cambridge MA: MIT Press; 2004. [Google Scholar]
- 27.Murphy SL, Xu JQ, Kochanek KD. National vital statistics reports. 4. Vol. 61. Hyattsville MD: National Center for Health Statistics; 2013. Deaths: final data for 2010. [PubMed] [Google Scholar]
- 28.Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA. 1996;275(16):1247–51. [PubMed] [Google Scholar]
- 29.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–78. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Choosing interventions that are cost effective (WHO-CHOICE)—cost-effectiveness thresholds. 2012 www.who.int/choice/costs/CER_thresholds/en/index.html.
- 31.U.S. Census Bureau Population Division. Annual estimates of the population for the United States, regions, states, and Puerto Rico. April 1, 2010 to July 1, 2012(NST-EST2012-01). www.census.gov/popest/data/national/totals/2012/index.html.
- 32.U.S. Bureau of Economic Analysis. GDP and the National Income and Product Account (NIPA) historical tables. www.bea.gov/national/index.htm.
- 33.Zhu SH, Wang JB, Hartman A, et al. Quitting cigarettes completely or switching to smokeless tobacco: do US data replicate the Swedish results? Tob Control. 2009;18(2):82–7. doi: 10.1136/tc.2008.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeager DS, Krosnik JA, Chang L, et al. Comparing the accuracy of RDD telephone surveys and internet surveys conducted with probability and non-probability samples. Public Opin Q. 2011;75(4):709–47. [Google Scholar]
- 35.Xiao D, Chen Z, Wang C. Effects of a short-term mass-media campaign against smoking. Lancet. 2013;382(9909):1964–6. doi: 10.1016/S0140-6736(13)61839-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


