Abstract
Alemtuzumab, fludarabine, and melphalan reduced-intensity conditioning (RIC) regimens are increasingly used for the hematopoietic cell transplantation (HCT) of pediatric and young adult patients with nonmalignant diseases. Early experience suggests that these regimens are associated with good survival but a high incidence of mixed chimerism, which we have previously shown to be influenced by the alemtuzumab schedule. We hypothesized that the underlying diagnosis and donor graft source would also affect the development of mixed chimerism and that the majority of patients would survive RIC HCT without graft loss. To examine this, we conducted a retrospective study of 206 patients with metabolic diseases, non-Fanconi anemia marrow failure disorders, and primary immune deficiencies who underwent 210 consecutive RIC HCT procedures at Cincinnati Children’s Hospital. Ninety-seven percent of the patients engrafted. Mixed donor and recipient chimerism developed in 46% of patients. Patients with marrow failure had a low risk of mixed chimerism (hazard ratio [HR], .208; 95% confidence interval [CI], .061 to .709; P = .012). The risk of mixed chimerism was high in patients who received a cord blood graft (HR, 3.122; 95% CI, 1.236 to 7.888; P = .016). As expected, patients who received a proximal or higher dose per kilogram of alemtuzumab schedule also experienced higher rates of mixed chimerism (all HR > 2, all P < .05). At the time of last follow-up (median, 654 days; range, 13 to 3337), over 75% of patients had greater than 90% whole blood donor chimerism. A second transplantation was performed in 5% of patients. Three-year survival without retransplantation was 84% (95% CI, 71% to 98%) for patients who underwent transplantation with an HLA-matched sibling donor. Survival without retransplantation was negatively affected by lack of a matched related donor, increasing age, and development of grades III and IV acute graft-versus-host disease. We conclude that alemtuzumab, fludarabine, and melphalan RIC HCT offers good results for many patients and that the risk of developing mixed chimerism is influenced by underlying diagnosis, graft source, and alemtuzumab dosing.
Keywords: Primary immune deficiency, Bone marrow transplantation, Hematopoietic cell transplantation, Reduced-intensity conditioning, Alemtuzumab, Mixed chimerism
INTRODUCTION
Reduced-intensity conditioning (RIC) hematopoietic cell transplantation (HCT) regimens have been introduced over the last 2 decades to avoid the acute toxicities and complications of myeloablative regimens. Several RIC regimens are reported in the literature, but in particular, RIC HCT regimens consisting of alemtuzumab, fludarabine, and melphalan are now commonly employed for pediatric and young adult patients with primary immune deficiencies and metabolic diseases, and its use is increasing for patients with marrow failure disorders. In addition to limited acute toxicities, RIC regimens that contain alemtuzumab are generally associated with a low incidence of acute graft-versus-host disease (GVHD). However, RIC regimens are often complicated by the development of mixed donor and recipient chimerism. Complete donor chimerism is not necessary to correct most nonmalignant diseases, but the development of mixed chimerism is disconcerting, because it can be associated with eventual graft loss.
To our knowledge, there are no prospective randomized studies comparing RIC to myeloablative conditioning regimens in pediatric patients with nonmalignant diseases. RIC HCT outcomes have been reported by several authors but in mostly small patient series or with heterogeneous approaches, and there are conflicting reports regarding the clinical significance of mixed chimerism [1–9]. Recent series using RIC regimens have reported high rates of primary or secondary graft failure, in 16% to 22% of patients, and the loss of cord blood grafts has been reported to occur in as many as one third to two thirds of patients [7,10,11]. In contrast, our group and the Great Ormond Street group have previously reported low rates of graft failure and retransplantation despite high rates of mixed chimerism in patients with hemophagocytic lymphohistiocytosis (HLH) and other primary immune deficiencies [2,9]. We have previously reported that the risk of mixed chimerism is influenced by the timing and dose per kilogram body weight of alemtuzumab in patients with HLH [3]. We hypothesized that the risk of mixed chimerism after alemtuzumab, fludarabine, and melphalan RIC HCT in broader patient groups would also be influenced by patient diagnosis and by the type of stem cell graft, and that like patients with HLH, the majority of patients would survive RIC HCT without graft loss. To examine this, we conducted a retrospective study of 206 patients with metabolic diseases, non-Fanconi anemia marrow failure disorders, and diverse primary immune deficiencies who underwent 210 consecutive RIC HCT procedures at Cincinnati Children’s Hospital. We observed that alemtuzumab, fludarabine, and melphalan RIC HCT offers good results for many patients and that the risk of developing mixed chimerism is influenced by underlying diagnosis, graft source, and alemtuzumab dosing.
METHODS
Patients
Institutional review board permission was granted for this study. Data were abstracted from the divisional database and we also retrospectively reviewed the charts of 206 pediatric and young adult patients who underwent 210 allogeneic hematopoietic cell transplantations using alemtuzumab, fludarabine, and melphalan at our center for treatment of an underlying nonmalignant disorder between August 8, 2004 and May 22, 2013. Patient demographics are summarized in Table 1. Median patient age at the time of HCT was 4.1 years (range, .24 to 27.2 years). Patients were classified into 6 groups based on underlying diagnosis: HLH and X-linked lymphoproliferative disease (n = 91), combined immune deficiency and common variable immunodeficiency (n = 23), severe combined immune deficiency (SCID) (n = 24), non-Fanconi anemia marrow failure disorders (n = 25), metabolic disorders (n = 22), or other disorders (n = 25). The other disorders category included immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome (n = 5), congenital enteropathy (n = 4), autoimmune lymphoproliferative syndrome (n = 3), chronic granulomatous disease (n = 2), CD25 deficiency (n = 1), DOCK8 deficiency (n = 1), hypereosinophilic syndrome (n = 1), interferon gamma receptor 2 deficiency (n = 1), Wiskott-Aldrich syndrome (n = 1), X-linked immunodeficiency with magnesium defect, Epstein-Barr virus infection, and neoplasia (n = 1), ill-defined immune deficiency (n = 2), sickle cell disease (n = 1), osteopetrosis (n = 1), and epidermolysis bullosa (n = 1). Seventy-four of the patients with HLH and X-linked lymphoproliferative disease have been reported previously [2,3].
Table 1.
Patient and Transplantation Information
| Characteristic | Value |
|---|---|
| No. of patients | 206 |
| No. of transplantations | 210 |
| Age at HCT, yr | |
| Minimum | .24 |
| Median | 4.1 |
| Maximum | 27.2 |
| Diagnosis | |
| HLH, XLP | 91 (43%) |
| CID, CVID | 23 (11%) |
| SCID | 24 (11%) |
| Marrow failure disorder | 25 (12%) |
| Metabolic disorder | 22 (10%) |
| Other | 25 (12%) |
| HLA match | |
| Matched related donor | 38 (18%) |
| Matched unrelated donor | 115 (55%) |
| 1 allele mismatch | 50 (24%) |
| 2 allele mismatch | 7 (3%) |
| Graft source | |
| Bone marrow | 189 (90%) |
| Cord blood | 10 (5%) |
| Bone marrow + cord blood | 1 (0%) |
| PBSC | 10 (5%) |
| Graft TNC dose, × 108/kg | |
| Minimum | .25 |
| Median | 6.80 |
| Maximum | 23.38 |
| Graft CD34 dose, × 106/kg | |
| Minimum | .06 |
| Median | 5.3 |
| Maximum | 63.0 |
| Alemtuzumab category | |
| Intermediate | 63 (30%) |
| Distal, 2.5 mg/kg or greater | 40 (19%) |
| Distal, less than 2.5 mg/kg | 35 (17%) |
| Proximal, greater than 1 mg/kg | 23 (11%) |
| Proximal, 1 mg/kg or less | 35 (17%) |
| Other | 14 (7%) |
| GVHD prophylaxis | |
| Methylprednisolone, cyclosporine | 147 (70%) |
| Methylprednisolone with tacrolimus or sirolimus | 11 (5%) |
| Methylprednisolone, cyclosporine, methotrexate | 42 (20%) |
| Other | 10 (5%) |
XLP indicates X-linked lymphoproliferative disease; CID, combined immune deficiency; CVID, common variable immune deficiency; PBSC, peripheral blood stem cells; TNC, total nucleated cell.
Data presented are n (%) unless otherwise indicated.
Transplantation Procedures
Transplantation details are summarized in Table 1. Transplantation procedures were based on published protocols [3,5,9]. Patients received fludarabine 150 mg/m2 (5 mg/kg in patients 10 kg or less) divided over days –8 to –4 or –7 or –3 and melphalan 140 mg/m2 (4.7 mg/kg in patients 10 kg or less) on day –3 or day –2. The dose of fludarabine was decreased by 20% for 2 patients with renal insufficiency. Because of concern for melphalan toxicity, the dose of melphalan was decreased by 25% to 50% for 2 patients with renal insufficiency, by 50% for 9 patients with a known underlying diagnosis characterized by DNA repair defects, or by 50% for 1 patient aged 2 months. The schedule of alemtuzumab was modified over the course of this report. Patients received alemtuzumab beginning on day –23, –22, or –21 (distal), –14 (intermediate), or –12, –11, –9, or –8 (proximal). Alemtuzumab was given either as a dose-escalation schedule of 3 mg,10 mg,15 mg, 20 mg (3 mg, 10 mg, 10 mg, 10 mg in patients less than 10 kg) or as a total of 1 mg/kg divided over 5 days, with the first dose being no more than 3 mg. Alemtuzumab was given subcutaneously in 79% of patients, as we began routine subcutaneous administration in 2007 to limit side effects of intravenous administration. For the purpose of analyses, patients were classified based on receipt of proximal, intermediate, or distal alemtuzumab schedules, with the proximal and distal categories further subdivided by receipt of above or below the median dose of alemtuzumab per kilogram into the following categories: proximal greater than 1 mg/kg, proximal 1 mg/kg or less, intermediate, distal 2.5 mg/kg or greater, and distal less than 2.5 mg/kg. Fourteen patients were classified as having received other alemtuzumab schedules because of acute illness, kidney injury, alemtuzumab reaction, active HLH, or another issue that prompted adjustment or temporary halt of alemtuzumab and/or the preparative regimen.
Donor and patient HLA match was determined using clinically available high-resolution molecular typing for HLA-A, -B, -C, and -DRB1. For the purpose of analyses, donor and patient HLA match were categorized as matched related donor (n = 38), matched unrelated donor (n = 115), donor with 1 allele mismatch (n = 50), and donor with 2 allele mismatch (n = 7). For GVHD analyses, 1 and 2 allele mismatches were grouped together. Most patients received bone marrow grafts (n = 189), whereas a minority received cord blood (n = 10) or peripheral blood stem cell grafts (n = 10). Peripheral blood stem cell grafts were selected on the basis of donor preference or inability to give marrow or large recipient size. One patient received a combined cord blood and bone marrow graft (from the same sibling donor) but was classified as having received bone marrow for all analyses. For patients who received a subsequent hematopoietic stem cell product “boost,” the product underwent CD34+ cell selection using the Miltenyi CliniMacs system (Miltenyi Biotec, Bergisch Gladbach, Germany) in all but 3 cases. T cell–depleted stem cell boosts were given with a maximum T cell dose limit of 5 × 104/kg. For patients who received donor lymphocyte infusions (DLI), an infusion was given from product stored from the original donation or obtained from a subsequent collection from the original donor, based on the CD3+ cell dose. GVHD prophylaxis consisted of methylprednisolone and cyclosporine (n = 147); methylprednisolone with tacrolimus or sirolimus (n = 11); methylprednisolone, cyclosporine, and methotrexate (n = 42); or other (n = 10).
All patients received granulocyte colony–stimulating factor, antimicrobial prophylaxis, intravenous immunoglobulin replacement, and nutritional and other supportive care per standard clinical practice. Engraftment studies were performed using XY fluorescence in situ hybridization in the case of opposite sex donors and short tandem repeat analysis in the case of same sex donors in the clinical genetics laboratory at Cincinnati Children’s Hospital. For analysis of lymphocyte subset and myeloid compartment chimerism, cell types of interest were sorted using the Miltenyi autoMACS Pro system (Miltenyi Biotec, Bergisch Gladbach, Germany) following a clinically adapted protocol based on the manufacturer’s instructions in the Diagnostic Immunology Lab at Cincinnati Children’s Hospital. Acute GVHD was graded based on consensus criteria [12]. Chronic GVHD was categorized as limited or extensive [13].
Definitions
Neutrophil engraftment was defined as the first of 3 consecutive days that the absolute neutrophil count was above 500 cells/μL. Platelet engraftment was defined as the first of 7 consecutive days with a platelet count > 20,000/μL without platelet transfusion support. Whole blood donor chimerism of 95% or greater was considered to be complete donor chimerism. Mixed chimerism was defined as whole blood donor chimerism of less than 95% on more than 2 consecutive occasions. Secondary graft failure was defined as a decline of donor chimerism to 0% after achieving neutrophil recovery with detection of donor chimerism in whole blood samples.
Statistical Analyses
Gray’s method was used to create cumulative incidence curves to evaluate the impact of several covariates on the development of mixed chimerism and acute GVHD before 180 or 365 days after HCT using the cmprsk package in R (version 3.0.1, R Foundation for Statistical Computing, Vienna, Austria). For the analysis of the cumulative incidence of acute GVHD in patients with and without an initial development of mixed chimerism, we separately analyzed the time to acute GVHD before or including any intervention for mixed chimerism. Multivariate cause-specific hazard models were created using Cox proportional hazards regression using the Survival package in R to evaluate the impact of covariates on the development of mixed chimerism and acute GVHD, where death was treated as a censoring event. Competing risk regression was also performed, where death was treated as a competing event. Covariates included in the initial models included age, diagnosis group, HLA match, alemtuzumab category, and graft source. GVHD prophylaxis and the occurrence of mixed chimerism were also included in the GVHD models. For the GVHD analyses, we analyzed the incidences of grades II to IV and III and IV acute GVHD after the initial infusion of the graft alone and also after interventions for mixed chimerism. Final models were selected using stepwise selection with forward and backward selection using the stepAIC function of the MASS package in R.
Kaplan-Meier curves were generated to study overall and event-free survival using XLStat (Addinsoft, Paris, France). Groups were compared using the log-rank test. An event was defined as death or retransplantation. Multivariate models were created using Cox proportional hazards regression using the Survival package in R to evaluate the impact of several covariates on survival. Covariates included in the initial models included age, diagnosis category, HLA match and relation, total nucleated cell dose, alemtuzumab category, occurrence of mixed chimerism, and occurrence of grades 3 and 4 acute GVHD. The final model was selected using stepwise selection with forward and backward selection. Statistical significance was considered for P < .05.
RESULTS
Initial Engraftment
Two hundred four of the 210 patients (97%) achieved neutrophil recovery at a median of 11 days after HCT (range, 2 to 48 days). Six patients (3%) died before achieving neutrophil recovery, at a median of 10 days after HCT (range, 7 to 15 days). Twenty-one patients failed to achieve platelet engraftment. Five patients did not have decreases in platelet counts to below 20 × 103/μL. Of the remaining patients, platelet recovery was achieved at a median of 20 days after HCT (range, 6 to 224 days).
Development of Mixed Chimerism
One patient with neutrophil recovery died before an engraftment study was performed. Of 203 evaluable patients, initial engraftment studies were performed at a median of 13 days after HCT (range, 4 to 134 days) and revealed 95% to 100% whole blood donor chimerism in 187 patients (92%). Over time, a total of 94 of the 203 evaluable patients (46%) developed whole blood mixed donor and recipient chimerism, with a median day of onset of 40 days after HCT (range, 10 to 914 days). Ninety-seven percent of patients who developed mixed chimerism did so before 6 months after HCT (Figure 1A).
Figure 1.
(A) Shows cumulative incidence of mixed chimerism in 203 evaluable patients during the first 6 months after HCT. (B) Violin plots showing the distribution of whole blood donor chimerism in all 203 evaluable patients at initial chimerism testing (median, 13 days; range, 4 to 134 days), time of lowest donor chimerism (median among patients with mixed chimerism, 149.5 days; range, 19 to 1804 days), and time of last follow-up (median, 654 days; range, 13 to 3337 days). (C) Violin plots showing the distribution of whole blood donor chimerism just among the 94 patients who developed mixed chimerism at initial chimerism testing (median, 14 days; range, 9 to 35 days), time of lowest donor chimerism (median, 149.5 days; range, 19 to 1804 days), and time of last follow-up (median, 863.5 days; range, 30 to 2968 days). (D) Stacked bar plots of the percentage of patients with >95%, 50% to 94%, 25% to 49%, 5% to 24%, and <5% donor chimerism over time. Of note, patients who experienced loss of graft (0%) before 3 months after HCT are only included in the 1-, 2-, and 3-month time points.
Among patients with mixed chimerism, whole blood donor chimerism declined to an overall median minimum of 30% (range, 0 to 93%) at a median time of 149.5 days after HCT (range, 19 to 1804) (Figure 1B,C). After interventions for mixed chimerism as described below, mixed chimerism resolved in 39 of the 94 patients (41%) (Figure 1B,C). At the time of last follow-up (median, 654 days; range, 13 to 3337), over 75% of patients had greater than 90% whole blood donor chimerism (Figure 1B). Considering all patients at any time during the first 2 years after HCT, approximately 70% of patients had greater than 95% donor chimerism, 15% had 50% to 95% donor chimerism, 5% had 25% to 50%, 5% had 5% to 25%, and 5% had less than 5% donor chimerism (Figure 1D).
Factors Influencing the Development of Whole Blood Mixed Chimerism
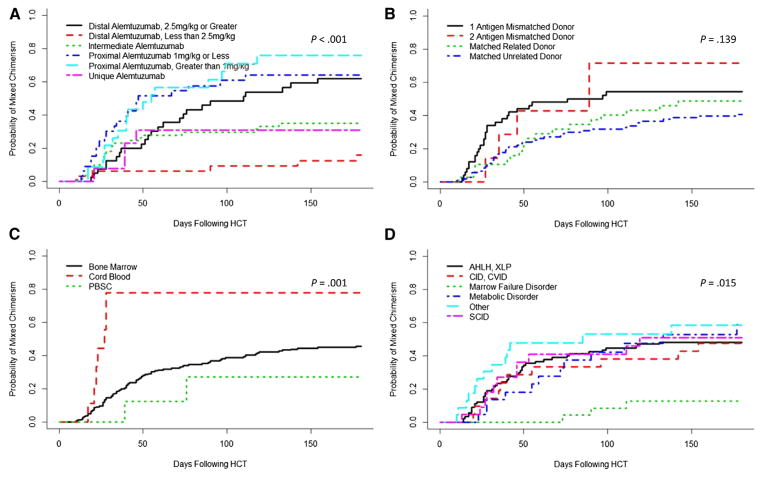
As previously reported, the cumulative incidence of mixed chimerism was highest in patients who received a proximal schedule of alemtuzumab or distal dosing of 2.5 mg/kg or greater (59% to 70%), versus those who had an intermediate schedule or distal dosing less than 2.5 mg/kg schedule (13% to 35%) (P < .001, Figure 2A). There was a suggestion of increased cumulative incidence of mixed chimerism in patients who received grafts from mismatched donors (P = .139, Figure 2B). Mixed chimerism was most common in patients who received cord blood grafts, 78% (P = .001, Figure 2C). Notably, the cumulative incidence of mixed chimerism was similar between all patient diagnosis groups except for marrow failure patients, who experienced a low cumulative incidence of 15% compared with that of the other diagnosis group rates of 43% to 58% (P = .015, Figure 2D). Multivariate analyses confirmed the increased risk of developing mixed chimerism in patients who receive a proximal alemtuzumab or distal alemtuzumab 2.5 mg/kg or greater schedule, patients who receive a cord blood graft, and patients with a diagnosis other than marrow failure (Table 2, Supplemental Table 1).
Figure 2.
Cumulative incidences of mixed chimerism among patients with (A) differing alemtuzumab schedules, (B) HLA match, (C) stem cell source, and (D) underlying diagnosis.
Table 2.
Cause-Specific Hazard Regression Model of the Impact of Factors Influencing the Development of Mixed Chimerism after HCT (Final Model)
| Risk Factor | HR (95% CI) | P Value |
|---|---|---|
| Diagnosis | ||
| HLH, XLP | 1.0 | |
| CID, CVID | .783 (.388–1.578) | .493 |
| Marrow failure | .208 (.061–.709) | .012 |
| Metabolic disorder | .509 (.198–1.304) | .159 |
| SCID | .887 (.444–1.771) | .733 |
| Other | 1.481 (.784–2.779) | .226 |
| Stem cell source | ||
| Bone marrow | 1.0 | |
| Cord blood | 3.122 (1.236–7.888) | .016 |
| PBSC | .478 (.115–1.988) | .310 |
| Alemtuzumab category | ||
| Intermediate | 1.0 | |
| Distal, 2.5 mg/kg or greater | 2.441 (1.174–5.079) | .017 |
| Distal, less than 2.5 mg/kg | .486 (.181–1.306) | .152 |
| Proximal, greater than 1 mg/kg | 2.589 (1.349–4.967) | .004 |
| Proximal, 1 mg/kg or less | 2.322 (1.259–4.281) | .007 |
| Other | .951 (.319–2.837) | .929 |
Age, diagnosis category, HLA match, graft source, and alemtuzumab category were included in the model selection. Death was treated as a censoring event.
Lineage-Specific Chimerism in Patients with Mixed Whole Blood Chimerism
Mixed chimerism was evident in the myeloid compartment in all patients for whom myeloid chimerism was evaluated (n = 25). Mixed chimerism was evident within the NK cell and B cell compartments in over 90% of evaluated patients (n = 41 and n = 25, respectively). The majority of patients with a diagnosis other than SCID were found to have mixed chimerism within the T cell compartment (31 of 34), whereas 6 of 7 evaluated SCID patients maintained greater than 90% donor-derived T cells.
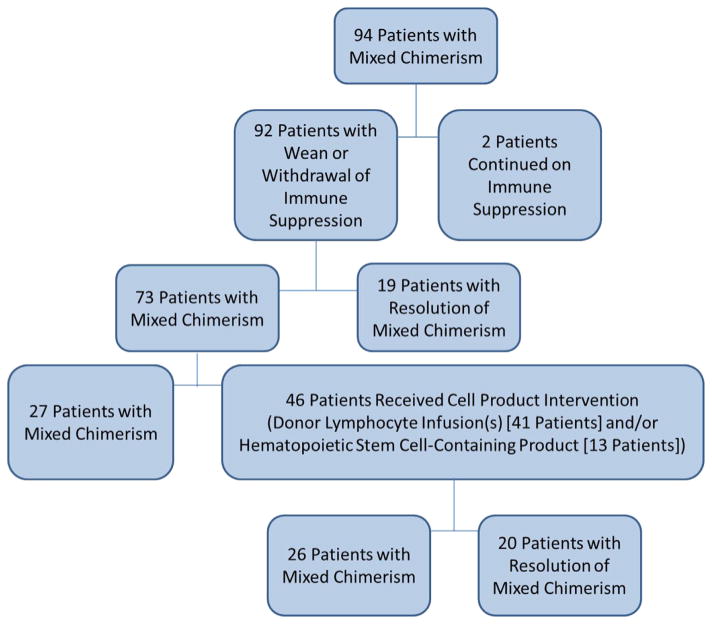
Interventions for Mixed Chimerism and Post-Intervention Acute GVHD
Interventions for mixed chimerism were directed at the discretion of the managing physician and are summarized in Figure 3. Immune suppression was weaned or withdrawn in most of the 94 cases of mixed chimerism, but 2 patients with a diagnosis of marrow failure were continued on cyclosporine. Nineteen patients (20%) experienced resolution of mixed chimerism with reduction or withdrawal of immune suppression alone. Ten patients developed acute GVHD grades I to IV after reduction of immune suppression (without later cell product interventions), and 9 of those 10 experienced resolution of mixed chimerism.
Figure 3.
Summary flow chart of interventions for mixed chimerism.
Almost one half of the patients (46 out of 94) with mixed chimerism were treated with 1 or more cell products as intervention for mixed chimerism. Mixed chimerism resolved in 20 of the 46 patients (43%). A hematopoietic stem cell–containing product was given to 13 patients at a median of 132 days after HCT (range, 36 to 815). One or more DLI were given to 41 patients at a median of 97 days after HCT (range, 36 to 955). The median T cell dose of the first or only DLI was 1 × 106 CD3+/kg (range, .02 to 22.8), and the median total cumulative T cell dose was 11 × 106 CD3+/kg (range, .1 to 1032). Further details of DLIs are shown in Supplemental Table 2. Twenty patients developed acute GVHD after DLI or non–T cell–depleted stem cell–containing product administration, and fifteen of those patients (75%) experienced resolution of mixed chimerism, versus 24% of patients who did not develop acute GVHD. We did not observe any correlation between cumulative DLI dose and the incidence or grade of GVHD (Supplemental Table 3), with the exception that no grade II to IV GVHD occurred in patients who received less than 1 × 106/kg CD3/kg.
Acute GVHD
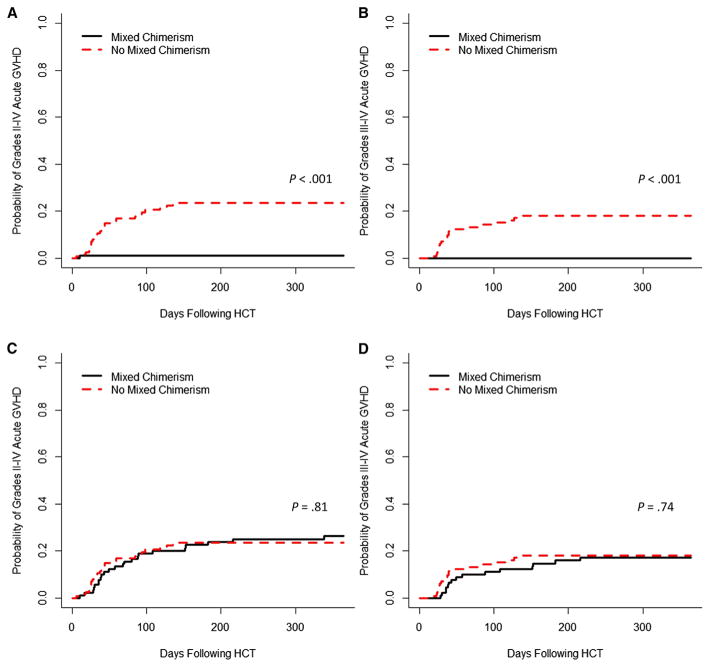
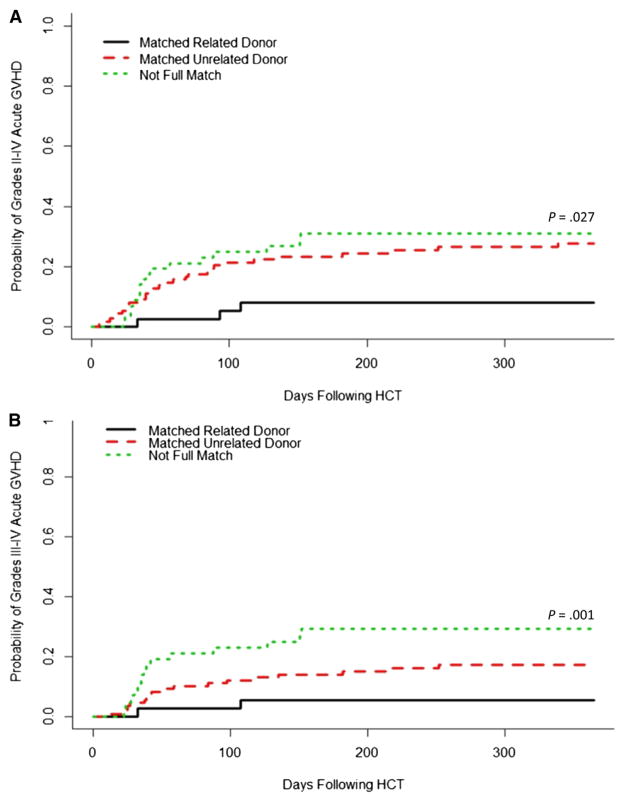
The development of mixed chimerism was associated with a lack of development of acute GVHD after the initial graft infusion. The cumulative incidences of grades II to IV and III and IV acute GVHD among patients with mixed chimerism were 1% and 0% before any interventions for mixed chimerism, respectively, compared with 23% and 18% among patients who did not develop mixed chimerism (P < .001 for both comparisons) (Figure 4A,B). However, after interventions for mixed chimerism as described above, the cumulative incidences of grades II to IV and III and IV acute GVHD increased to 25% and 17% in the patients with mixed chimerism, respectively, essentially equivalent to the rates observed in the patients who did not develop mixed chimerism (P =.81 and P =.74) (Figure 4C,D). The cumulative incidences of overall acute grades II to IV GVHD and III and IV GHVD among all patients were 25% (95% confidence interval [CI], 20% to 32%) and 18% (95 CI%, 14% to 25%), respectively. Though mixed chimerism protected against the development of acute GVHD after the initial graft infusion, the only covariate that significantly influenced the cumulative incidence of overall acute GVHD grades II to IV and III and IV was HLA match (Figure 5). This was confirmed by multivariate analysis (Table 3).
Figure 4.
Cumulative incidence of acute GVHD (A) grades II to IV and (B) grades III and IV in patients with and without mixed chimerism, before any interventions for mixed chimerism. Cumulative incidence of acute GVHD (C) grades II to IV and (D) grades III and IV in patients with and without mixed chimerism including acute GVHD which occurred after interventions for mixed chimerism (wean or withdrawal of immune suppression, with or without subsequent donor lymphocyte infusion and/or hematopoietic stem cell–containing product).
Figure 5.
Cumulative incidence of overall (A) grades II to IV and (B) grades III and IV acute GVHD among patients with differing donor and recipient HLA-match categories.
Table 3.
Multivariate Analysis of Factors Influencing Development of Overall Grades II to IV Acute GVHD (Final Model)
| Risk Factor | HR (95% CI) | P Value |
|---|---|---|
| HLA match and relation | ||
| Matched related donor | 1.0 | |
| Matched unrelated donor | 3.798 (1.318–10.776) | .028 |
| 1–2 allele mismatched donor | 4.539 (1.094–11.198) | .016 |
GVHD prophylaxis, HLA match, graft source, patient age, and alemtuzumab category were included in the model selection.
Chronic GVHD
In total, 33 patients (16%) developed chronic GVHD. Ten patients developed limited chronic GVHD and 23 patients developed extensive chronic GVHD.
Retransplantation
Three patients experienced secondary graft failure before 30 days after HCT, and 4 patients experienced secondary graft failure between 37 and 731 days after HCT, all with autologous recovery. Ten patients (5%) underwent retransplantation at a median of 305 days after the first transplantation (range, 69 to 848 days) due to either graft failure or low level donor chimerism with reoccurrence of primary disease symptoms. Six patients underwent retransplantation using busulfan, cyclophosphamide, and antithymocyte globulin; 5 are surviving. Alemtuzumab, fludarabine, and melphalan RIC HCT was used again for 4 patients; 1 is surviving.
Overall and Event-Free Survival
Fifty-nine patients died at a median of 181 days after HCT (range, 7 to 2170 days), with a 1-year probability of overall survival of 78% (95% CI, 72% to 83%) and a 3-year probability of overall survival of 69% (95% CI, 62% to 75%).
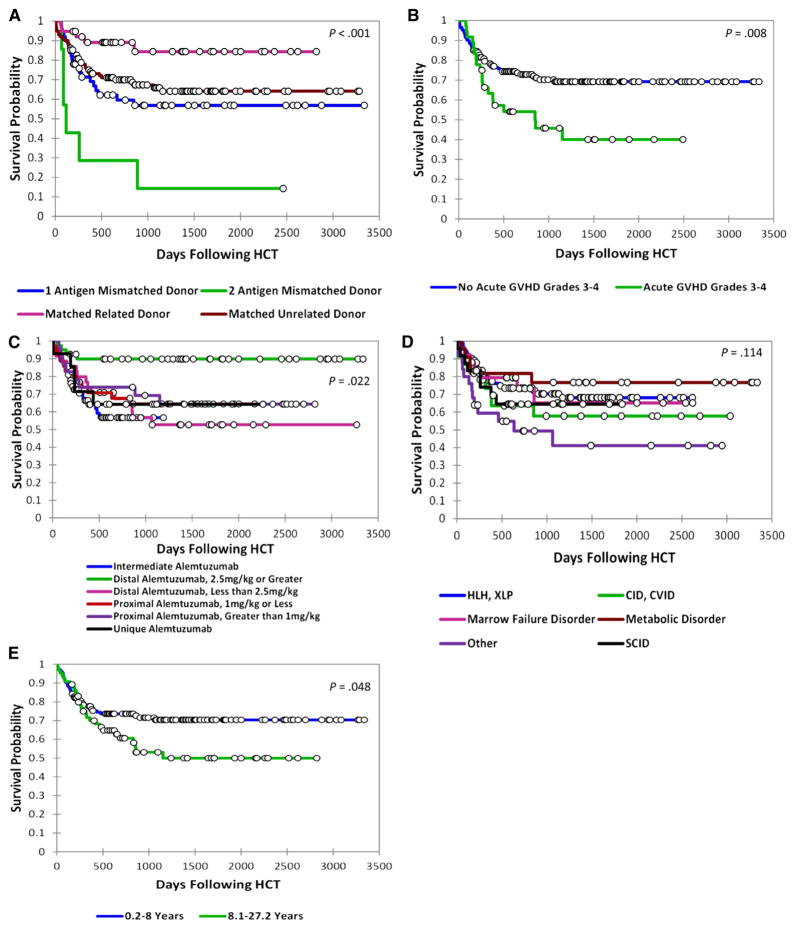
Because of the 5% incidence of retransplantation, we focused on analysis of event-free survival (EFS) outcomes, where an event is defined as death or retransplantation. Three-year EFS was 84% (95% CI, 71% to 98%) for patients who underwent transplantation with an HLA-matched related donor, compared with 64% (95% CI, 55% to 74%), 57% (95% CI, 42% to 71%), and 14% (95% CI, 0 to 40%) for patients who underwent transplantation with a matched unrelated donor, 1 allele mismatched donor, or 2 allele mismatched donor, respectively (P < .001) (Figure 6A). Patients who developed grades III and IV acute GVHD fared worse than patients without, 40% EFS versus 69% (P = .008) (Figure 6B). Unexpectedly, patients who received distal alemtuzumab greater than 2.5 mg/kg appeared to experience better survival compared with the other groups (P = .022) (Figure 6C). Patients with combined immune deficiency/common variable immunodeficiency and the heterogeneous group of patients classified as having other disorders experienced the lowest survival, whereas patients with metabolic disorders experienced the highest survival (P = .114) (Figure 6D). Three-year EFS was lower for patients in the oldest age quartile (older than 8 years) versus the lower 3 quartiles (.2 to 8 years), 53% versus 70% (P = .048) (Figure 6E).
Figure 6.
Comparisons of event-free survival of patients based on (A) donor, match and relation, HLA. (B) occurrence of grade III and IV acute GVHD, (C) receipt of different alemtuzumab schedules, (D) underlying diagnosis, or (E) age group (oldest age quartile versus the lower 3 quartiles).
Multivariate analysis confirmed the significant impact of donor-patient HLA match and use of a related or unrelated donor on EFS (Table 4). Patients who underwent transplantation with a matched unrelated donor (hazard ratio [HR], 3.04; 95% CI, 1.18 to 7.85; P =.022), 1 allele mismatched donor (HR, 4.91; 95% CI, 1.78 to 13.55; P = .002), or 2 allele mismatched donor (HR, 32.19; 95% CI, 8.16 to 127.03; P < .001) had poorer outcomes compared with patients with matched sibling donors. Mortality was increased in recipients with grades III and IV acute GVHD (HR, 1.68; 95% CI, .97 to 2.90; P = .062). The distal alemtuzumab 2.5 mg/kg or greater group again appeared to have better EFS compared with the other alemtuzumab groups (HR, .17; 95% CI, .06 to .53; P = .002). A detrimental effect of older age at transplantation (HR, 1.04; 95% CI, 1.00 to 1.09; P = .037) was also observed.
Table 4.
Multivariate Analysis of Event-Free Survival (Final Model)
| Risk Factor | HR (95% CI) | P Value |
|---|---|---|
| Age | 1.04 (1.00–1.09) | .037 |
| TNC dose | 1.07 (.98–1.16) | .136 |
| Match | ||
| Matched related donor | 1.00 | |
| Matched unrelated donor | 3.04 (1.18–7.85) | .022 |
| 1 allele mismatched donor | 4.91 (1.78–13.55) | .002 |
| 2 allele mismatched donor | 32.19 (8.16–127.03) | <.001 |
| Alemtuzumab category | ||
| Intermediate | 1.00 | |
| Distal, 2.5 mg/kg or greater | .17 (.06–.53) | .002 |
| Distal, less than 2.5 mg/kg | .99 (.50–1.95) | .980 |
| Proximal, greater than 1 mg/kg | .43 (.17–1.05) | .065 |
| Proximal, 1 mg/kg or less | .57 (.27–1.18) | .137 |
| Unique | .65 (.24–1.70) | .376 |
| Occurrence of acute GVHD grades III–IV | ||
| No acute GVHD grades III–IV | 1.00 | |
| Acute GVHD grades III–IV | 1.68 (.97–2.90) | .062 |
Age, diagnosis category, HLA match, total nucleated cell dose, alemtuzumab category, occurrence of mixed chimerism, and occurrence of grades III–IV acute GVHD were included in the model selection.
Among the patients who died, only 2 deaths were clearly related to acute toxicity (Table 5). The predominant cause of death at all time points after HCT was infection (n = 30, 51% of patients who died). Other causes of death included organ or multiorgan failure (n = 6), acute GVHD with or without concurrent infection (n = 4), chronic GVHD with or without bronchiolitis obliterans and/or pneumonia (n = 6), hemorrhage (n = 3), or other causes (n = 8).
Table 5.
Causes of Death among 59 Patients
| Cause | n (%) |
|---|---|
| Infection and complications | 30 (51%) |
| Bacterial | |
| Pseudomonas aeruginosa | 7 (12%) |
| Enterococcus faecium | 3 (5%) |
| Klebsiella pneumonia and Staphylococcus aureus | 1 (2%) |
| Klebsiella pneumonia and Enterococcus faecium | 1 (2%) |
| Acinetobacter baumannii | 1 (2%) |
| Stenotrophomonas maltophilia and Pseudomonas aeruginosa | 1 (2%) |
| Pneumonia with negative bronchoalveolar lavage | 1 (2%) |
| Fungal | |
| Aspergillus fumigatus | 2 (3%) |
| Scedosporium apiospermum | 1 (2%) |
| Scedosporium prolificans | 1 (2%) |
| Coccidiomycosis | 1 (2%) |
| Pneumocystis jirovecii | 1 (2%) |
| Candida glabrata | 1 (2%) |
| Fungalinfection without identification | 3 (5%) |
| Viral | |
| Adenovirus | 2 (3%) |
| Human metapneumovirus and adenovirus | 1 (2%) |
| CMV | 2 (3%) |
| Acute GVHD with or without concurrent infection | 4 (7%) |
| Chronic GVHD with or without bronchiolitis obliterans and/or infection | 6 (10%) |
| MOF | 6 (10%) |
| Hemorrhage | 3 (5%) |
| Toxicity: VOD, hemorrhage, MOF; or severe mucositis and MOF | 2 (3%) |
| Other | 8 (14%) |
MOF indicates multiorgan failure; VOD, veno-occlusive disease.
DISCUSSION
Our data represent the largest cohort reported to date of pediatric and young adult patients who consecutively underwent transplantation with alemtuzumab, fludarabine, and melphalan RIC regimens. For all patients, the cumulative incidence of mixed chimerism was 46%. However, only 5% of patients required retransplantation, and notably, over 75% of patients possessed greater than 90% whole blood donor chimerism at the time of last follow-up. Our data support the continued use of alemtuzumab, fludarabine, and melphalan RIC HCT for patients with nonmalignant diseases, but there is a need for frequent analysis of whole blood donor chimerism. We routinely monitor whole blood chimerism studies weekly for the first several months after HCT. In patients who develop mixed chimerism, interventions including withdrawal of immune suppression and the use of additional cell products (most notably DLI) can lead to resolution of mixed chimerism. Planning for possible DLI should begin at the time of graft harvest, as lymphocytes can be stored at that time for later use if the total cell dose allows.
Differences in risk of mixed chimerism were observed in patients within 2 diagnosis subgroups. Patients with marrow failure experienced less whole blood mixed chimerism, likely due to poor hematopoietic capability of the patient’s marrow before HCT. Patients with SCID experienced less mixed chimerism within the T cell compartment, presumably due to an intrinsic survival advantage for donor-derived T cells. Alemtuzumab, fludarabine, and melphalan regimens can, therefore, be used in these patients with less concern regarding graft loss or loss of chimerism specifically in the cell compartment that was deficient. Differences in risk of mixed chimerism were also observed with regard to graft source. Almost all patients who received cord blood grafts developed mixed chimerism. Because additional cell products are not available when cord blood is used, alemtuzumab, fludarabine, and melphalan should likely be avoided in patients for whom a cord blood graft is the best or only option.
As we have noted previously, the highest incidences of mixed chimerism occurred after proximal schedules and higher doses of alemtuzumab. The terminal half-life of alemtuzumab ranges from 6 to 21 days but has significant interpatient variability due to differences in patient quantities of CD52 antigen [14–17]. Because of the long half-life, alemtuzumab is often still present at the time of graft infusion (and up to 2 months after HCT) and lowers the risk of GVHD by lymphocyte/T cell depletion of the graft [18–21]. The development of mixed chimerism is likely influenced by the extent of T cell depletion of the graft by alemtuzumab, coupled with the propensity for autologous recovery of patient bone marrow after the nonablative fludarabine and melphalan regimen. Alemtuzumab dose de-escalation studies in adults have resulted in decreased incidences of mixed chimerism and graft failure, though sometimes with increased rates of acute GVHD; dose de-escalation can also improve immune recovery [17,22–25]. As demonstrated here and in our previous report [3], an intermediate dosing regimen of alemtuzumab consisting of 1 mg/kg divided over days –14 to –10 can be used in pediatric and young adult patients to decrease the incidence of mixed chimerism. It is possible that correlation of peritransplantation alemtuzumab levels with pediatric outcomes may lead to optimized individualized dosing protocols to minimize the incidence of mixed chimerism. Alternatively, efforts to individualize melphalan dosing based on patient pharmacokinetics may help ensure adequate drug exposure and minimize the propensity for recipient marrow recovery. The inclusion of additional myelosuppressive agents could also be considered to further prohibit the propensity for recipient marrow recovery, and thiotepa and hydroxyurea have already been added to an alemtuzumab, fludarabine, and lower dose melphalan (70 mg/m2) regimen in the cord blood setting with some success [26].
Three-year survival without retransplantation in our cohort was 84% for patients who underwent transplantation with a matched related donor graft. Survival was negatively affected by several factors, including lack of a matched related donor, development of grades III and IV acute GVHD, and older age. Unexpectedly, we observed that patients treated with distal alemtuzumab 2.5 mg/kg or greater appeared to have better survival. The clinical significance of this observation is uncertain, as despite multivariate analysis, this group of patients is intrinsically confounded by young age (only 1 patient was older than 7 years), and is also confounded by a preponderance of metabolic disorders. The high rate of mixed chimerism in this group (59%) argues against the use of dose escalation schedules of alemtuzumab in young (small) patients that result in high total doses of alemtuzumab per kilogram body weight.
Despite the unique complications inherent to alemtuzumab, fludarabine, and melphalan RIC HCT, we conclude that this approach offers good outcomes for many patients with nonmalignant diseases, and that the risk of developing mixed chimerism is influenced by underlying diagnosis, graft source, and alemtuzumab dosing. Further advancements will continue to improve this RIC HCT approach.
Supplementary Material
Acknowledgments
The authors thank Linda Carl, Mathew Goodridge, Cherie Short, Angie Bonavita, Christine Sper, Melanie Jordan, and Ashely Teusink for assistance with data collection. We wish to thank all of the Cincinnati Children’s Hospital Medical Center nurses, physicians, and other caregivers who provided clinical care for the patients.
Footnotes
Financial disclosure: The authors have nothing to disclose.
Conflict of interest: There are no conflicts of interest to report.
Authorship contributions: R.A.M. designed the research, collected data, performed statistical analyses, and wrote the manuscript. M.B.R. designed, supervised, and performed statistical analyses and edited the manuscript. A.G. and D.B. collected data and edited the manuscript. A.H.F. and S.M.D. designed the research, directed statistical analyses, and edited the manuscript. All remaining authors contributed to the study design and edited the manuscript.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbmt.2015.04.009.
References
- 1.Rzepecki P, Sarosiek T, Szczylik C. Alemtuzumab, fludarabine and melphalan as a conditioning therapy in severe aplastic anemia and hypoplastic myelodysplastic syndrome–single center experience. Jpn J Clin Oncol. 2006;36:46–49. doi: 10.1093/jjco/hyi211. [DOI] [PubMed] [Google Scholar]
- 2.Marsh RA, Vaughn G, Kim MO, et al. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010;116:5824–5831. doi: 10.1182/blood-2010-04-282392. [DOI] [PubMed] [Google Scholar]
- 3.Marsh RA, Kim MO, Liu C, et al. An intermediate alemtuzumab schedule reduces the incidence of mixed chimerism following reduced-intensity conditioning hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transplant. 2013;19:1625–1631. doi: 10.1016/j.bbmt.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen MD, Filipovich AH, Davies SM, et al. Allogeneic hematopoietic cell transplantation (HCT) in Hurler’s syndrome using a reduced intensity preparative regimen. Bone Marrow Transplant. 2008;41:349–353. doi: 10.1038/sj.bmt.1705926. [DOI] [PubMed] [Google Scholar]
- 5.Shenoy S, Grossman WJ, DiPersio J, et al. A novel reduced-intensity stem cell transplant regimen for nonmalignant disorders. Bone Marrow Transplant. 2005;35:345–352. doi: 10.1038/sj.bmt.1704795. [DOI] [PubMed] [Google Scholar]
- 6.Bhatla D, Davies SM, Shenoy S, et al. Reduced-intensity conditioning is effective and safe for transplantation of patients with Shwachman-Diamond syndrome. Bone Marrow Transplant. 2008;42:159–165. doi: 10.1038/bmt.2008.151. [DOI] [PubMed] [Google Scholar]
- 7.Oshrine BR, Olson TS, Bunin N. Mixed chimerism and graft loss in pediatric recipients of an alemtuzumab-based reduced-intensity conditioning regimen for non-malignant disease. Pediatr Blood Cancer. 2014;61:1852–1859. doi: 10.1002/pbc.25113. [DOI] [PubMed] [Google Scholar]
- 8.Satwani P, Cooper N, Rao K, et al. Reduced intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases. Bone Marrow Transplant. 2008;41:173–182. doi: 10.1038/sj.bmt.1705923. [DOI] [PubMed] [Google Scholar]
- 9.Rao K, Amrolia PJ, Jones A, et al. Improved survival after unrelated donor bone marrow transplantation in children with primary immunodeficiency using a reduced-intensity conditioning regimen. Blood. 2005;105:879–885. doi: 10.1182/blood-2004-03-0960. [DOI] [PubMed] [Google Scholar]
- 10.Satwani P, Jin Z, Duffy D, et al. Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant. 2013;19:552–561. doi: 10.1016/j.bbmt.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Kamani NR, Walters MC, Carter S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: results of one cohort from the phase II study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biol Blood Marrow Transplant. 2012;18:1265–1272. doi: 10.1016/j.bbmt.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 13.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 14.Rebello P, Cwynarski K, Varughese M, et al. Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy. 2001;3:261–267. doi: 10.1080/146532401317070899. [DOI] [PubMed] [Google Scholar]
- 15.Hale G, Rebello P, Brettman LR, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104:948–955. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- 16.Mould DR, Baumann A, Kuhlmann J, et al. Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Clin Pharmacol. 2007;64:278–291. doi: 10.1111/j.1365-2125.2007.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraverty R, Orti G, Roughton M, et al. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116:3080–3088. doi: 10.1182/blood-2010-05-286856. [DOI] [PubMed] [Google Scholar]
- 18.Kottaridis PD, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96:2419–2425. [PubMed] [Google Scholar]
- 19.van Besien K, Kunavakkam R, Rondon G, et al. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. 2009;15:610–617. doi: 10.1016/j.bbmt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgado J, Pillai S, Benjamin R, et al. The effect of in vivo T cell depletion with alemtuzumab on reduced-intensity allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2008;14:1288–1297. doi: 10.1016/j.bbmt.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Hale G, Zhang MJ, Bunjes D, et al. Improving the outcome of bone marrow transplantation by using CD52 monoclonal antibodies to prevent graft-versus-host disease and graft rejection. Blood. 1998;92:4581–4590. [PubMed] [Google Scholar]
- 22.Shore T, Harpel J, Schuster MW, et al. A study of a reduced-intensity conditioning regimen followed by allogeneic stem cell transplantation for patients with hematologic malignancies using Campath-1H as part of a graft-versus-host disease strategy. Biol Blood Marrow Transplant. 2006;12:868–875. doi: 10.1016/j.bbmt.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Juliusson G, Theorin N, Karlsson K, et al. Subcutaneous alemtuzumab vs ATG in adjusted conditioning for allogeneic transplantation: influence of Campath dose on lymphoid recovery, mixed chimerism and survival. Bone Marrow Transplant. 2006;37:503–510. doi: 10.1038/sj.bmt.1705263. [DOI] [PubMed] [Google Scholar]
- 24.Gartner F, Hieke S, Finke J, Bertz H. Lowering the alemtuzumab dose in reduced intensity conditioning allogeneic hematopoietic cell transplantation is associated with a favorable early intense natural killer cell recovery. Cytotherapy. 2013;15:1237–1244. doi: 10.1016/j.jcyt.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Lane JP, Evans PT, Nademi Z, et al. Low-dose serotherapy improves early immune reconstitution after cord blood transplantation for primary immunodeficiencies. Biol Blood Marrow Transplant. 2014;20:243–249. doi: 10.1016/j.bbmt.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh SH, Mendizabal A, Benjamin CL, et al. A novel reduced-intensity conditioning regimen for unrelated umbilical cord blood transplantation in children with nonmalignant diseases. Biol Blood Marrow Transplant. 2014;20:326–336. doi: 10.1016/j.bbmt.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.