Abstract
Objective
To assess trends in elective single ET and identify factors associated with a good perinatal outcome.
Design
Retrospective cohort study.
Setting
Clinic-based data.
Patient(s)
A total of 886,686 fresh, nondonor cycles reported to the National Assisted Reproductive Technology Surveillance System during 1999–2010, of which 17,166 met criteria for elective single ET.
Intervention(s)
None.
Main Outcome Measure(s)
Rates of elective single ET and good perinatal outcome (term, singleton infant with normal birth weight).
Result(s)
In 2010, elective single ET comprised 5.6% of all fresh transfers, representing an eightfold increase since publication of first guidelines in 2004 recommending elective single ET. Compared with other ETs, elective single ETs were nearly twice as likely to result in a good perinatal outcome (37.1% vs. 18.9%, respectively). Among women using elective single ET, those aged <35 and 35–37 years had a good perinatal outcome (40.2% and 32.5%, respectively). In multivariable, log-binomial analyses, factors positively associated with a good perinatal outcome included male factor infertility, day 5 ET, and having ≥3 supernumerary embryos for cryopreservation.
Conclusion(s)
Between 1999 and 2010, national rates of elective single ET increased. Given the frequency of good perinatal outcomes among women aged 35–37 years, guidelines for elective single ET could be expanded to include patients in this age group with favorable prognoses.
Keywords: Elective single ET, single ET, in vitro fertilization, singleton pregnancy, good perinatal outcome
Multiple gestations, and their associated complications, remain the most common adverse outcome associated with assisted reproductive technology (ART). The most effective method for reducing the risk of multiple births after ART is to limit the number of embryos transferred. Evolving practice guidelines from the American Society for Reproductive Medicine (ASRM) and the Society of Assisted Reproductive Technologies (SART) have led to a steady decline in the frequency of higher order (≥3) ETs during the past decade (1). This downward trend has caused an increased number of double ETs, resulting in an unchanged, or even slightly increased, rate of twin gestation resulting from ART (1). Elective single ET, defined as the transfer of only one embryo when more than one high-quality embryo is available, has been proposed as the only means of avoiding multiple gestations after IVF (2).
As observed in several studies (3–9), elective single ET successfully reduces the risk of multiple gestations, without significantly compromising live birth rates. However, in prior studies, the population had been restricted to select subsets of patients with the most “favorable prognosis,” thereby limiting the generalizability of the findings (3–9). In a study of unselected patients (10), use of elective single ET effectively eliminated multiple gestations, but nearly halved pregnancy rates (PRs), compared with double ETs. Due to low rates of elective single ET in the United States, there has only been one small, single center analysis evaluating factors associated with birth outcomes after fresh elective single ET (11). An analysis of national data would add to this study, which found that younger maternal age and blastocyst expansion were positively associated with clinical pregnancy and live birth among patients with favorable prognosis.
Given the limited information on use of elective single ET in the United States, we analyzed data from the Centers for Disease Control and Prevention National ART Surveillance System (NASS) to estimate national trends in elective single ET from 1999 through 2010. Furthermore, because the motivation behind promoting elective single ET is to increase the rate of healthy, singleton infants after ART, we sought to identify characteristics associated with a good perinatal outcome after elective single ET.
MATERIALS AND METHODS
The data used for this analysis were obtained from NASS, which was established after the enactment of the Fertility Clinic Success Rate and Certification Act of 1992. This law mandates that ART clinics report data annually to the Centers for Disease Control and Prevention for all cycles initiated during that year (12). The data include patient demographics, medical and obstetric history, infertility diagnosis, and information regarding resultant pregnancies and births. Approximately 6.5% of ART clinics did not provide data to Centers for Disease Control and Prevention in 2010 (13). Because most nonreporting clinics are small, we estimate that NASS contains information on more than 97% of all ART cycles performed in the United States (13). The study was reviewed and approved by the Institutional Review Board of the Centers for Disease Control and Prevention.
The elective single ET study population was defined as fresh, nondonor cycles in which a single embryo was transferred and at least one supernumerary embryo was available for cryopreservation. The comparison group consisted of all other fresh, nondonor cycles. These included cycles in which only one embryo was available for transfer (single ET without additional embryos available for cryopreservation), as well as transfers of more than one embryo.
The characteristics assessed included maternal age, race/ethnicity, infertility diagnosis, number of prior pregnancies, number of prior spontaneous abortions, number of prior live births, number of prior ART cycles, year of cycle treatment, insurance mandate status for state of residence, number of oocytes retrieved, use of intracytoplasmic sperm injection (ICSI) (± male factor infertility), use of assisted hatching, use of preimplantation genetic diagnosis (for years 2004 and later), embryo stage at transfer, number of embryos transferred, and number of supernumerary embryos cryopreserved. Because race/ethnicity was unknown or missing in nearly 40% of the study population, an unknown/missing category was included to allow evaluation of these data in multivariable analyses. Also, as patients may have had more than one infertility diagnosis, these diagnoses were not mutually exclusive. To assess potential temporal differences in elective single ET practices, we compared transfers performed in 2005–2010 with those performed in 1999–2004, as the first ASRM/SART guidelines recommending elective single ET were published in September 2004 (14). With regard to embryo stage at transfer, we chose to restrict our analysis to the two most common days for embryo transfer (day 3 for cleavage stage and day 5 for blastocyst stage), which together represented 82.5% of all transfers.
A “good perinatal outcome” was defined as the live birth of a singleton infant born at term (≥37 completed weeks of gestation) and at a normal birth weight (≥2,500 g) (15). Gestational age was calculated by subtracting the date of oocyte retrieval from the date of delivery, then adding 14 days to adjust for the theoretical date of the last menstrual period.
We used SAS statistical software version 9.3 (SAS Institute) to conduct all analyses. For all transfers and selected maternal age strata, we calculated the percentage of all ETs meeting elective single ET criteria for each year. We used Mantel-Haenszel statistics to assess trends in the proportion of elective single ET during the study period, and two-tailed χ2 tests to compare the distribution of maternal and cycle characteristics for the elective single ET group with all other fresh, nondonor transfers during the study period.
To evaluate factors associated with good perinatal outcome among the cycles with elective single ET, we calculated the frequency of good outcomes for each of these maternal and cycle characteristics. Log-binomial models were used to calculate unadjusted and adjusted risk ratios (RR) for the association between maternal and cycle characteristics and a good perinatal outcome after elective single ET. The number of previous pregnancies was not included as a predictor in the adjusted models because it represented the sum of number of previous abortions and number of previous live births, two variables that were already included in the model. Preimplantation genetic diagnosis was also excluded from the models because this information was not ascertained for all study years. We also conducted stratified analyses to examine potential effect modification by maternal age using a variety of different maternal age groupings; no differential effects were noted thus stratified results were not presented. Due to the high proportion of missing or unknown race data, we compared the findings for adjusted models with and without the race variable. Because differences between the two models were nominal, we retained race in the final model.
RESULTS
During the study years 1999–2010, a total of 1,541,825 ART cycles were included in NASS. Of the 1,111,766 cycles using fresh, nondonor oocytes, 886,686 (79.8%) proceeded to transfer at least 1 embryo, and 17,166 (1.9%) met elective single ET criteria.
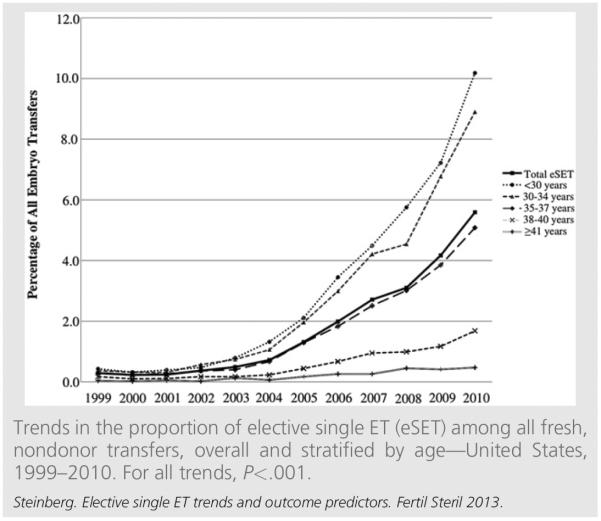
Until 2004, less than 1% of all transfers in the United States were elective single ET (Fig. 1). Since that time, there has been an eightfold increase in national elective single ET rates (from 0.72% in 2004 to 5.6% in 2010). In 2010, 9.3% of all transfers in women less than 35 years of age were elective single ET, as were 5.1% of ETs in women aged 35–37 years. Since 1999, each age group has shown a significant positive trend in rates of elective single ET (all P for trend <.001).
FIGURE 1.
With the exception of number of prior pregnancies, the distribution of all characteristics assessed differed significantly between the elective single ET group and all other transfers (Table 1). For example, patients undergoing elective single ET were more likely to be younger than 35 years, have a diagnosis of ovulatory disorder, and have no history of prior ART cycles, when compared to all other transfers. Cycles resulting in elective single ET were more likely to have taken place after 2004, have more than 16 oocytes retrieved, and have transferred a blastocyst-stage embryo.
TABLE 1.
Characteristics of the fresh, nondonor elective single ET compared with all other fresh, nondonor transfers—United States, 1999–2010.
| Characteristic | Elective single ET (%) | All others (%) |
|---|---|---|
| Total | 17,166 (100) | 869,520 (100) |
| Maternal age, ya | ||
| <30 | 3,821 (22.2) | 108,689 (12.5) |
| 30–34 | 8,406 (49.0) | 281,923 (32.4) |
| 35–37 | 3,599 (21.0) | 198,192 (22.8) |
| 38–40 | 1,097 (6.4) | 172,089 (19.8) |
| ≥41 | 243 (1.4) | 108,627 (12.5) |
| Race/ethnicitya | ||
| Non-Hispanic white | 7,731 (45.0) | 392,460 (45.1) |
| Non-Hispanic black | 600 (3.5) | 31,213 (3.6) |
| Hispanic | 1,454 (8.5) | 47,024 (5.4) |
| Asian/Pacific Islander | 626 (3.7) | 38,574 (4.4) |
| Other race | 28 (0.2) | 923 (0.1) |
| Unknown or missing | 6,727 (39.2) | 359,326 (41.3) |
| Infertility diagnosisa,b | ||
| Tubal factor | 2,950 (17.2) | 185,540 (21.3) |
| Endometriosis | 1,706 (10.0) | 123,789 (14.2) |
| Uterine factor | 1,004 (5.9) | 44,193 (5.1) |
| Ovulatory disorder | 3,717 (21.7) | 122,997 (14.2) |
| Diminished ovarian reserve | 953 (5.6) | 142,034 (16.3) |
| Male factor | 6,143 (35.8) | 329,417 (37.9) |
| Unexplained | 2,620 (15.3) | 107,924 (12.4) |
| No. of prior pregnancies | ||
| 0 | 8,113 (47.4) | 409,625 (47.2) |
| 1 | 4,547 (26.5) | 226,411 (26.1) |
| ≥2 | 4,472 (26.1) | 232,140 (26.7) |
| No. of prior spontaneous abortionsa | ||
| 0 | 12,943 (75.4) | 603,721 (69.5) |
| 1 | 2,864 (16.7) | 169,080 (19.5) |
| ≥2 | 1,356 (7.9) | 95,783 (11.0) |
| No. of prior live birthsa | ||
| 0 | 11,156 (65.5) | 628,104 (72.6) |
| 1 | 4,325 (25.4) | 174,947 (20.2) |
| ≥2 | 1,548 (9.1) | 61,680 (7.1) |
| No. of prior ART cyclesa | ||
| 0 | 12,882 (75.0) | 486,083 (55.9) |
| 1 | 2,068 (12.1) | 177,067 (20.4) |
| ≥2 | 2,216 (12.9) | 205,832 (23.7) |
| Year cycle treatment starteda | ||
| 1999–2004 (first guidelines September 2004) | 1,597 (9.3) | 393,050 (45.2) |
| 2005–2010 | 15,569 (90.7) | 476,470 (54.8) |
| Insurance mandate statusa | ||
| State not providing coverage for ART | 5,830 (36.1) | 311,713 (39.0) |
| State providing coverage | 10,312 (63.9) | 487,996 (61.0) |
| No. of oocytes retrieveda | ||
| 1–10 | 3,523 (20.5) | 406,726 (46.8) |
| 11–15 | 4,391 (25.6) | 217,602 (25.0) |
| ≥16 | 9,252 (53.9) | 245,192 (28.2) |
| Use of intracytoplasmic sperm injection (ICSI)a | ||
| Did not use ICSI | 5,946 (34.7) | 287,588 (33.1) |
| Used ICSI | 11,201 (65.3) | 581,551 (66.9) |
| With male factor | 5,625 (50.2)c | 280,366 (48.2)c |
| Without male factor | 5,576 (49.8)c | 301,185 (51.8)c |
| Use of assisted hatchinga | ||
| Did not use assisted hatching | 14,124 (82.3) | 477,975 (55.0) |
| Used assisted hatching | 3,042 (17.7) | 391,545 (45.0) |
| Use of preimplantation genetic diagnosis (PGD)a,d | ||
| Did not use PGD | 14,491 (91.4) | 496,167 (95.4) |
| Used PGD | 1,356 (8.6) | 23,857 (4.6) |
| Missing | 1,319 | 349,496 |
| Embryo stage at transfera | ||
| Cleavage stage (day 3) | 2,667 (22.0) | 537,074 (74.6) |
| Blastocyst stage (day 5) | 9,476 (78.0) | 182,554 (25.4) |
| Missing/out of range | 5,023 | 149,892 |
| No. of embryos transferreda | ||
| 1 | 17,166 (100) | 70,024 (8.0) |
| 2 | 0 | 364,723 (42.0) |
| ≥3 | 0 | 434,773 (50.0) |
| No. of supernumerary embryos cryopreserveda | ||
| Unknown | 0 | 4,226 (0.5) |
| 0 | 0 | 594,623 (68.4) |
| ≥1 | 17,166 (100) | 270,671 (31.1) |
Note: ART = assisted reproductive technology.
P<.001 for χ2 test of distribution of variable in the elective single ET group versus all other transfers.
percentages do not sum to 100 because groups are not mutually exclusive (patient may carry multiple diagnoses).
Among those using ICSI.
Data only available for 2004 and later.
Steinberg. Elective single ET trends and outcome predictors. Fertil Steril 2013.
More than one-third of elective single ET resulted in a good perinatal outcome (37.1%, compared with 18.9% of all other transfers during the same period [data not shown]). Approximately 40% of elective single ET in the two youngest age groups (<30 years and 30–34 years) resulted in a good perinatal outcome, whereas 32.5% of elective single ET in the 35- to 37-year-old age group reported the same outcome (Table 2). Among all other transfers, approximately 22% and 20% of transfers in women <35 and 35–37 years, respectively, resulted in a good perinatal outcome. The findings were similar for women with no prior ART cycles (23% and 21%, respectively) (data not shown). Patients undergoing ART for the first time also had a higher-than-average rate of good outcomes (38.4%). Among cycles where ET occurred on day 5, 39.7% resulted in a good perinatal outcome. Similarly, when five or more supernumerary embryos were available for cryopreservation 41.0% of cycles resulted in a good perinatal outcome.
TABLE 2.
Predictors of good perinatal outcome after fresh, nondonor elective single ET—United States, 1999–2010.
| % Elective single ET with good perinatal outcome | ||||
|---|---|---|---|---|
| Exposure | N | % | Unadjusted risk ratio (95% CI) | Adjusted risk ratioa (95% CI) |
| Total | 6,361 | 37.1 | ||
| Maternal age, yb | ||||
| <30 | 1,592 | 41.7 | Reference | Reference |
| 30–34 | 3,321 | 39.5 | 0.95 (0.91–0.99) | 0.97 (0.92–1.03) |
| 35–37 | 1,169 | 32.5 | 0.78 (0.73–0.83) | 0.85 (0.79–0.92) |
| 38–40 | 251 | 22.9 | 0.55 (0.49–0.62) | 0.60 (0.52–0.70) |
| ≥41 | 28 | 11.5 | 0.28 (0.19–0.39) | 0.36 (0.23–0.57) |
| Race/ethnicity | ||||
| Non-Hispanic white | 3,127 | 40.5 | Reference | Reference |
| Non-Hispanic black | 157 | 26.2 | 0.65 (0.56–0.74) | 0.60 (0.50–0.72) |
| Hispanic | 202 | 32.2 | 0.80 (0.71–0.90) | 0.80 (0.69–0.92) |
| Asian/Pacific Islander | 439 | 30.2 | 0.75 (0.69–0.81) | 0.77 (0.69–0.85) |
| Other race | 10 | 35.7 | 0.88 (0.54–1.45) | 1.11 (0.68–1.81) |
| Unknown/missing | 2,426 | 36.1 | 0.89 (0.86–0.93) | 0.94 (0.89–0.98) |
| Infertility diagnosis | ||||
| No tubal factor | 5,365 | 37.7 | Reference | Reference |
| Tubal factor | 996 | 33.8 | 0.89 (0.85–0.95) | 0.97 (0.91–1.03) |
| No endometriosis | 5,752 | 37.2 | Reference | Reference |
| Endometriosis | 609 | 35.7 | 0.96 (0.90–1.03) | 0.94 (0.86–1.01) |
| No uterine factor | 6,114 | 37.8 | Reference | Reference |
| Uterine factor | 247 | 24.6 | 0.65 (0.58–0.73) | 0.64 (0.55–0.73) |
| No ovulatory disorder | 4,850 | 36.1 | Reference | Reference |
| Ovulatory disorder | 1,511 | 40.7 | 1.13 (1.08–1.18) | 1.00 (0.94–1.06) |
| No diminished ovarian reserve | 6,104 | 37.6 | Reference | Reference |
| Diminished ovarian reserve | 257 | 27.0 | 0.72 (0.64–0.80) | 0.95 (0.83–1.08) |
| No male factor | 3,961 | 35.9 | Reference | Reference |
| Male factor | 2,400 | 39.1 | 1.09 (1.04–1.13) | 1.08 (1.02–1.14) |
| No. of prior spontaneous abortionsb | ||||
| 0 | 5,014 | 38.7 | Reference | Reference |
| 1 | 955 | 33.3 | 0.86 (0.81–0.91) | 0.90 (0.84–0.96) |
| ≥2 | 392 | 28.9 | 0.75 (0.68–0.81) | 0.87 (0.79–0.97) |
| No. of prior live births | ||||
| 0 | 4,205 | 37.7 | Reference | Reference |
| 1 | 1,644 | 38.0 | 1.01 (0.96–1.05) | 1.11 (1.05–1.17) |
| ≥2 | 468 | 30.2 | 0.80 (0.74–0.87) | 0.90 (0.81–0.99) |
| No. of prior ART cycles | ||||
| 0 | 4,946 | 38.4 | Reference | Reference |
| 1 | 706 | 34.1 | 0.90 (0.83–0.95) | 0.96 (0.89–1.04) |
| ≥2 | 709 | 32.0 | 0.83 (0.78–0.89) | 0.96 (0.89–1.04) |
| Year cycle treatment started | ||||
| 1999–2004 | 471 | 29.5 | Reference | Reference |
| 2005–2010 | 5,890 | 37.8 | 1.28 (1.19–1.39) | 1.23 (1.13–1.34) |
| Insurance mandate status | ||||
| State not providing coverage for ART | 2,330 | 40.0 | Reference | Reference |
| State providing coverage | 3,717 | 36.1 | 0.90 (0.86–0.94) | 0.96 (0.91–1.00) |
| No. of oocytes retrieved | ||||
| 1–10 | 1,083 | 30.7 | Reference | Reference |
| 11–15 | 1,646 | 37.5 | 1.22 (1.15–1.30) | 1.05 (0.97–1.13) |
| ≥16 | 3,632 | 39.3 | 1.28 (1.21–1.35) | 0.99 (0.92–1.07) |
| Use of intracytoplasmic sperm injection (ICSI) | ||||
| Did not use ICSI | 2,299 | 38.7 | Reference | Reference |
| Used ICSI | 4,058 | 36.2 | 0.94 (0.90–0.98) | 0.88 (0.84–0.93) |
| Use of assisted hatching | ||||
| Did not use assisted hatching | 5,464 | 38.7 | Reference | Reference |
| Used assisted hatching | 897 | 29.5 | 0.76 (0.72–0.81) | 0.88 (0.82–0.95) |
| Embryo stage at transfer | ||||
| Cleavage stage (day 3) | 767 | 28.8 | Reference | Reference |
| Blastocyst stage (day 5) | 3,765 | 39.7 | 1.38 (1.30–1.47) | 1.33 (1.24–1.43) |
| No. of supernumerary embryos cryopreservedb | ||||
| 1–2 | 1,768 | 31.4 | Reference | Reference |
| 3–4 | 1,750 | 38.0 | 1.21 (1.15–1.27) | 1.11 (1.04–1.19) |
| ≥5 | 2,843 | 41.0 | 1.30 (1.24–1.37) | 1.20 (1.12–1.27) |
Note: ART = assisted reproductive technology, CI = confidence interval.
Regression model adjusted for all other variables listed in the table.
P<.001 for linear trends in risk ratios.
Steinberg. Elective single ET trends and outcome predictors. Fertil Steril 2013.
In the adjusted model, a primary diagnosis of male factor infertility, a treatment start date of 2005 or later, and an ET on day 5 were positively associated with a good perinatal outcome after elective single ET. The following characteristics were found to be negatively associated with a good perinatal outcome: race/ethnicity (specifically, non-Hispanic black, Hispanic, or Asian/Pacific Islander); primary infertility diagnosis of uterine factor; use of ICSI; and use of assisted hatching.
The strongest positive association with good perinatal outcome was for the use of day 5 ET, compared with day 3 ET (adjusted RR 1.33, confidence interval [CI] 1.24–1.43). Conversely, the strongest negative associations were estimated for maternal age of 41 years or older, compared with age less than 30 years (adjusted RR 0.36, CI 0.23–0.57), and non-Hispanic black race, compared with non-Hispanic white race (adjusted RR 0.60, CI 0.50–0.72).
Among the predictors with ordinal categories, significant linear trends in the risk ratios were noted for maternal age, number of prior spontaneous abortions, and number of super-numerary embryos cryopreserved.
DISCUSSION
Using national data on cycles of fresh, nondonor ETs, we found that rates of elective single ET increased significantly across all age groups from 1999–2010, with elective single ET comprising 5.6% of ART cycles in 2010. This trend is likely due to continually evolving guidelines from ASRM and SART promoting fewer embryos per transfer, as well as improvements in embryo culture that have facilitated the selection of higher quality embryos for transfer (1, 16). Although we observed an eightfold increase in elective single ET rates during the study period, the proportion of elective single ET in the United States remains low compared with other developed countries (1). Although the greatest increases in the present study were observed among patients less than 35 years of age, for whom ET guidelines currently recommend elective single ET, rates of elective single ET among patients aged 35–37 years have increased by an average of 0.7% per year since 2004, and comprised more than 5% of all transfers in this age group in 2010. This trend suggests that clinicians are expanding the current elective single ET guidelines to include a slightly broader patient population, although it is unclear what prognostic criteria are being used to identify good candidates.
Previous studies investigating prognostic criteria for elective single ET evaluated the outcomes of clinical pregnancy or live birth (1, 11, 17–19). We chose to focus on having a good perinatal outcome. This measure, a variation of the Birth Emphasizing a Successful Singleton at Term outcome, which was first proposed by Min and colleagues (20), attempts to redefine “success” after ART as the delivery of a healthy singleton infant. We found that elective single ETs were nearly twice as likely to result in a good perinatal outcome compared with all other fresh, nondonor ETs. This finding adds to the growing body of literature supporting elective single ET as an effective means of avoiding the adverse outcomes commonly associated with ART (15, 21).
Within the elective single ET group, good perinatal outcomes were most common among women less than 35 years of age or those undergoing their first ART cycle–the women considered to be of “a more favorable prognosis” by ASRM/SART guidelines on number of embryos transferred (22). However, 32.5% of elective single ET in women aged 35–37 years resulted in a good perinatal outcome (vs. 40.2% in the under-35-years cohort). These findings, like those of Veleva and co-workers (23) suggest that the ET guidelines could potentially be expanded to include the age group 35–37 years as candidates for elective single ET. The increasing rates of elective single ET observed in this age group suggest that clinicians may already be practicing based on anecdotal evidence supporting this conclusion.
In our analysis, the strongest independent predictor of a good perinatal outcome after elective single ET was ET on day 5. This finding builds on a study in which Papanikolaou and co-workers (24) found higher rates of pregnancy and delivery among a group of favorable prognosis patients undergoing transfer of a single blastocyst-stage embryo, compared with those undergoing transfer of a single cleavage-stage embryo. We also found having three or more supernumerary embryos available for cryopreservation is associated with a good outcome. The survival of one or more embryos in culture until day 5, for transfer or cryopreservation, may be an indirect measure of “good embryo quality,” which current ET guidelines include as a characteristic associated with a more favorable prognosis (22).
We found significant racial/ethnic disparities with respect to good perinatal outcomes after elective single ET. Specifically, non-Hispanic black, Hispanic, and Asian/Pacific Islander race/ethnicity were less likely to have a good perinatal outcome after elective single ET. Although these findings are consistent with other analyses of national ART data (25–29), they should be interpreted with caution; in nearly 40% of cycles, data regarding maternal race/ethnicity was missing or unknown. A recent systematic review of national race/ethnicity reporting for ART suggested that it is not possible to draw definitive conclusions regarding disparities due to lack of universal reporting of this race/ethnicity (30).
Although the diagnosis of male factor infertility has yet to be reported as a prognostic factor for elective single ET, we found it to be a positive predictor of good perinatal outcome. A potential explanation for this finding may be that, among couples where male factor is the only infertility diagnosis, the female is otherwise healthy and therefore more likely to have a good perinatal outcome. Furthermore, we found uterine factor infertility to be inversely associated with good perinatal outcome, which is consistent with previous studies that demonstrated worsened pregnancy outcomes among patients with uterine factor (11, 15). Interestingly, in a previous analysis of national data, Luke and colleagues (31) suggested that the diagnosis of uterine factor was more common among women undergoing elective single ET; however, our data do not support their conclusion.
Strengths of our study include the use of nationwide surveillance data with nearly universal reporting of ART procedures, which allowed for the analysis of the largest population of elective single ET in the United States to date. Furthermore, the use of multiple years of national data allowed for the first comprehensive analysis of elective single ET trends in the United States. Unlike previous studies evaluating prognostic criteria for elective single ET, we did not restrict our study population to patients with a favorable prognosis. Given the large sample size and increased representativeness of national data, our findings can be applied broadly by clinicians to all patients considering ART.
Our findings are subject to several limitations. As indicated in the SART reporting guidelines, elective single ET is generally defined as “an embryo transfer in which more than one high-quality embryo exists but it was decided to transfer only one embryo.” Because NASS does not collect information on embryo morphology, our definition of elective single ET did not include such parameters, and, thus, we were unable to directly evaluate whether better embryo quality is associated with a good perinatal outcome after elective single ET, particularly among women 35–37 years of age. In addition, we could not evaluate the potential impact of clinic policies on elective single ET use. A clinician's decision to recommend elective single ET may be affected by the clinic's PRs, an element of elective single ET selection criteria that we did not assess. Finally, NASS validation results indicate that infertility diagnosis has a relatively high discrepancy rate (approximately 18% in 2010) (13), with about half of the discrepancies due to report of a single cause of infertility when multiple causes were noted in the medical record.
Although rates of elective single ET increased significantly during the 12-year study period, our findings suggest there may be additional patients who could be considered strong candidates for elective single ET. Continuing to redefine a “successful” ART outcome as a healthy singleton birth, may further motivate clinicians to consider elective single ET for their patients. By investigating factors associated with a good perinatal outcome, we aimed to help identify those patients who may be good candidates for elective single ET but are not included in the “favorable prognosis” group by the current ET guidelines. Our findings support better birth outcomes associated with younger age, day 5 transfer, race/ethnicity (non-Hispanic white), and the absence of uterine factor infertility. However, our results also indicate that the use of elective single ET may be appropriate for women aged 35–37 years, and couples affected by male factor infertility. Consideration of these factors may further continue the downward trend in number of embryos transferred, thereby decreasing the current rate of twin births among patients receiving ART.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
M.L.S. has nothing to disclose. S.B. has nothing to disclose. D.K. has nothing to disclose. L.W. has nothing to disclose. D.J.J. has nothing to disclose.
REFERENCES
- 1.Practice Committee of Society for Assisted Reproductive Technologies. Practice Committee of American Society for Reproductive Medicine Elective single-embryo transfer. Fertil Steril. 2012;97:835–42. doi: 10.1016/j.fertnstert.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 2.Coetsier T, Dhont M. Avoiding multiple pregnancies in in-vitro fertilization: who's afraid of single embryo transfer? Hum Reprod. 1998;13:2663–4. doi: 10.1093/humrep/13.10.2663. [DOI] [PubMed] [Google Scholar]
- 3.Mullin CM, Fino ME, Talebian S, Krey LC, Licciardi F, Grifo JA. Comparison of pregnancy outcomes in elective single blastocyst transfer versus double blastocyst transfer stratified by age. Fertil Steril. 2010;93:1837–43. doi: 10.1016/j.fertnstert.2008.12.137. [DOI] [PubMed] [Google Scholar]
- 4.Thurin A, Hausken J, Hillensjo T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392–402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 5.Bechoua S, Astruc K, Thouvenot S, Girod C, Chiron A, Jiminez C, et al. How to demonstrate that eSET does not compromise the likelihood of having a baby? Hum Reprod. 2009;24:3073–81. doi: 10.1093/humrep/dep321. [DOI] [PubMed] [Google Scholar]
- 6.Styer AK, Wright DL, Wolkovich AM, Veiga C, Toth TL. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil Steril. 2008;89:1702–8. doi: 10.1016/j.fertnstert.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 7.Criniti A, Thyer A, Chow G, Lin P, Klein N, Soules M. Elective single blastocyst transfer reduces twin rates without compromising pregnancy rates. Fertil Steril. 2005;84:1613–9. doi: 10.1016/j.fertnstert.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81:551–5. doi: 10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Gerris J, de Neubourg D, Mangelschots K, van Royen E, Vercruyssen M, Barudy-Vasquez J, et al. Elective single day 3 embryo transfer halves the twinning rate without decrease in the ongoing pregnancy rate of an IVF/ICSI programme. Hum Reprod. 2002;17:2626–31. doi: 10.1093/humrep/17.10.2626. [DOI] [PubMed] [Google Scholar]
- 10.Van Montfoort AP, Fiddelers AA, Janssen JM, Derhaag JG, Dirksen CD, Dunselman GA, et al. In unselected patients, elective single embryo transfer prevents all multiples, but results in significantly lower pregnancy rates compared with double embryo transfer: a randomized controlled trial. Hum Re-prod. 2006;21:338–43. doi: 10.1093/humrep/dei359. [DOI] [PubMed] [Google Scholar]
- 11.Kresowik JD, Sparks AE, van Voorhis BJ. Clinical factors associated with live birth after single embryo transfer. Fertil Steril. 2012;98:1152–6. doi: 10.1016/j.fertnstert.2012.07.1141. [DOI] [PubMed] [Google Scholar]
- 12.United States Fertility Clinic Success Rates and Certification Act of 1992: Public Law 102–493. US Statut Large. 1992;106:3146–52. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. American Society for Reproductive Medicine. Society for Assisted Reproductive Technologies . 2010 Assisted reproductive technology success rates: fertility clinic success rates report. United States Department of Health and Human Services; Atlanta, GA: 2012. [Google Scholar]
- 14.Practice Committee SART. American Society for Reproductive Medicine Guidelines on the number of embryos transferred. Fertil Steril. 2004;82:773–4. doi: 10.1016/j.fertnstert.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Joshi N, Kissin D, Anderson JE, Session D, Macaluso M, Jamieson DJ. Trends and correlates of good perinatal outcomes in assisted reproductive technology. Obstet Gynecol. 2012;120:843–51. doi: 10.1097/AOG.0b013e318269c0e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern JE, Cedars MI, Jain T, Klein NA, Beaird CM, Grainger DA, et al. Assisted reproductive technology practice patterns and the impact of embryo transfer guidelines in the United States. Fertil Steril. 2007;88:275–82. doi: 10.1016/j.fertnstert.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Dessolle L, Freour T, Ravel C, Jean M, Colombel A, Darai E, et al. Predictive factors of healthy term birth after single blastocyst transfer. Hum Reprod. 2011;26:1220–6. doi: 10.1093/humrep/der039. [DOI] [PubMed] [Google Scholar]
- 18.Sifer C, Sermondade N, Poncelet C, Hafhouf E, Porcher R, Cedrin-Durnerin I, et al. Biological predictive criteria for clinical pregnancy after elective single embryo transfer. Fertil Steril. 2011;95:427–30. doi: 10.1016/j.fertnstert.2010.07.1055. [DOI] [PubMed] [Google Scholar]
- 19.Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289–96. doi: 10.1093/humrep/der325. [DOI] [PubMed] [Google Scholar]
- 20.Min JK, Breheny SA, MacLachlan V, Healy DL. What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: the [Birth Emphasizing a Successful Singleton at Term] BESST endpoint for assisted reproduction. Hum Reprod. 2004;19:3–7. doi: 10.1093/humrep/deh028. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan EA, Wang YA, Hayward I, Chambers GM, Illingworth P, McBain J, et al. Single embryo transfer reduces the risk of perinatal mortality, a population study. Hum Reprod. 2012;27:3609–15. doi: 10.1093/humrep/des315. [DOI] [PubMed] [Google Scholar]
- 22.Practice Committee of the American Society for Reproductive M. Practice Committee of the Society for Assisted Reproductive Technologies Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–9. doi: 10.1016/j.fertnstert.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 23.Veleva Z, Vilska S, Hyden-Granskog C, Tiitinen A, Tapanainen JS, Martikainen H. Elective single embryo transfer in women aged 36–39 years. Hum Reprod. 2006;21:2098–102. doi: 10.1093/humrep/del137. [DOI] [PubMed] [Google Scholar]
- 24.Papanikolaou EG, Camus M, Kolibianakis EM, van Landuyt L, van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–46. doi: 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 25.Seifer DB, Frazier LM, Grainger DA. Disparity in assisted reproductive technologies outcomes in black women compared with white women. Fertil Steril. 2008;90:1701–10. doi: 10.1016/j.fertnstert.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto VY, Luke B, Brown MB, Jain T, Armstrong A, Grainger DA, et al. Racial and ethnic disparities in assisted reproductive technology outcomes in the United States. Fertil Steril. 2010;93:382–90. doi: 10.1016/j.fertnstert.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifer DB, Zackula R, Grainger DA, Society for Assisted Reproductive Technologies Writing Group R Trends of racial disparities in assisted reproductive technology outcomes in black women compared with white women: Society for Assisted Reproductive Technologies 1999 and 2000 vs. 2004–2006. Fertil Steril. 2010;93:626–35. doi: 10.1016/j.fertnstert.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 28.Baker VL, Luke B, Brown MB, Alvero R, Frattarelli JL, Usadi R, et al. Multivariate analysis of factors affecting probability of pregnancy and live birth with in vitro fertilization: an analysis of the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril. 2010;94:1410–6. doi: 10.1016/j.fertnstert.2009.07.986. [DOI] [PubMed] [Google Scholar]
- 29.Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. Racial and ethnic disparities in assisted reproductive technology pregnancy and live birth rates within body mass index categories. Fertil Steril. 2011;95:1661–6. doi: 10.1016/j.fertnstert.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Wellons MF, Fujimoto VY, Baker VL, Barrington DS, Broomfield D, Catherino WH, et al. Race matters: a systematic review of racial/ethnic disparity in Society for Assisted Reproductive Technology reported outcomes. Fertil Steril. 2012;98:406–9. doi: 10.1016/j.fertnstert.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luke B, Brown MB, Grainger DA, Cedars M, Klein N, Stern JE, et al. Practice patterns and outcomes with the use of single embryo transfer in the United States. Fertil Steril. 2010;93:490–8. doi: 10.1016/j.fertnstert.2009.02.077. [DOI] [PubMed] [Google Scholar]