Abstract
Group sequential trials are one important instance of studies for which the sample size is not fixed a priori but rather takes one of a finite set of pre-specified values, dependent on the observed data. Much work has been devoted to the inferential consequences of this design feature. Molenberghs et al (2012) and Milanzi et al (2012) reviewed and extended the existing literature, focusing on a collection of seemingly disparate, but related, settings, namely completely random sample sizes, group sequential studies with deterministic and random stopping rules, incomplete data, and random cluster sizes. They showed that the ordinary sample average is a viable option for estimation following a group sequential trial, for a wide class of stopping rules and for random outcomes with a distribution in the exponential family. Their results are somewhat surprising in the sense that the sample average is not optimal, and further, there does not exist an optimal, or even, unbiased linear estimator. However, the sample average is asymptotically unbiased, both conditionally upon the observed sample size as well as marginalized over it. By exploiting ignorability they showed that the sample average is the conventional maximum likelihood estimator. They also showed that a conditional maximum likelihood estimator is finite sample unbiased, but is less efficient than the sample average and has the larger mean squared error. Asymptotically, the sample average and the conditional maximum likelihood estimator are equivalent.
This previous work is restricted, however, to the situation in which the the random sample size can take only two values, N = n or N = 2n. In this paper, we consider the more practically useful setting of sample sizes in a the finite set {n1, n2, …, nL}. It is shown that the sample average is then a justifiable estimator , in the sense that it follows from joint likelihood estimation, and it is consistent and asymptotically unbiased. We also show why simulations can give the false impression of bias in the sample average when considered conditional upon the sample size. The consequence is that no corrections need to be made to estimators following sequential trials. When small-sample bias is of concern, the conditional likelihood estimator provides a relatively straightforward modification to the sample average. Finally, it is shown that classical likelihood-based standard errors and confidence intervals can be applied, obviating the need for technical corrections.
Keywords: Exponential Family, Frequentist Inference, Generalized Sample Average, Joint Modeling, Likelihood Inference, Missing at Random, Sample Average
1 Introduction
Principally for ethical and economic reasons, group sequential clinical trials are in common use (Wald, 1945; Armitage, 1975; Whitehead, 1997; Jennison and Turnbull, 2000). Tools for constructing such designs, and for testing hypotheses from the resulting data, are well established both in terms of theory and implementation. By contrast, issues still surround the problem of estimation (Siegmund, 1978; Hughes and Pocock, 1988; Todd, Whitehead, and Facey, 1996; Whitehead, 1999) following such trials. In particular, various authors have reported that standard estimators such as the sample average are biased. In response to this, various proposals have been made to remove or at least alleviate this bias and its consequences (Tsiatis, Rosner, and Mehta, 1984; Rosner and Tsiatis, 1988; Emerson and Fleming, 1990). An early suggestion was to use a conditional estimator for this Blackwell (1947).
To successfully address the bias issue, it is helpful to understand its origins. Lehman (1950) showed that it stems from the so-called incompleteness of the sufficient statistics involved, which in turn implies that there can be no minimum variance unbiased linear estimator. Liu and Hall (1999) and Liu et al (2006) explored this incompleteness in group sequential trials, for outcomes with both normal and one-parameter exponential family distributions. For these distributions, Molenberghs et al (2012) and Milanzi et al (2012) embedded the problem in the broader class with random sample size, which includes, in addition to sequential trials, incomplete data, completely random sample sizes, censored time-to-event data, and random cluster sizes. In so doing, they were able to link incompleteness to the related concepts of ancillarity and ignorability in the missing-data sense. By considering the conventional sequential trial with a deterministic stopping rule as a limiting case of a stochastic stopping rule, these authors were able to derive properties of families of linear estimators as well as likelihood-based estimators. The key results are as follows:(1) there exists a maximum likelihood estimator that conditions on the realized sample size (CL), which is finite sample unbiased, but has slightly larger variance and mean square error (MSE) than the SA ;(2) the sample average (SA) exhibits finite sample bias, although it is asymptotically unbiased; (3) apart from the exponential distribution setting, there is no optimal linear estimator, although the sample average is asymptotically optimal; (4) the validity of the sample average as an estimator also follows from standard ignorable likelihood theory.
Evidently, the CL is unbiased both conditionally and marginally with respect to the sample size. In contrast, the CL is marginally unbiased, but there exist classes of stopping rules where, conditionally on the sample size, there is asymptotic bias for some values of the sample sizes. Surprisingly, this is not of concern. Milanzi et al (2012) showed this for the case of two possible sample sizes, N = n and N = 2n. With such a stopping rule, it is possible that, for example when N = n, the bias grows unboundedly with n; when this happens though, the probability that N = n shrinks to 0 at the same rate. If strict finite sample unbiasedness is regarded as essential, the conditional MLE can be used, which, like the MLE, also admits the standard likelihood-based precision measures, although it is computationally intensive. This is a very important result and should be contrasted with the various precision estimators that have been developed in the past.
On the other hand, developments in Molenberghs et al (2012) and Milanzi et al (2012) show that despite finite sample bias, a correction may not be strictly necessary for SA. Further, its likelihood basis, implies it can be used in conjunction with standard likelihood-based measures of precision, such as standard errors and associated confidence intervals to provide valid inferences.
A major limitation of Molenberghs et al (2012) and Milanzi et al (2012) is the restriction to two looks of equal size. It is the main aim of this paper to extend this work to the practically more useful setting of multiple looks of potentially different sample sizes.
In Section 2, we introduce notation, describe the setting, the models, and the associated generic problem. In Section 3, we study the problems of incompleteness when using a stochastic stopping rule. The class of generalized sample averages in introduced in Section 6, and conditional and joint maximum likelihood estimators are derived. Their asymptotic properties are studied in Section 7. A simulation study is described in the Supplementary Materials, Section A.
2 Problem and Model Formulation
Consider a sequential trial with L pre-specified looks, with sample sizes n1 < n2 < … , < nL. Assume that there are nj i.i.d. observations Y1, …, Ynj, from the jth look that follow an exponential family distribution with density
| (1) |
for θ the natural parameter, a(θ) the mean generating function, and h(y) the normalizing constant. Subsequent developments are based on a generic data-dependent stochastic stopping rule, which we write
| (2) |
where also has an exponential family density:
| (3) |
Our inferential target is the parameter θ, or a function of this.
2.1 Stochastic Rule As A Group Sequential Stopping Rule
While the stopping rule seems different from the ones frequently used, it will later on be clear as to how it can be specified to conform to the commonly used stopping rules in the sequential trials. For instance, when the conditional probability of stopping of an exponential family form is chosen, e.g.,
| (4) |
then an appealing form for the marginal stopping probability can be derived. Here can be seen as an exponential family member, underlying the stopping process. When the outcomes Y and hence K do not range over the entire real line, the lower integration limit in (4) should be adjusted accordingly, and the function A(k) should be chosen so as to obey the range restrictions. It is convenient to assume that has no free parameters; should there be the need for such, then they can be absorbed into A(k). Hence, we can write
| (5) |
Using (3) and (5), the marginal stopping probability becomes:
| (6) |
where
Milanzi et al (2012) studied in detail the behavior of stopping rules where
with αj, β and m are constants specific to a design.
Choosing β → ∞ and β → −∞ results into deterministic stopping or continuing thus corresponding to the stopping rules commonly used in sequential trials. The trial is stopped when (6) is greater than a randomly generated number from a Uniform(0,1). Note that the higher the evidence against (for) the null hypothesis, the higher the probability to stop. In the specific example of normally distributed responses, (1) can be chosen as standard normal. The value of α is paramount to deciding the behavior of stopping boundaries. Consider, for example, O’Brien and Fleming stopping boundaries where it is difficult to stop in early stages; one can then specify αj such that the probability of stopping increases with the stages. In addition to the computational advantages and the associated practicality, we use the stochastic rule to maintain the focus of this paper, which is estimation.
3 Incomplete Su cient Statistics
Several concepts play a crucial role in determining the properties of estimators following sequential trials: incompleteness, a missing at random (MAR) mechanism, ignorability, and ancillarity (Molenberghs et al, 2012). We consider the role of incompleteness first: a statistic s(Y) of a random variable Y, with Y belonging to a family Pθ, is complete if, for every measurable function g(·), E[g{s(Y)}] = 0 for all θ, implies that Pθ[g{s(Y)} = 0] = 1 for all θ (Casella and Berger, 2001, pp. 285–286). Incompleteness is central to the various developments (Liu and Hall, 1999; Liu et al, 2006; Molenberghs et al, 2012) because of the the Lehman-Scheffé theorem which states that “if a statistic is unbiased, complete, and sufficient for some parameter θ, then it is the best mean-unbiased estimator for θ,” (Casella and Berger, 2001). In the present setting, the relevant sufficient statistic is not complete, and so the theorem cannot be applied here.
In line with extending the work of Molenberghs et al (2012) and Milanzi et al (2012) to a general number of looks, we explore incompleteness and its consequences in studies with more than two looks, using the stochastic rule.
In a sequential setting, a convenient sufficient statistic is (K, N). Following the developments in the above papers, the joint distribution for (K, N) is:
| (7) |
| (8) |
| (9) |
If (K, N) were complete, then there would exist a function g(K, N) such that E[g(K, N)] = 0 if and only if g(K, N) = 0, implying that
| (10) |
with
Tedious but straightforward algebra results into:
Assigning, for example, arbitrary constants to g(n1, kn1), …, g(nL–1, knL–1), a value can be found for g(nL, knL) ≠ 0, contradicting the requirement for (K, N) to be complete, hence establishing incompleteness. From applying the Lehmann-Scheffé theorem, it follows that no best mean-unbiased estimator is guaranteed to exist. The practical consequence of this is that even estimators as simple as a sample average need careful consideration and comparison with alternatives. Nevertheless, the situation is different for non-linear mean estimators as illustrated for the conditional likelihood estimator.
4 Unbiased Estimation: Conditional Likelihood
An important drawback of linear mean estimators in the context of sequential trials is their finite-sample bias. In connecting missing data and sequential trials theory, Molenberghs et al (2012) provided a factorization for the joint distribution of observed data and sample size that leads to an unbiased conditional likelihood mean estimator. For an arbitrary number of looks, the conditional distribution for N = n1 is:
from which the log-likelihood, score, Hessian, and information follow as:
| (11) |
| (12) |
| (13) |
where
Similarly for N = nj where j > 1, we have the conditional distribution:
| (14) |
| (15) |
| (16) |
The following expressions for the likelihood, score, Hessian, and information are:
| (17) |
| (18) |
| (19) |
| (20) |
where
The overall information for the conditional likelihood estimator is given by
| (21) |
From scores (12) and (18), it can be seen that conditional likelihood estimator is unbiased. Clearly, the bias correction in the CLE mirrors the bias expression of the SA, as can be seen from (33). Upon writing (12) and (18), as
the bias-correction factor in the CLE becomes even more apparent.
In contrast to the case of a fixed sample size, conditioning on the sample size in this case leads to loss of information, as can be seen by the subtraction of a positive factor in (21). This is a consequence of conditioning on a non-ancillary statistic, as discussed in Casella and Berger (2001).
Additionally, despite having the appealing property of finite sample unbiasedness, its non-linear nature comes with computational problems. Note that maximization of (11) and (14) requires simultaneous optimization and solution of multiple integrals. Unless small-sample unbiasedness is of paramount importance, consideration has to be given to the time and complexity of implementing the conditional likelihood.
5 Joint Likelihood Estimation
Likelihood methods, while allowing for a unified treatment across a variety of settings (e.g., data types, stopping rules), they do rely heavily on correct parametric specification. This should be taken into account when opting for a particular approach.
Selection model factorization for the joint distribution of observed data and sample size also leads to joint likelihood estimation (JLE). Employing the separability and ignorability concepts from the missing data theory, it is known that under a missing at random (MAR) assumption, maximizing the joint likelihood is equivalent to maximizing the likelihood of the observed data only. This is crucial when considered against the background of Kenward and Molenberghs (1998), where it was shown that for frequentist inference and under the missing at random (MAR) assumption, the observed information matrix gives valid inferences. Other properties of joint likelihood estimation are explored below.
The joint distribution of the sufficient statistics (K, N) is given by:
| (22) |
Because our stopping rule is independent of the parameter of interest, the log-likelihood, the score, the Hessian, and the expected information simplify as follows:
| (23) |
| (24) |
| (25) |
| (26) |
In deriving the score (24) from (23) the rightmost term drops out, i.e., conventional ignorability applies. As a consequence, the maximum likelihood estimator (MLE) reduces to , the SA.
Because of the bias, a finite sample comparison among estimators needs to be based on the MSE. For , this is
| (27) |
For the conditional likelihood estimate (CLE) the MSE is:
| (28) |
where
The condition that MSE is equivalent to the requirement that
holds. For the special case of equal sample sizes this can never be true, hence the SA has the smaller MSE. More generally, neither is uniformly superior in terms of MSE.
6 Generalized Sample Averages
To get a broad picture of the properties of the SA, which follows from JLE, we embed it into a broader class of linear estimator. Extending the definition in Molenberghs et al (2012), the generalized sample average (GSA) can be be defined as:
| (29) |
for a set of constants a1, …, aL. The SA follows as the special case where each aj = 1. To explore the properties of the GSA, we make use of the fact that:
and derive three useful identities:
| (30) |
| (31) |
Using identities (30) and (31), the expectation of (29) can then be formulated as
| (32) |
establishing the bias as a function of the difference between the marginal and conditional means. When (32) is unbiased, at least one value among a1, …, aL will depend on μ. This means that none of the GSA can be uniformly unbiased. Focusing on the SA, the expectation reduces to
| (33) |
from which we get the bias as
| (34) |
Thus, the SA is unbiased when the conditional and marginal means are equal.
7 Asymptotic Properties
We now turn to the large-sample properties of the estimators discussed in the previous sections. When N → ∞, approximately K ~ N(Nμ, Nσ2), so normal-theory arguments can be used. Considering a first-order Taylor series expansion of F(knj) around njμ results in F(knj) ≈ F(njμ)+F’(njμ)(knj – njμ). Without loss of generality, consider a class of stopping rules for which . In this setting, the expressions derived above can be approximated by
These approximations will be useful in what follows.
7.1 Asymptotic Bias
Conditional Likelihood Estimation
We turn now to the asymptotic conditional behavior of the bias of the sample average given the sample size. Two cases are considered:
Case I
and . For this case , for j = 1, … , L.
Case II
Here, both the function F(·) and its first derivative F(·) converge to zero. When this happens, it does so for all but one of the sample sizes that can possibly be realized. The one exception is the sample size that will be realized, asymptotically, with probability one. Without loss of generality, we illustrate this case for stopping at the first look, assuming that the sample size realized at the first look corresponds to a set of values for μ that do not contain the true one. Thus, and . This case can correspond for particular forms of F(knj). Given that K is asymptotically normally distributed, letting F(K) = Φ(k) is a mathematically convenient choice from which it follows that F(njμ) = Φ(njμ). Consider first N = n1. Then,
of which the right hand term approaches 0/0. We therefore apply l’Hopital’s rule and obtain:
with the sign opposite to that of μ. Hence, conditional on the fact that stopping occurs after the first look, the estimate may grow in an unbounded way. However, recalling that F(nμ), the probability of stopping when N = n1, also approaches zero, these extreme estimates are a the same time also extremely rare. In the same case, for N = nj (j > 1), . So for these sample sizes no asymptotic bias occurs.
Milanzi et al (2012) showed that a large class of stopping rules corresponds to either Case I or Case II. For example, for stopping rule Φ(α + βk/n), they found that Case I applies. Switching to Φ(α + βk), F’(nμ) = βϕ(α + βnμ) which again tends to zero. However, Φ(α + βnμ) may tend to either zero or one. For a general rule F(k) = Φ(α + βknm), with m any real number, F’(nμ) converges to zero whatever m is. Further, F(nμ) converges to Φ(α + βμ) for m = −1, Φ(α) for m < −1, and Φ(±∞) (i.e., 0 or 1) for m > −1.
Joint Likelihood Estimation
Recall that the bias for the SA was given by (34), which asymptotically tends to the limit
Although the sample average is generally finite-sample biased for data-dependent stopping rules, it is asymptotically unbiased and hence can be considered an appropriate candidate for practical use following a sequential trial. Emerson (1988) established the same result for two possible looks and further noted that this property is not relevant in group sequential trials, because large sample sizes are unethical, hence making the study of small sample properties crucial. On the other hand, results from a comprehensive analysis, comparing randomized controlled trials (RCTs) stopped for early benefit (truncated) and RCTs not stopped for early benefit (non-truncated), indicated that treatment effect was over-estimated in most of truncated RCTs regardless of the pre-specified stopping rule used (Bassler et al , 2010). They further advocate stopping rules that demand large number of events. In their exploration of properties of estimators, Milanzi et al (2012) showed that in the general class of linear mean estimators, only the sample average has the asymptotic unbiasedness property, thus giving it an advantage in cases where asymptotic unbiasedness would play a role. The sample average is asymptotically unbiased in all cases, and additionally conditionally asymptotically unbiased, even in the case of an arbitrary number of looks. Further, under the usual likelihood regularity conditions, the SA is then consistent and asymptotically normally distributed, and the likelihood-based precision estimator and its corresponding confidence intervals are valid. Care has to be exercised when working under the MAR assumption, as is the case here, because the observed information matrix rather than the expected information matrix should be used to obtain precision estimators to ensure their validity. Kenward and Molenberghs (1998) noted that, provided that use is made of the likelihood ratio, Wald, or score statistics based on the observed information, then reference to a null asymptotic χ2 distribution will be appropriate.
This conventional asymptotic behavior contrasts with the idiosyncratic small-sample properties of the SA derived in Section 6.
7.2 Asymptotic Mean Square Error
Given that the bias for the sample average tends to zero as the sample size increases and that , it follows that
8 Simulation Study
8.1 Design
The simulation study has been designed to corroborate the theoretical findings on the behavior of the likelihood estimators, in comparison to commonly used biased adjusted estimators. Assume a clinical trial comparing a new therapy to a control, designed to follow O’Brien and Fleming’s group sequential plan with four interim analyses.
The objective of the trial is to show that the mean response from the new therapy is higher than that of the control group. Let Yit ~ N(μt, 1) and Yic ~ N(μc, 1) be the responses from subject i in the therapy and control groups, respectively. The null hypothesis is formulated as H0 : θ = μt – μc = 0 vs. H1 : θ = θ1 > 0. Further, allow a type I error of 2.5% and 90% power to detect the clinically meaningful difference.
Given that we are interested in asymptotic behavior, different values of the clinically meaningful difference, θ1 = 0.5, 0.25, and 0.15 are considered to achieve different sample sizes, with smaller θ1 corresponding to larger sample size.
With the settings described above, datasets are generated as follows; at each stage, Yit ~ N(2, 1), i = 1 …nj, j = 1 …4 and Yic ~ N(μc, 1), where μc = 1.5, 1.75, and 1.85 for the first, second, and third setting, respectively. These also serve as the true mean values under which the bias is being considered.
Estimation proceeds by obtaining the maximum likelihood estimator (sample average: ) at each stage and applying the stopping rule:
where β = 100 to represent the rules applied to the group sequential trials case (Milanzi et al, 2012). To follow the behavior of O’Brien and Fleming boundaries (where early stopping is difficult), a value of α is chosen to make sure that the probability of stopping increases with the increase in number of looks, i.e., αj = [2(h – j + 1)]/hα1, where α1 = −50, −25, and −15, for θ1 = 0.5, 0.25, and 0.15, respectively, and h is the number of planned looks. Obviously, the choice of αj depends on the design and goals of the trial. In this setting, α1 was chosen such that P(N = n3∣θ = 1) ≥ 0.5 and to make early stopping difficult. The decision to stop is made when F(knj) > U, where U ~ Uniform(0, 1); otherwise, we continue. For example if F(knj) = 0.70, then the probability of continuing is 30% and for large values of β, F(knj) ∈ {0, 1}.
The objective of the simulation is to show that the performance of the CLE as the mean estimator after a group sequential trial and compare MLE to other bias adjusted estimators. We further show that MLE confidence intervals obtained by using the observed information matrix, lead to valid conclusions.
Other estimators obtained include: the mean unbiased estimator (MUE), the bias adjusted estimator (BAM; Todd, Whitehead, and Facey 1996), and Rao’s bias-adjusted estimator (RBADJ; Emerson and Fleming 1990).
Additional simulations with two possible looks and a smaller value of β for both joint and conditional likelihood are presented in the Appendix.
8.2 Results
Table 1 gives the mean estimates for different estimators of θ. On average, the MLE exhibits large relative bias compared to the bias adjusted estimates, for example, for θ1 = 0.15, which corresponds to a maximum sample size of 1949, relative bias for MLE is 6% compared to 0.7% for CLE. The conditional likelihood estimator performs as expected with consistently small bias under all the three scenarios. On the other hand, the MLE shows asymptotically unbiased behavior, seen by the reduction (though small) in relative bias as sample size increases. This is not the same for BAM and RBADJ. While point estimates are useful in giving the picture of the magnitude of the difference, confidence intervals (CI) are highly important in decision making. A comparison of adjusted confidence intervals provided with the RCT design package in R (Emerson et al, 2012), to the likelihood based confidence intervals, obtained by using observed variance as precision estimates, indicates that their coverage probabilities are comparable. The coverage probabilities were (94.6%, 94.6%, 97.6%) for the adjusted CI and (93.8%, 92.8%, 96.8%) for MLE based CI, for the three settings in the order of increasing sample size. Using the same design parameters, we also investigated the type I error rate for MLE and adjusted estimators, by setting θ1 = 0 and obtaining the percentage times the confidence interval does not contain zero. Type I error rates for likelihood based CI were (5.6%,6.4%,2.8%), which are similar to those based on adjusted CIs, (5.4%,4.8%,2.8%) for the three settings in the order of increasing sample size. Certainly, using either of the CIs will lead to similar conclusions, which makes the simpler and well known sample average a good estimator candidate for analysis after group sequential trials.
Table 1.
Mean estimates (Est.) and relative bias (R.Bias) for the three different settings of O’Brien and Fleming’s design. Parameters common to all the three settings include, power=90%, type I error=0.025, H0 : θ = 0 vs. H1 : θ = θ1 > 0, where only the detectable difference (θ1) was changed to initiate change in maximum sample size (Size). MLE is the maximum likelihood estimate, BAM is the bias-adjusted maximum likelihood estimator, RBADJ is the Rao bias-adjusted estimator, MUE is the median unbiased estimator, and CLE is the conditional likelihood estimator.
| MLE |
BAM |
RBADJ |
MUE |
CLE |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size | Est. | R.Bias | Est. | R.Bias | Est. | R.Bias | Est. | R.Bias | Est. | R.Bias |
| 176 | 0.5448 | (0.0895) | 0.5142 | (0.0285) | 0.5019 | (0.0037) | 0.5251 | (0.0502) | 0.5026 | (0.0052) |
| 702 | 0.2665 | (0.0661) | 0.2508 | (0.0031) | 0.2473 | (0.0108) | 0.2557 | (0.0228) | 0.2476 | (0.0094) |
| 1949 | 0.1595 | (0.0635) | 0.1489 | (0.0070) | 0.1469 | (0.0209) | 0.1520 | (0.0130) | 0.1511 | (0.0071) |
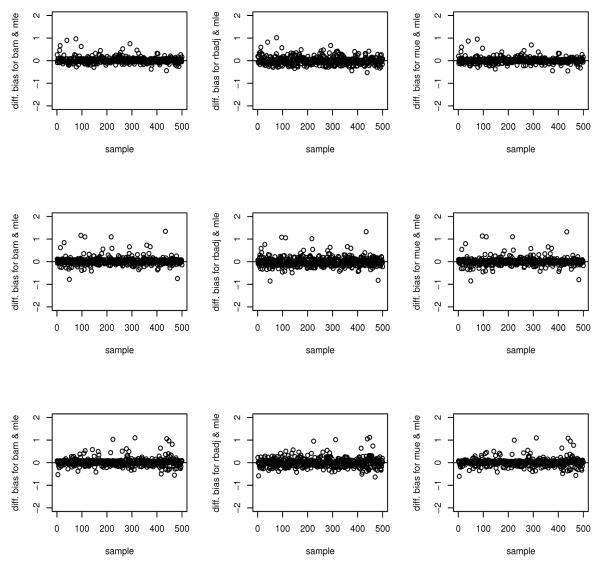
At first sight, it looks like there may be less practical interest, given this similarity. However, the implications should not be underestimated. There is a general feeling that adjustments need to be made. As is clear from earlier work in the literature and from this manuscript, corrections are computationally challenging. In contrast, the standard joint likelihood estimator being the ordinary sample average, is extremely simple. Thus, our results may simplify calculations in important ways. We also explore the bias of each of the estimators at the sample level in contrast to the averaged bias as presented in Table 1. Recall that we had 500 samples for each setting, Table 2 gives the proportion of samples whose estimates’ relative bias fell into a specified category. The CLE had a reverse trend of the other estimators where a only few estimated had large bias. Indeed, it is hard to pick a preferred estimator among the others estimators based on these results since each of the estimator has about 75% of the estimates having relative bias of > 10%. It is also clear from Figure 1, which plot the difference in relative bias, between each of the bias adjusted estimates and MLE, that none of the estimates discussed above is uniformly unbiased in comparison to MLE, i.e is some instances MLE may do better.
Table 2.
Results from three different settings of O’Brien and Fleming’s design. Parameters common to all three settings include: power=90%, type I error=0.025, H0 : θ = 0 vs. H1 : θ = θ1 > 0, where only the detectable difference (θ1) was changed to initiate change in maximum sample size (Size). Out of 500 datasets generated for each setting, we compare the percentage of estimates (Prop. as a percentage) whose relative bias falls in the specified range (R.Bias as a percentage). MLE is the maximum likelihood estimate, BAM is the biased adjusted maximum likelihood estimator, RBADJ is Rao’s bias-adjusted estimator, MUE is the median unbiased estimator, and CLE is the conditional likelihood estimator.
| Prop.(%) |
||||||
|---|---|---|---|---|---|---|
| θ1(Size) | R.Bias(%) | BAM | RBADJ | MUE | MLE | CLE |
| 0.5(176) | ≤0.99 | 2.6 | 2.0 | 2.2 | 2.2 | 76.3 |
| 1 – 4.99 | 8.4 | 11.4 | 11.0 | 10.6 | 13.2 | |
| 5 – 10 | 10.6 | 11.6 | 12.6 | 15.0 | 7.9 | |
| >10 | 78.4 | 75.0 | 74.2 | 72.2 | 2.6 | |
|
| ||||||
| 0.25(702) | ≤0.99 | 2.0 | 3.2 | 1.4 | 2.6 | 81.3 |
| 1 – 4.99 | 7.2 | 9.0 | 8.8 | 9.0 | 12.5 | |
| 5 – 10 | 9.4 | 9.8 | 10.8 | 9.8 | 2.1 | |
| > 10 | 81.4 | 78.0 | 79.0 | 78.6 | 4.2 | |
|
| ||||||
| 0.15(1949) | ≤0.99 | 2.6 | 1.8 | 1.4 | 2.2 | 55.6 |
| 1 – 4.99 | 7.4 | 13.2 | 8.0 | 13.2 | 33.3 | |
| 5 – 10 | 9.2 | 9.0 | 11.8 | 11.0 | 8.9 | |
| > 10 | 80.8 | 76.0 | 77.6 | 74.4 | 2.2 | |
Figure 1.
Difference in relative bias between MLE and each the biased adjusted estimates (BAM, RBADJ and MUE). The first row is for θ1 = 0.5, second row, θ1 = 0.25 and third row, ∣
9 Concluding Remarks
As a result of the bias associated with joint maximum likelihood estimators following sequential trials, much work has been applied to providing alternative estimators. The origin of the problem lies with the incompleteness of the sufficient statistic for the mean parameter (Lehman, 1950), implying, among others, that there is no best unbiased linear mean estimator.
Using stochastic stopping rules, which encompass the deterministic stopping rules used in sequential trials as special cases, we have studied the properties of joint maximum likelihood estimators afresh, in an attempt to enhance our understanding of the behavior of estimators (for both bias and precision) based on data from such studies. We have focused on one-parameter exponential family distributions, which encompasses several response types, including but not limited to binary, normal, Poisson, exponential, and time-to-event data.
First, the incompleteness of the sufficient statistic when using a stochastic stopping rule has been established. Using a generalized sample average, it is noted that in almost no case is there an unbiased estimator. Even when such an estimator does exist, with a completely random sample size, it cannot be uniformly best.
Second, there exist an unbiased estimator resulting from the likelihood of the observed data conditional on the sample size. While appealing, the conditional estimator is computationally more involved, because there is no closed-form solution. Although for a sequential trial with a deterministic stopping rule, the ordinary sample average is finite-sample biased, it can be been shown both directly and through likelihood arguments, that it is asymptotically unbiased and so remains a good candidate for practical use. Further, it is computationally trivial, has a correspondingly simple estimator of precision, derived from the observed information matrix and hence a well behaved asymptotic likelihood-based confidence interval. In addition, the mean square error of the sample average is smaller than that of the estimator based on the conditional likelihood. Asymptotically, the mean square errors of both estimators converge.
Third, there is the subtle issue that the sample average may be asymptotically biased for certain stopping rules, when its expectation is considered conditionally on certain values of the sample size. However, this is not a real practical problem because this occurs only for sample sizes that have asymptotic probability zero of being realized. We placed emphasis on joint and conditional likelihood estimators. While in the former the stopping rule is less present than sometimes thought, it is not in the latter. Also, when alternative frequentist estimators are considered, the stopping rule is likely to play a role in synchrony with the rule’s influence on hypothesis testing due to the duality between hypothesis testing and confidence intervals.
While in some circumstances other sources of inaccuracy may overwhelm the issue studied here, we believe that it is useful to bring forward implications of our findings for likelihood-based estimation.
Our findings, especially for the simulations in the Appendix, indicate that bias decreases relatively rapidly with sample size, but there are subtle differences depending on stopping rule considered. In this sense, fixed rules are different from Z-statistic based rules (Emerson 1988, p. 5; Jennison and Turnbull, 2000).
In conclusion, the sample average is a very sensible choice for point, precision, and interval estimation following a sequential trial.
Supplementary Material
Acknowledgments
Elasma Milanzi, Geert Molenberghs, Michael G. Kenward, and Geert Verbeke gratefully acknowledge support from IAP research Network P7/06 of the Belgian Government (Belgian Science Policy). The work of Anastasios Tsiatis and Marie Davidian was supported in part by NIH grants P01 CA142538, R37 AI031789, R01 CA051962, and R01 CA085848.
References
- Armitage P. Sequential Medical Trials. Blackwell; Oxford: 1975. [Google Scholar]
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, Heels-Ansedell D, Walter SD, Guyatt GH, the STOPIT-2 Study Group Stopping randomized trials early for benefit and estimation of treatment effects. Systematic review and meta-regression analysis. Journal of the American Medical Association. 2010;303:1180–1187. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- Blackwell D. Conditional expectation and unbiased sequential estimation. Annals of Mathematical Statistics. 1947;18:105–110. [Google Scholar]
- Casella G, Berger RL. Statistical Inference. Duxbury Press; Pacific Grove: 2001. [Google Scholar]
- Emerson SS. PhD dissertation. University of Washington; 1988. Parameter estimation following group sequential hypothesis testing. [Google Scholar]
- Emerson SS, Fleming TR. Parameter estimation following group sequential hypothesis testing. Biometrika. 1990;77:875–892. [Google Scholar]
- Emerson SS, Gillen DL, Kittelson JK, Emerson SC, Levin GP. RCTdesign: Group Sequential Trial Design. R package version 1.0. 2012 [Google Scholar]
- Hughes MD, Pocock SJ. Stopping rules and estimation problems in clinical trials. Statistics in Medicine. 1988;7:1231–1242. doi: 10.1002/sim.4780071204. [DOI] [PubMed] [Google Scholar]
- Jennison C, Turnbull BW. Group Sequential Methods With Applications to Clinical Trials. Chapman & Hall/CRC; London: 2000. [Google Scholar]
- Kenward MG, Molenberghs G. Likelihood based frequentist inference when data are missing at random. Statistical Science. 1998;13:236–247. [Google Scholar]
- Lehmann EL, Stein C. Completeness in the sequential case. Annals of Mathematical Statistics. 1950;21:376–385. [Google Scholar]
- Liu A, Hall WJ. Unbiased estimation following a group sequential test. Biometrika. 1999;86:71–78. [Google Scholar]
- Liu A, Hall WJ, Yu KF, Wu C. Estimation following a group sequential test for distributions in the one-parameter exponential family. Statistica Sinica. 2006;16:165–81. [Google Scholar]
- Milanzi E, Molenberghs G, Alonso A, Kenward MG, Aerts M, Verbeke G, Tsiatis AA, Davidian M. Properties of estimators in exponential family settings with observation-based stopping rules. 2012. Submitted for publication. [DOI] [PMC free article] [PubMed]
- Molenberghs G, Kenward MG, Aerts M, Verbeke G, Tsiatis AA, Davidian M, Rizopoulos D. On random sample size, ignorability, ancillarity, completeness, separability, and degeneracy: sequential trials, random sample sizes, and missing data. Statistical Methods in Medical Research. 2012 doi: 10.1177/0962280212445801. 00, 000–000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner GL, Tsiatis AA. Exact confidence intervals following a group sequential trial: A comparison of methods. Biometrika. 1988;75:723–729. [Google Scholar]
- Siegmund D. Estimation following sequential tests. Biometrika. 1978;64:191–199. [Google Scholar]
- Tsiatis AA, Rosner GL, Mehta CR. Exact confidence intervals following a group sequential test. Biometrics. 1984;40:797–803. [PubMed] [Google Scholar]
- Todd S, Whitehead J, Facey KM. Point and interval estimation following a sequential clinical trial. Biometrika. 1996;83:453–461. [Google Scholar]
- Wald A. Sequential tests of statistical hypotheses. The Annals of Mathematical Statistics. 1945;16:117–186. [Google Scholar]
- Whitehead J. The Design and Analysis of Sequential Clinical Trials. 2nd ed John Wiley & Sons; New York: 1997. [Google Scholar]
- Whitehead J. A unified theory for sequential clinical trials. Statistics in Medicine. 1999;18:2271–2286. doi: 10.1002/(sici)1097-0258(19990915/30)18:17/18<2271::aid-sim254>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.