Abstract
Amino acid residues D24/D25, E99/E100, E360/E361, and D363/E364 in subdomain 1 of Dictyostelium actin were replaced with histidine residues by site-directed mutagenesis. Mutant actins were expressed in Dictyostelium cells and purified to homogeneity. The sliding movement of mutant actin filaments on heavy meromyosin attached to a glass surface was measured to assess the effect of the mutation on the motility of actin. For two C-terminal mutants, force generated by a single actin filament and myosin was also measured. These measurements indicated that both D24/D25 and E99/E100 are involved in ATP-driven sliding, whereas E360/E361/D363/E364 are not essential for ATP-driven sliding and force generation.
Full text
PDF
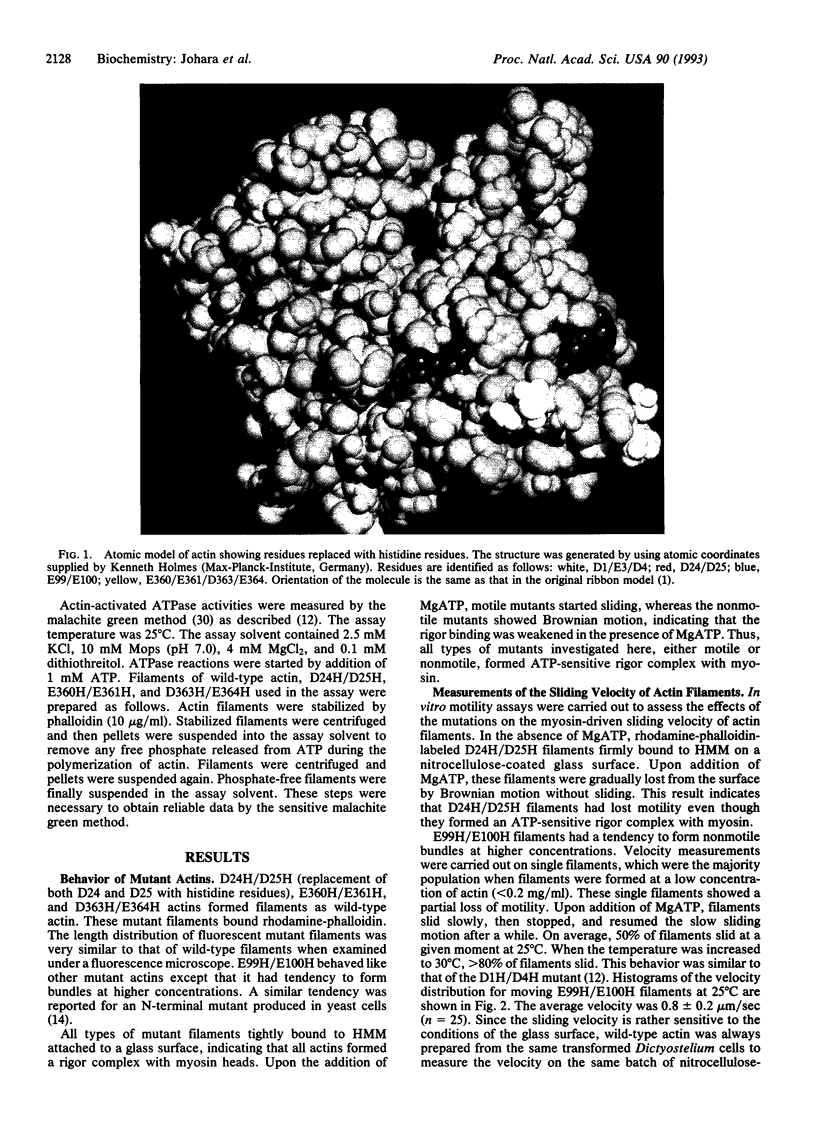
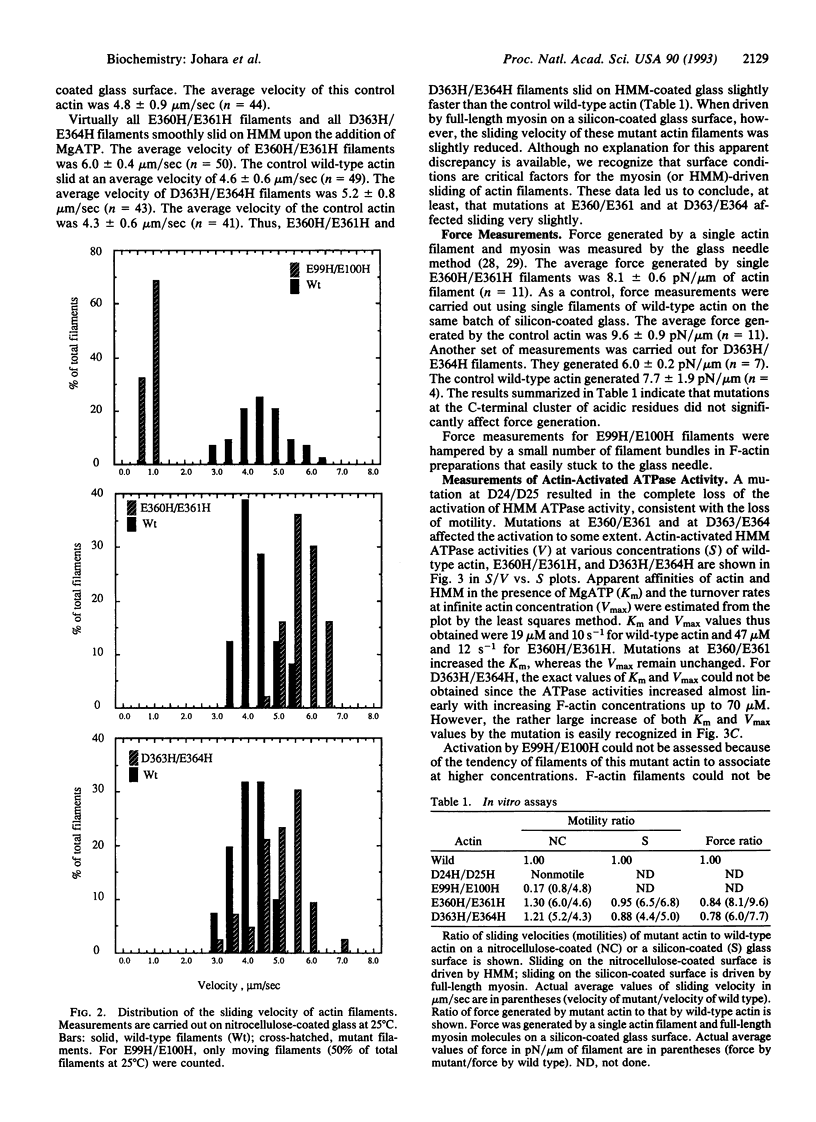
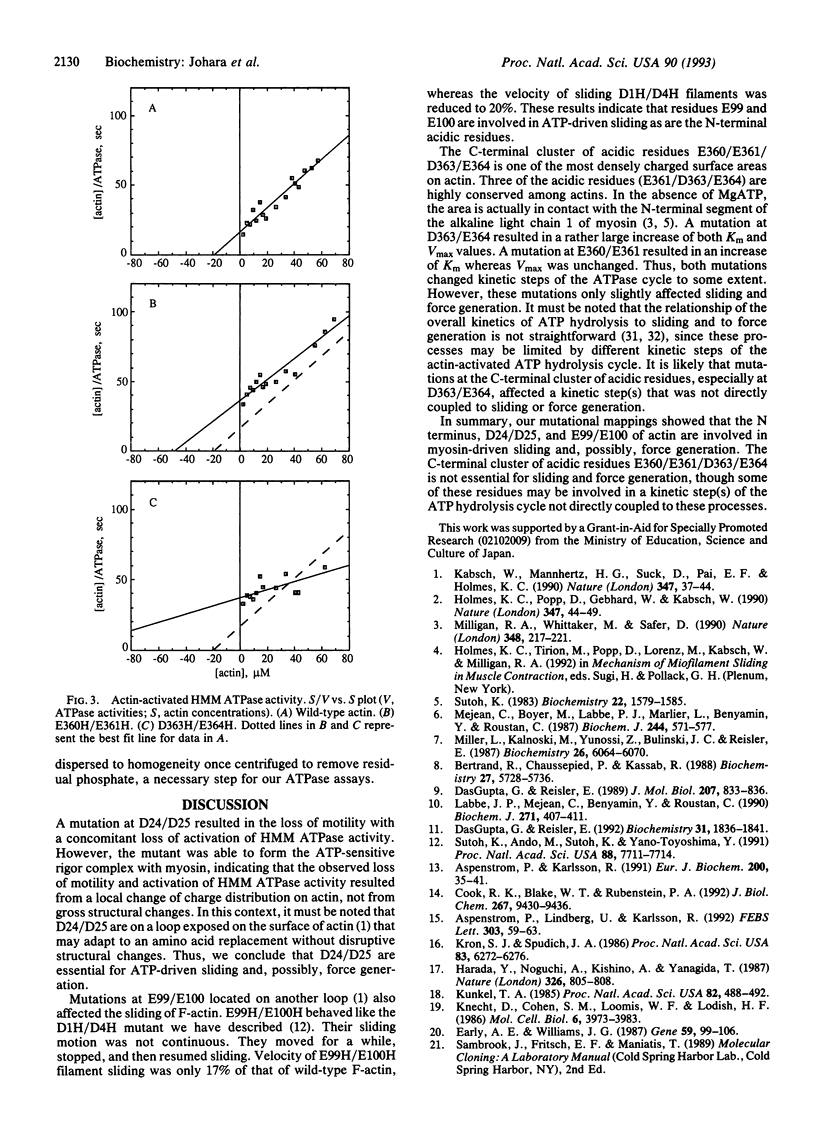

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspenström P., Karlsson R. Interference with myosin subfragment-1 binding by site-directed mutagenesis of actin. Eur J Biochem. 1991 Aug 15;200(1):35–41. doi: 10.1111/j.1432-1033.1991.tb21045.x. [DOI] [PubMed] [Google Scholar]
- Aspenström P., Lindberg U., Karlsson R. Site-specific amino-terminal mutants of yeast-expressed beta-actin. Characterization of the interaction with myosin and tropomyosin. FEBS Lett. 1992 May 25;303(1):59–63. doi: 10.1016/0014-5793(92)80477-x. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Chaussepied P., Kassab R., Boyer M., Roustan C., Benyamin Y. Cross-linking of the skeletal myosin subfragment 1 heavy chain to the N-terminal actin segment of residues 40-113. Biochemistry. 1988 Jul 26;27(15):5728–5736. doi: 10.1021/bi00415a050. [DOI] [PubMed] [Google Scholar]
- Cook R. K., Blake W. T., Rubenstein P. A. Removal of the amino-terminal acidic residues of yeast actin. Studies in vitro and in vivo. J Biol Chem. 1992 May 5;267(13):9430–9436. [PubMed] [Google Scholar]
- DasGupta G., Reisler E. Actomyosin interactions in the presence of ATP and the N-terminal segment of actin. Biochemistry. 1992 Feb 18;31(6):1836–1841. doi: 10.1021/bi00121a036. [DOI] [PubMed] [Google Scholar]
- DasGupta G., Reisler E. Antibody against the amino terminus of alpha-actin inhibits actomyosin interactions in the presence of ATP. J Mol Biol. 1989 Jun 20;207(4):833–836. doi: 10.1016/0022-2836(89)90249-0. [DOI] [PubMed] [Google Scholar]
- Early A. E., Williams J. G. Two vectors which facilitate gene manipulation and a simplified transformation procedure for Dictyostelium discoideum. Gene. 1987;59(1):99–106. doi: 10.1016/0378-1119(87)90270-8. [DOI] [PubMed] [Google Scholar]
- Harada Y., Noguchi A., Kishino A., Yanagida T. Sliding movement of single actin filaments on one-headed myosin filaments. Nature. 1987 Apr 23;326(6115):805–808. doi: 10.1038/326805a0. [DOI] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990 Nov 5;216(1):49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Holmes K. C., Popp D., Gebhard W., Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Howard P. K., Ahern K. G., Firtel R. A. Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res. 1988 Mar 25;16(6):2613–2623. doi: 10.1093/nar/16.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima A., Doi T., Sakurada K., Yanagida T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature. 1991 Jul 25;352(6333):301–306. doi: 10.1038/352301a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Cohen S. M., Loomis W. F., Lodish H. F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986 Nov;6(11):3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Fukui K., Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem. 1986 May;99(5):1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. P., Méjean C., Benyamin Y., Roustan C. Characterization of an actin-myosin head interface in the 40-113 region of actin using specific antibodies as probes. Biochem J. 1990 Oct 15;271(2):407–413. doi: 10.1042/bj2710407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejean C., Boyer M., Labbé J. P., Marlier L., Benyamin Y., Roustan C. Anti-actin antibodies. An immunological approach to the myosin-actin and the tropomyosin-actin interfaces. Biochem J. 1987 Jun 15;244(3):571–577. doi: 10.1042/bj2440571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Kalnoski M., Yunossi Z., Bulinski J. C., Reisler E. Antibodies directed against N-terminal residues on actin do not block acto-myosin binding. Biochemistry. 1987 Sep 22;26(19):6064–6070. doi: 10.1021/bi00393a018. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Nellen W., Datta S., Reymond C., Sivertsen A., Mann S., Crowley T., Firtel R. A. Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 1987;28:67–100. doi: 10.1016/s0091-679x(08)61637-4. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Prochniewicz E., Yanagida T. Inhibition of sliding movement of F-actin by crosslinking emphasizes the role of actin structure in the mechanism of motility. J Mol Biol. 1990 Dec 5;216(3):761–772. doi: 10.1016/0022-2836(90)90397-5. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Ando M., Sutoh K., Toyoshima Y. Y. Site-directed mutations of Dictyostelium actin: disruption of a negative charge cluster at the N terminus. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7711–7714. doi: 10.1073/pnas.88.17.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K. Mapping of actin-binding sites on the heavy chain of myosin subfragment 1. Biochemistry. 1983 Mar 29;22(7):1579–1585. doi: 10.1021/bi00276a009. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Umemoto S., Bengur A. R., Sellers J. R. Effect of multiple phosphorylations of smooth muscle and cytoplasmic myosins on movement in an in vitro motility assay. J Biol Chem. 1989 Jan 25;264(3):1431–1436. [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]