Abstract
Objectives:
Our objective was to determine the extent of testing and costs solely related to diagnosis (the diagnostic odyssey) in a cohort of children with inherited leukodystrophies.
Methods:
We determined all inpatient and outpatient laboratory testing, including brain MRIs obtained for the purpose of diagnosis, over an 8-year time period in a retrospective population cohort of children with inherited leukodystrophies. Costs were determined from an activity-based cost accounting system and were standardized to 2013 constant US dollars.
Results:
Each patient had on average 20 tests (range 2–42 tests), with costs of $4,200 (range $357–$15,611). Diagnostic yield plateaued after 25 tests, and costs increased significantly after 32 tests. Fifty-three percent of patients were diagnosed in 20 or fewer tests, compared with 17% if more than 20 tests were performed.
Conclusions:
Our findings provide details on the amount and costs of testing in children who often undergo a diagnostic odyssey. Our results suggest that diagnostic testing is a relatively modest contributor to the overall health care costs in patients with leukodystrophy, and offer insights into the diagnostic odyssey of children with neurologic impairment.
Inherited leukodystrophies affecting the myelin are a subgroup of diseases causing neurologic impairment.1 Children with neurologic impairment constitute the single largest cohort of inpatient admissions at children's hospitals, accounting for 10% of admissions and 40% of hospital charges in the United States.2,3 For children with inherited leukodystrophies, a specific diagnosis is often difficult to determine, leading to time- and money-consuming testing with low yields and a multiyear “diagnostic odyssey.” Health care costs for patients with leukodystrophy average more than $100,000, and in some patients are higher than $1 million.4 However, a definitive diagnosis is reached in only approximately 50% of patients.1,5 Newer testing approaches based on next-generation sequencing may offer higher testing yields in a shorter time. However, there are no studies to determine what costs are directly related to the determination of a diagnosis. Furthermore, there are no data on the costs of the diagnostic odyssey for any group of disorders. Understanding the costs for diagnosis could clarify options for testing and provide a context for understanding the significant costs and health care burden of leukodystrophies. Our objective was to determine the extent of testing and costs solely related to the diagnostic odyssey in a cohort of children with leukodystrophies.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the institutional review boards at the University of Utah and Intermountain Healthcare (IH).
A retrospective population cohort of 269 patients with inherited leukodystrophies (see criteria below) presenting at Primary Children's Hospital or the University of Utah Pediatric Neurology clinic over an 8-year period from 2002 to 2010 was followed through 2014.1,6 Patients were included if all of their diagnostic care was obtained at IH, if they did not have diagnostic testing at another health care system, and if they had no prior family members with the same diagnosis. Cases were identified through a computerized search of ICD-9-CM diagnosis codes and confirmed by manual chart review and review of laboratory results and MRIs. Criteria for the diagnosis of an inherited leukodystrophy were the same as previously used1: age younger than 19 years at their initial presentation for evaluation of their symptoms that led to the diagnosis of leukodystrophy; if they had brain MRI findings showing abnormalities of white matter signal consistent with the diagnosis of a leukodystrophy; and if the MRI results were obtained before determination of an alternative diagnosis not typically considered a leukodystrophy. Primary Children's Hospital is part of IH, which maintains clinical electronic records and charge codes captured from multiple information systems related to admissions, emergency room, and clinic visits in an enterprise data warehouse.
We manually determined all inpatient and outpatient laboratory testing related to diagnosis, including brain MRIs performed after the MRI performed at presentation. We confirmed the manual data by comparison with data extracted via a computerized search of the enterprise data warehouse. The initial MRI was not counted in the cost since it formed the basis of the inclusion criteria we used. Testing and associated costs related to patient care, evaluation and treatment for infections, or for monitoring of drug levels were not included. Tests (and associated costs) included general screening labs, disease screening labs, and disease-specific testing (for example, respectively: blood chemistry, hemoglobin; leukocyte lysosomal enzymes, chromosome karyotype; very long-chain fatty acids, Pelizaeus-Merzbacher gene testing) (see table e-1 on the Neurology® Web site at Neurology.org for listing of all tests performed). If a test was obtained more than once, only the first instance was included; however, costs for repeat MRIs were included. Complete cost data were extracted for each patient from IH's activity-based cost accounting system and were standardized to 2013 constant US dollars.4 We did not include costs for hospital care or professional fees.
RESULTS
We identified 269 patients with leukodystrophy over an 8-year time period. Patients were determined to have an inherited leukodystrophy if they had abnormal signal on MRI in the brain myelin, and if they had no other explanation for their leukodystrophy, for example, prematurity or multiple sclerosis (see full criteria in the methods section). Sixty-four patients ultimately met inclusion and exclusion criteria. For the 64 patients, we then determined all testing performed for the purposes of diagnosis, including repeated brain MRIs, and determined costs for that testing.
Patients with leukodystrophy had on average 20 tests each for the purpose of diagnosis, but the number of tests ranged from 2 to 42 tests (table 1). Patients had on average 2.5 brain MRIs after their MRI on presentation (range 0–8). Average diagnostic testing cost per patient was $4,209, but ranged from $357 to $15,611. A final specific etiologic diagnosis was determined in 31% of patients. In approximately one-fourth of diagnosed patients, an additional gene-specific test was performed (such as sequencing of the ARSA gene for patients with metachromatic leukodystrophy), but which contributed typically less than 10% to 20% of their overall costs.
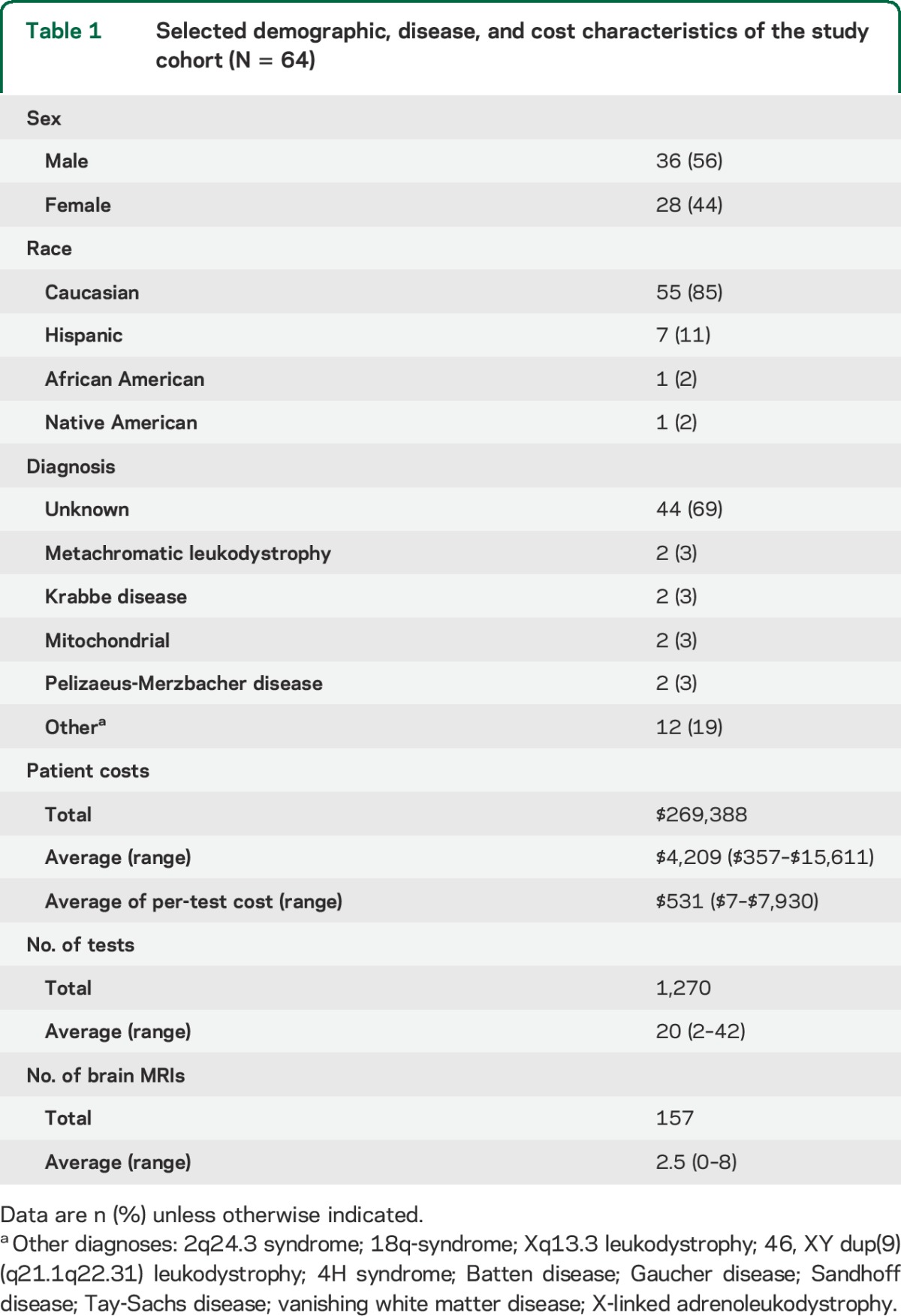
Table 1.
Selected demographic, disease, and cost characteristics of the study cohort (N = 64)
An increase in the number of tests ordered was associated with an increase in cost, most noticeably after 32 tests were ordered (figure, A). The overall diagnosis rate plateaued when 25 or more tests had been ordered. Few patients had more than 30 tests; numbers of tests were otherwise relatively evenly distributed (figure, B). Fifty-three percent of patients were diagnosed in 20 or fewer tests, compared with 17% if more than 20 tests were performed.
Figure. Costs, testing, and diagnosis in the diagnostic odyssey.
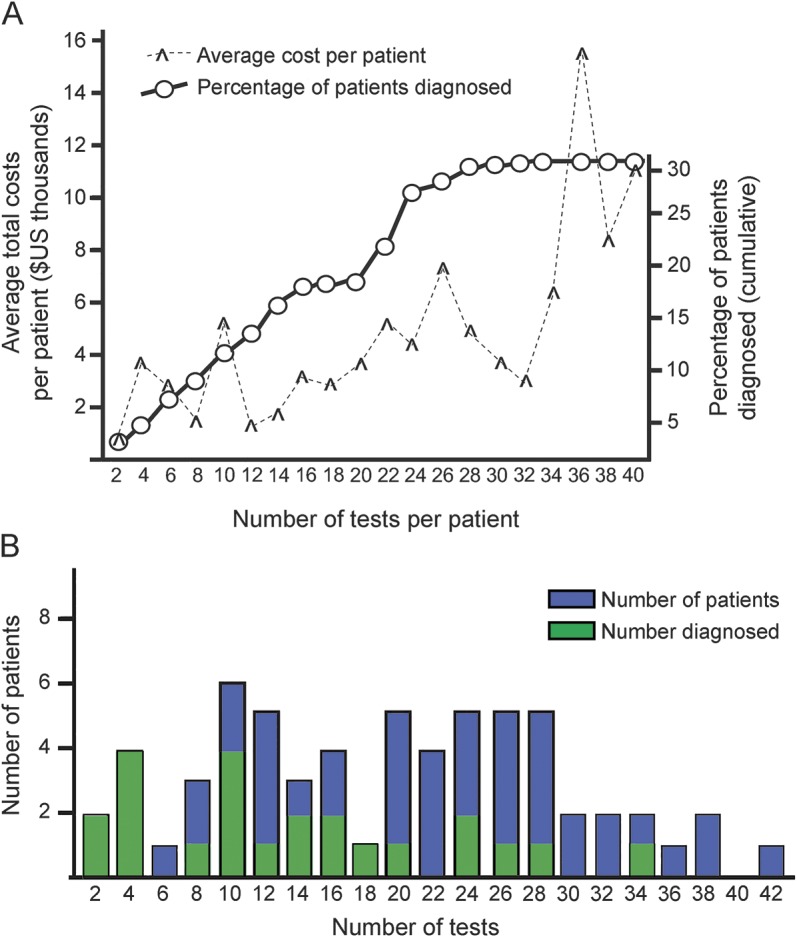
(A) Average costs per patient based on number of tests ordered (hash), arranged by number of tests obtained (x-axis), and cumulative percentage of patients diagnosed (circles). (B) Number of patients (y-axis) viewed by number of tests obtained (x-axis); green bar indicates number of patients with a final diagnosis.
DISCUSSION
The diagnostic odyssey in children with leukodystrophies can be prolonged and frustrating for patients, their families, and providers. We have determined the financial costs directly associated with testing to make a diagnosis. We used costs, since charges reflect hospital billing variability and differences in contractual relationships with insurers. We are the first to determine costs of the diagnostic odyssey in any disease group; prior studies focused on charges and only included results in patients ultimately diagnosed using next-generation sequencing, thereby biasing charge amounts to a subset of patients.7
Limitations of this study include that it represents a single health care system, that data were collected retrospectively, and that cost data may have been affected by total duration of the patient in the study. Costs may continue to increase if a patient remains undiagnosed, although our personal experience suggests that many families and their providers do not continue testing after a certain point, depending on both the provider and the family. Patients who remained undiagnosed had between 6 and 42 tests performed. The low overall rate of a specific etiologic diagnosis may reflect our restrictive inclusion criteria, which excluded patients with known affected family members, or patients who had outside diagnostic testing performed. Another limitation is that we did not include social and psychological burdens on the patient and family; the costs of additional hospitalization that could be associated with trying to obtain a diagnosis; and the adverse costs of missed opportunity to diagnose potentially treatable disorders.
Diagnosis costs in our cohort averaged $4,209. In comparison, overall costs of care for patients with leukodystrophy averaged more than $107,000, and in some patients more than $1 million.1,4,8 Thus, financial costs for the diagnostic odyssey and diagnostic testing are relatively low compared to the overall costs of care for patients with leukodystrophy. It would seem that financial costs of the diagnostic odyssey should not limit pursuit of diagnosis. In some patients, a diagnosis was reached almost immediately with only a few tests obtained. In most of those patients, the clinical presentation and initial brain MRI had findings that suggested the ultimate diagnosis; for example, contrast enhancement and abnormal T2 signal in a boy consistent with X-linked adrenoleukodystrophy. Future studies could examine whether there are logical algorithms to efficiently find a diagnosis in children with leukodystrophies. Our findings on the costs and yields of testing offer insights into the diagnostic odyssey of children with neurologic impairment.
Supplementary Material
ACKNOWLEDGMENT
The authors thank R. Rasmussen and T. Pysher for assistance with obtaining costs data.
GLOSSARY
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IH
Intermountain Healthcare
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
All authors assisted with data analysis, writing, and revising the manuscript for content. J.R., E.K.K., and J.L.B. were involved in data acquisition. J.R., R.S., and J.L.B. conceived the study.
STUDY FUNDING
Supported by NIH DP2 MH100008 to J.L.B.
DISCLOSURE
J. Richards, E. Korgenski, and R. Srivastava report no disclosures relevant to the manuscript. J. Bonkowsky is funded by NIH grant DP2 MH100008. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bonkowsky JL, Nelson CR, Kingston JL, et al. The burden of inherited leukodystrophies in children. Neurology 2010;75:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry J, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med 2012;9:e1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics 2010;126:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson C, Mundorff MB, Korgenski EK, et al. Determinants of health care use in a population-based leukodystrophy cohort. J Pediatr 2013;162:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiffmann R, van der Knaap MS. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 2009;72:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson HM, Wilkes J, Korgenski EK, et al. Preventable infections in children with leukodystrophy. Ann Clin Transl Neurol 2014;1:370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soden SE, Saunders CJ, Willig LK, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med 2014;6:265ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brimley CJ, Lopez J, van Haren K, et al. National variation in costs and mortality for leukodystrophy patients in US children's hospitals. Pediatr Neurol 2013;49:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


