Abstract
Objective:
We aimed to investigate whether the efficacy and safety of clopidogrel plus aspirin vs aspirin alone were consistent between patients with and without intracranial arterial stenosis (ICAS), in the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial.
Methods:
We assessed the interaction of the treatment effects of the 2 antiplatelet therapies among patients with and without ICAS, identified by magnetic resonance angiography (MRA) in CHANCE (ClinicalTrials.gov identifier NCT00979589).
Results:
Overall, 1,089 patients with MRA images available in CHANCE were included in this subanalysis, 608 patients (55.8%) with ICAS and 481 (44.2%) without. Patients with ICAS had higher rates of recurrent stroke (12.5% vs 5.4%; p < 0.0001) at 90 days than those without. But there was no statistically significant treatment by presence of ICAS interaction on either the primary outcome of any stroke (hazard ratio for clopidogrel plus aspirin vs aspirin alone: 0.79 [0.47–1.32] vs 1.12 [0.56–2.25]; interaction p = 0.522) or the safety outcome of any bleeding event (interaction p = 0.277).
Conclusions:
The results indicated higher rate of recurrent stroke in minor stroke or high-risk TIA patients with ICAS than in those without. However, there was no significant difference in the response to the 2 antiplatelet therapies between patients with and without ICAS in the CHANCE trial.
Classification of evidence:
This study provides Class II evidence that for patients with acute minor stroke or TIA with and without ICAS identified by MRA, clopidogrel plus aspirin is not significantly different than aspirin alone in preventing recurrent stroke.
Previous trials indicated that clopidogrel plus aspirin might be more effective than aspirin alone in reducing microembolic signals in patients with ischemic stroke due to carotid or intracranial arterial stenoses (ICAS).1,2 However, whether such dual antiplatelet therapy could be more effective in reducing the risk of recurrence in stroke patients with ICAS is still uncertain.
The risk of recurrent stroke was reduced by dual antiplatelet therapy of clopidogrel and aspirin, as compared with aspirin alone, among all the Chinese patients with acute noncardioembolic minor stroke or high-risk TIA enrolled in the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial.3 However, there were concerns that the positive findings in CHANCE might be attributable to the fact that the rate of ICAS in the study population in China is higher than that in Western populations.4,5 Thus, in this subgroup analysis, we aimed to investigate whether the efficacy and safety of clopidogrel plus aspirin vs aspirin alone were consistent among the subgroups of patients with and without ICAS in the CHANCE trial, so as to establish the generalizability of the findings of the CHANCE trial.
METHODS
Primary research question.
Were the effects of clopidogrel plus aspirin vs aspirin alone in reducing stroke recurrence and leading to bleeding events different between patients with and without ICAS in the CHANCE trial? The current subgroup analysis of the CHANCE trial provides Class II evidence that the effects of clopidogrel plus aspirin vs aspirin alone in reducing the risk of any stroke recurrence were not significantly different between patients with and without ICAS (hazard ratio [HR] 0.79 [0.47–1.32] vs 1.12 [0.56–2.25]; interaction p = 0.522) in the CHANCE trial; in addition, the effects of the 2 antiplatelet therapies on any bleeding event were not statistically different (HR 2.83 [0.57–14.11] vs 1.02 [0.35–2.97]; interaction p = 0.277).
Overview of the CHANCE trial.
The detailed design and methods of the CHANCE trial (ClinicalTrials.gov identifier NCT00979589) have been previously described.3,6 It was a randomized, double-blind, placebo-controlled clinical trial conducted at 114 centers in China to test the effects of clopidogrel plus aspirin vs aspirin alone on reducing the 90-day risk of any stroke (ischemic or hemorrhagic) when initiated within 24 hours of symptom onset in high-risk patients with acute minor stroke or TIA.
Standard protocol approvals, registrations, and patient consents.
The CHANCE trial was approved by the ethics committee at each study center. All of the participants or their legal proxies provided written informed consent.3
Patients were recruited to CHANCE if they met the following criteria: (1) older than 40 years; (2) diagnosed with acute minor ischemic stroke (NIH Stroke Scale score of 0–3) or moderate- to high-risk TIA (ABCD2 score of ≥4) at the time of randomization; (3) able to initiate the study medications within 24 hours of ictus; and (4) able to provide written informed consent. All eligible patients were randomly assigned to either of the 2 treatment groups with a double-blind, double-dummy design: the clopidogrel plus aspirin group and the placebo plus aspirin group. Patients in the dual antiplatelet group were treated with clopidogrel plus aspirin for the first 21 days, and clopidogrel alone on days 22 through 90. Patients in the mono-antiplatelet group were treated with placebo plus aspirin for 90 days.3
Efficacy and safety outcomes of the CHANCE trial.
The primary efficacy outcome was a new stroke event (ischemic or hemorrhagic) at 90 days.3 Secondary efficacy outcomes included a new clinical vascular event at 90 days (ischemic stroke, hemorrhagic stroke, myocardial infarction, or vascular death), analyzed as a composite outcome and individual outcomes as well, and modified Rankin Scale score at 90 days, dichotomized as favorable and poor functional outcomes with scores of 0–2 and 3–6, respectively. Primary safety outcome was a moderate to severe bleeding event at 90 days, as per the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) definition.7 Other safety outcomes included mild bleeding by the GUSTO definition,7 and any bleeding event. The detailed definitions of all the efficacy and safety outcomes have been previously described.3
Patient screening for the current subgroup analysis.
Patients recruited to the CHANCE trial who underwent baseline magnetic resonance examinations (3.0 or 1.5 tesla) with the following sequences were analyzed in the current subgroup analysis: T1-weighted imaging, T2-weighted imaging, diffusion-weighted imaging, and 3-dimensional (3D) time-of-flight magnetic resonance angiography (MRA). Those without baseline magnetic resonance examinations or without any of the sequences as mentioned above were excluded from the current subgroup analysis (figure e-1 on the Neurology® Web site at Neurology.org).
Image analysis and interpretation.
All MRIs were collected from individual centers in digital format and were read centrally by 2 readers (Xinying Zou and J.J.) who were blinded to patients' baseline and outcome information. Presence of ICAS was defined as 50% to 99% stenosis (Warfarin-Aspirin Symptomatic Intracranial Disease trial criteria8) or occlusion of at least one of the following arterial segments on maximum intensity projections of 3D time-of-flight MRA: intracranial portion of internal carotid arteries, middle cerebral arteries (M1/M2), intracranial portion of vertebral arteries, and basilar artery. For each potential ICAS lesion, disagreement in the degree of stenosis of >10% was resolved by consulting with a third reader. The intra- and interrater reliabilities of detecting ICAS on MRA images at the reading center were previously tested, which were 0.793 and 0.815, respectively.9
Statistical analysis.
In the current subgroup analysis of the CHANCE trial, efficacy analyses were performed in the intention-to-treat population, including all patients randomized to either treatment group. Safety analyses were performed in the on-treatment population, including all patients who received ≥1 dose of a study drug. Since every individual randomized in the CHANCE trial had at least one study drug, the intention-to-treat population was equal to the on-treatment population in this subgroup analysis. All statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC). Two-sided p values <0.05 were considered statistically significant.
Baseline characteristics were compared between patients with and without ICAS (regardless of treatment allocation), using χ2 tests and Wilcoxon rank sum tests for categorical and continuous variables, respectively. The rates of primary and secondary efficacy and safety outcomes at 90 days were also compared between patients with and without ICAS by using χ2 tests. Cox proportional hazards regression was performed with the presence of ICAS as the sole covariate, to obtain the HRs and 2-sided 95% confidence intervals (CIs) of presence of ICAS for the primary efficacy outcome of any stroke and the safety outcome of any bleeding, regardless of the treatment allocation.
Cox proportional hazards regression was also performed with treatment (clopidogrel plus aspirin, or placebo plus aspirin), presence of ICAS, and the treatment by presence of ICAS interaction as covariates, to test the interaction between the differential effects of dual vs mono-antiplatelet therapies on the primary efficacy outcome (any stroke) among patients with and without ICAS. The time to the primary efficacy outcome event for each group was presented by the Kaplan-Meier curves. The interactions of treatment by presence of ICAS on secondary efficacy outcomes and safety outcomes as mentioned above were also assessed. Estimates and 2-sided 95% CIs of the HRs of clopidogrel plus aspirin vs aspirin alone for efficacy and safety outcomes were respectively presented for patients with and without ICAS.
Classification of evidence.
The current subgroup analysis of the CHANCE trial provides Class II evidence that the effects of clopidogrel plus aspirin vs aspirin alone in reducing the risk of stroke recurrence and leading to bleeding events were not significantly different between acute minor stroke or TIA patients with and without ICAS identified by MRA in the CHANCE trial. The study lacks the precision to exclude important differences between clopidogrel plus aspirin and aspirin alone in these subgroups.
RESULTS
Patient demographics and baseline characteristics.
Among the 5,170 patients recruited to CHANCE between October 2009 and July 2012, 1,089 patients at 45 centers undergoing magnetic resonance examinations at baseline with all the sequences as required were included in this subgroup analysis (figure e-1). With each of these patients having at least one study drug, the intention-to-treat population was equal to the on-treatment population in this subgroup analysis (n = 1,089; figure e-1). Baseline characteristics of patients in the CHANCE trial with and without the magnetic resonance sequences as described above who were included in this subgroup analysis or not were basically similar, except that patients included in this subgroup analysis were slightly older, had higher systolic blood pressure, lower body mass index, and longer time to randomization, fewer of them had prior ischemic stroke, and more of them had minor stroke as a qualifying event (table e-1). In addition, the rates of using antihypertensive and lipid-lowering medications at baseline were higher in patients included in the current study (table e-1).
Among the 1,089 patients included in the current subgroup analysis, 481 (44.2%) had ICAS of at least one of the intracranial arterial segments as mentioned above as detected on time-of-flight MRA, while 608 (55.8%) did not have ICAS. Of the 1,089 patients, 531 (48.8%) and 558 (51.2%), respectively, were allocated to receive clopidogrel plus aspirin and placebo plus aspirin. Patients with ICAS were older and more of them had prior ischemic stroke, TIA, and diabetes compared with those without ICAS (table 1). Other baseline characteristics were not significantly different between those with and without ICAS.
Table 1.
Baseline characteristics of patients with and without ICAS in the current subgroup analysis of the CHANCE trial

Efficacy outcomes.
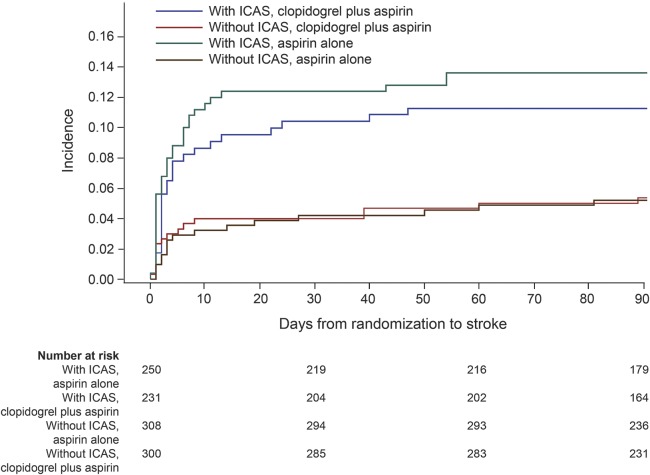
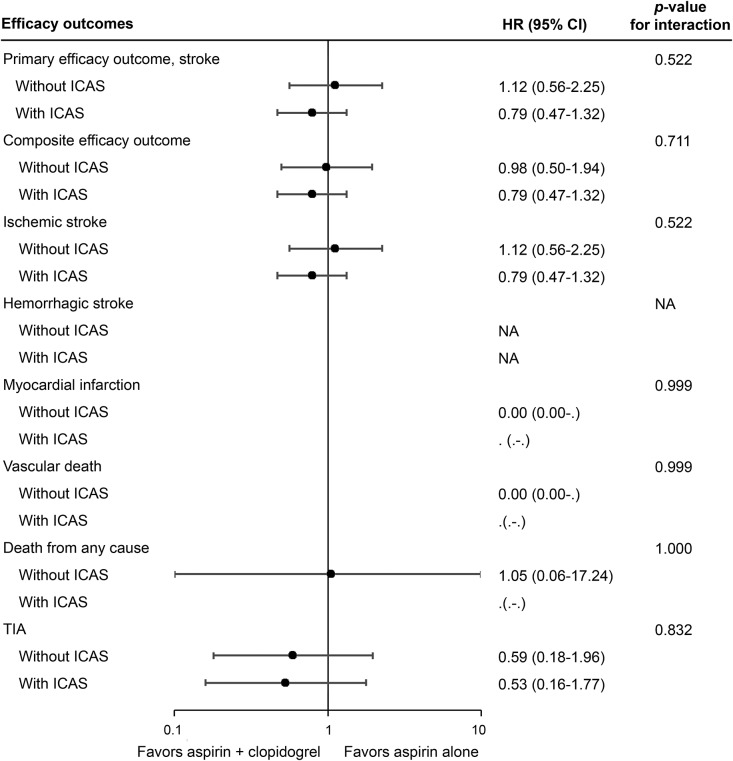
Overall, 93 patients (8.5%) in the current subgroup analysis had a primary efficacy outcome of recurrent stroke at 90 days. More patients with ICAS than those without had a primary efficacy outcome (12.5% vs 5.4%; p < 0.0001) at 90 days (table 2), and the incidence of event over time was significantly higher in patients with ICAS than in those without (HR 2.39, 95% CI 1.57–3.66; p < 0.0001), regardless of treatment allocation. The effects of dual and mono-antiplatelet therapies in preventing recurrent stroke were not significantly different among the patients included in the current subgroup analysis, irrespective of the presence of stenosis (HR 0.86, 95% CI 0.57–1.30; p = 0.481). Moreover, there was no statistically significant evidence for the existence of the treatment by presence of ICAS interaction on the primary outcome of any stroke, with HRs for clopidogrel plus aspirin vs aspirin alone in patients with and without ICAS, respectively, being 0.79 (95% CI 0.47–1.32) and 1.12 (95% CI 0.56–2.25) (interaction p = 0.522; figures 1 and 2).
Table 2.
Efficacy and safety outcomes of patients with and without ICASa
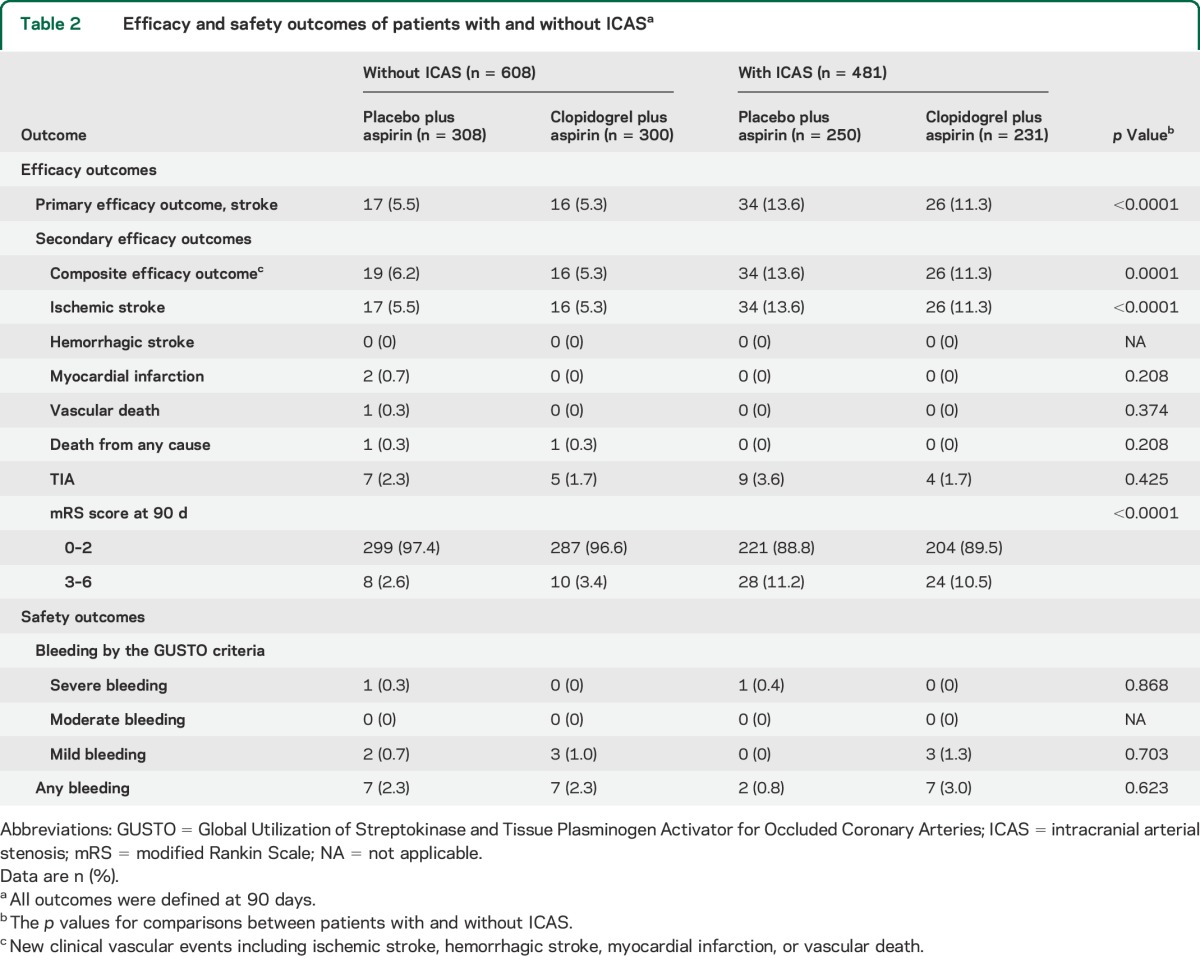
Figure 1. Kaplan-Meier curves for the primary efficacy outcome of any stroke.
Kaplan-Meier curves showing the time to the primary efficacy outcome event (any stroke) in patients with and without ICAS, treated with clopidogrel plus aspirin, or placebo plus aspirin. ICAS = intracranial arterial stenosis.
Figure 2. Forest plot for intention-to-treat analyses of the efficacy outcomes at 90 days.
Intention-to-treat analyses showed no statistically significant treatment by presence of ICAS interaction on the effects of clopidogrel plus aspirin vs aspirin alone in the primary outcome of any stroke at 90 days (interaction p = 0.522), or other efficacy outcomes. Composite efficacy outcome indicated any new clinical vascular events including ischemic stroke, hemorrhagic stroke, myocardial infarction, or vascular death. CI = confidence interval; HR = hazard ratio; ICAS = intracranial arterial stenosis; NA = not applicable.
For secondary efficacy outcomes, more patients with ICAS had a composite efficacy outcome, ischemic stroke, and poor functional outcome (modified Rankin Scale score 3–6) at 90 days compared with patients without ICAS. The rates of all other secondary efficacy outcomes were not significantly different between those with and without ICAS (table 2). The efficacy of clopidogrel plus aspirin compared with aspirin alone on each of the secondary efficacy outcomes was not statistically significant between patients with and without ICAS (figure 2).
Safety outcomes.
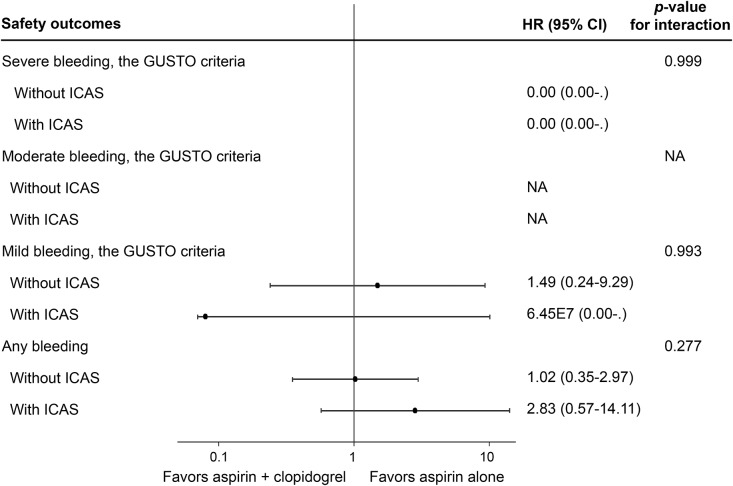
There was no moderate bleeding event in either treatment group. Thus, the rates of the primary safety outcome of moderate to severe bleeding events at 90 days equaled the rates of severe bleeding events, which were both extremely low in patients with and without ICAS at, respectively, 0.21% and 0.16% (table 2). There was no statistically significant evidence for the interaction on the effects of clopidogrel plus aspirin vs aspirin alone on moderate and severe bleeding events among patients with and without ICAS (figure 3).
Figure 3. Forest plot for on-treatment analyses of safety outcomes at 90 days.
On-treatment analyses showed no statistically significant treatment by presence of ICAS interaction on the effects of clopidogrel plus aspirin vs aspirin alone on the safety outcome of any bleeding at 90 days (interaction p = 0.277), or other safety outcomes. CI = confidence interval; GUSTO = Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries7; HR = hazard ratio; ICAS = intracranial arterial stenosis; NA = not applicable.
The rates of the safety outcome of any bleeding at 90 days were not significantly different between those with and without ICAS (1.9% vs 2.3%, p = 0.623; table 2). The incidences of any bleeding event over time were not significantly different between those with and without ICAS (HR 0.75, 95% CI 0.31–1.78; p = 0.508) regardless of treatment allocation. The effect of clopidogrel plus aspirin compared with aspirin alone on any bleeding was not statistically different between patients with (HR 2.83, 95% CI 0.57–14.11) and without ICAS (HR 1.02, 95% CI 0.35–2.97; interaction p = 0.277; figure 3). For the rates of mild bleeding at 90 days, there was no significant difference between patients with and without ICAS (table 2). The effect of clopidogrel plus aspirin compared with aspirin alone on mild bleeding was not statistically significant between patients with and without ICAS (figure 3).
DISCUSSION
In this subgroup analysis of the CHANCE trial, we found that patients with ICAS had higher rates of recurrent stroke and poor functional outcome at 90 days than patients without ICAS, consistent with previous findings.9,10 However, we did not identify any statistically significant treatment by presence of ICAS interaction on the efficacy and safety of clopidogrel plus aspirin vs aspirin alone among patients with and without ICAS in the CHANCE trial.
Patients with ICAS were older and more had histories of stroke, TIA, and diabetes than those without ICAS in the current study, which were well established in previous studies.11 However, the different compositions of patients with and without ICAS regarding age and histories of stroke, TIA, and diabetes probably would not interfere with the analysis of the effects of the 2 antiplatelet therapies in the current study, based on our previous findings that there were no significant interactions in subgroups of patients in the CHANCE trial dichotomized by these specific factors.3
Doubts had aroused that the positive beneficial effects of dual antiplatelets documented in the CHANCE trial might be partly attributable to the unique profile of distribution of cervicocerebral large artery diseases among ischemic stroke patients in China, in contrast to that among Western populations.4,5 For instance, for patients with established vascular diseases or multiple vascular risk factors, the previous 2 large, multicenter, randomized clinical trials, Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) and Secondary Prevention of Small Subcortical Strokes (SPS3), did not show any significant beneficial effects but showed increasing bleeding risk of clopidogrel plus aspirin compared with aspirin alone as mentioned above.12,13 However, the different results between CHANCE and CHARISMA and SPS3 may be possibly explained by the different onset-to-treatment intervals and the inclusion of lacunar stroke patients who are at higher risk of bleeding (SPS3).14
As for the effects of clopidogrel plus aspirin vs aspirin alone in acute ischemic stroke or TIA patients early after ictus, we have recently performed a systematic review and meta-analysis.14 Among the 5 published trials with at least a portion of the patients initiating antiplatelet treatment within 3 days of symptom onset (Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis,1 CHARISMA,12 Fast Assessment of Stroke and TIA to Prevent Early Recurrence,15 Clopidogrel Plus Aspirin Versus Aspirin Alone for Reducing Embolization in Patients with Acute Symptomatic Cerebral or Carotid Artery Stenosis,2 and CHANCE3), only the CHANCE trial showed significant reduction in stroke recurrence in those treated with clopidogrel plus aspirin compared with aspirin alone.14 In addition, we did not identify significant beneficial effects of such dual antiplatelet therapy over aspirin alone in the current subgroup analysis. This was probably because the numbers of patients in the other 4 trials, as well as this subgroup analysis, were underpowered to detect any significant difference between the effects of dual vs mono-antiplatelet therapies.
To our knowledge, the current subgroup analysis was the first to compare the effects of clopidogrel plus aspirin vs aspirin alone initiated early after ictus in secondary prevention of ischemic stroke or TIA patients with and without ICAS. It did not provide statistically significant evidence for the existence of treatment by presence of ICAS interaction on the effects of the 2 treatment strategies in the CHANCE trial. In addition, there were no statistically significant effects of clopidogrel plus aspirin vs aspirin alone in those with and without ICAS according to the current analyses. Therefore, it is unlikely that the overall positive results of the CHANCE trial were driven by the high prevalence of ICAS in Chinese stroke patients. However, there might be selection bias in the current analyses because of only including patients with a baseline magnetic resonance examination. Therefore, our findings should be further validated in other populations because of this limitation and other limitations as follows.
Only approximately 20% of patients in the CHANCE trial were analyzed in this secondary analysis, and there were small numbers of outcome events, especially for the safety outcomes of bleeding events. In addition, the rate of recurrent stroke was lower in the current substudy than that in the CHANCE trial (8.5% vs 10.0%), which might indicate potential selection bias of the current study, considering the fact that we only included cases from 45 of 114 participating centers providing MRIs, and that antihypertensive and lipid-lowering medications were more frequently used in patients included in this substudy. Therefore, the current study had limited power to detect heterogeneities of the treatment effects of dual vs mono-antiplatelet therapies among patients with and without ICAS. Thus, extrapolation of findings from CHANCE to other populations with different profiles of cervicocerebral large artery diseases should occur with caution. At present, a similar trial is being conducted in the United States—the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trial (ClinicalTrials.gov identifier NCT00991029)—with dual or mono-antiplatelet treatment initiated within 12 hours of symptom onset.16 Results of the POINT trial and future similar trials will verify the generalizability of the effects of early initiated, short-term clopidogrel plus aspirin therapy in this patient subset. Another limitation of the current study was that we did not define symptomatic or responsible ICAS lesions, since considerable percentages of patients had a TIA as the qualifying event to be enrolled in CHANCE or had watershed or multiple infarcts, which made it difficult to define the responsible ICAS lesions, especially in TIA patients with multiple or tandem lesions.
The current subgroup analysis of the CHANCE trial revealed a higher rate of recurrent stroke in minor stroke or high-risk TIA patients with ICAS than in those without. The efficacy and safety of clopidogrel plus aspirin vs aspirin alone, initiated early after ictus and lasting for a short period, in reducing the risk of any stroke and not increasing the risk of bleeding events might be consistent among patients with and without ICAS in the CHANCE trial. However, more studies are needed before more confirmative conclusions can be drawn because of the limitations of the current subgroup analysis.
Supplementary Material
GLOSSARY
- CHANCE
Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events
- CHARISMA
Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance
- CI
confidence interval
- GUSTO
Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries
- HR
hazard ratio
- ICAS
intracranial arterial stenosis
- MRA
magnetic resonance angiography
- POINT
Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke
- SPS3
Secondary Prevention of Small Subcortical Strokes
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Zhimin Wang, Yongjun Wang, Haiqin Xia, Bin Li, Guiru Zhang, Xudong Ren, Chunling Ji, Guohua Zhang, Jianhua Li, Bohua Lu, Liping Wang, Shutao Feng, Dali Wang, Weiguo Tang, Juntao Li, Hongtian Zhang, Guanglai Li, Baojun Wang, Yuhua Chen, Ying Lian, Bin Liu, Junfang Teng, Rubo Sui, Lejun Li, Zhiling Yuan, Dawei Zang, Zuneng Lu, Li Sun, Dong Wang, Liying Hou, Dongcai Yuan, Yongliang Cao, Hui Li, Xiuge Tan, Huicong Wang, Haisong Du, Mingyi Liu, Suping Wang, Qiuwu Liu, Zhong Zhang, Qifu Cui, Runqing Wang, Jialin Zhao, Jiewen Zhang, Jianping Zhao, Qi Bi, Xiyou Qi, Junyan Liu, Changxin Li, Ling/Lv Li, Xiaoping Pan, Junling Zhang, Derang Jiao, Zhao Han, Dawei Qian, Jin Xiao, Yan Xing, Huishan Du, Guang Huang, Yongqiang Cui, Yan Li, Lianyuan Feng, Lianbo Gao, Bo Xiao, Yibin Cao, Yiping Wu, Jinfeng Liu, Zhiming Zhang, Zhengxie Dong, Limin Wang, Li He, Xinchen Wang, Xueying Guo, Ming Wang, Xiaosha Wang, Jiandong Jiang, Renliang Zhao, Shengnian Zhou, Hao Hu, Maolin He, Fengchun Yu, Quping Ouyang, Jingbo Zhang, Anding Xu, Xiaokun Qi, Lei Wang, Fuming Shi, Fuqiang Guo, Jianfeng Wang, Fengli Zhao, Ronghua Dou, Dongning Wei, Qingwei Meng, Yilu Xia, Shimin Wang, Zhangcang Xue, Yuming Xu, Liping Ma, Chun Wang, Jiang Wu, Yifeng Du, Yinzhou Wang, Lijun Xiao, Fucong Song, Wenli Hu, Zhigang Chen, Qingrui Liu, Jiemin Zhang, Mei Chen, Xiaodong Yuan, Zhihui Liu, Guozhong Li, Xiaohong Li, and Tingchen Tian
AUTHOR CONTRIBUTIONS
Dr. Liping Liu and Dr. Ka Sing Lawrence Wong contributed to study design, literature search, data analysis, data interpretation, and manuscript preparation. Dr. Xinyi Leng contributed to literature search, data analysis and interpretation, figures, and manuscript preparation. Dr. Yuehua Pu contributed to data collection and interpretation and manuscript preparation. Dr. Yilong Wang contributed to study design and data interpretation, and provided critical and essential comments on the manuscript. Dr. Jing Jing and Dr. Xinying Zou contributed to data collection and image analysis and interpretation. Dr. Yuesong Pan and Mr. Anxin Wang contributed to statistical analysis, data interpretation, and figures. Dr. Xia Meng contributed to data collection and image interpretation. Dr. Chunxue Wang, Dr. Xingquan Zhao, and Dr. Yannie Soo contributed to study design and provided critical and essential comments on the manuscript. Dr. S. Claiborne Johnston and Dr. Yongjun Wang were the principal investigators of the CHANCE study, and contributed to study design, data analysis and interpretation, and provided critical comments on the manuscript.
STUDY FUNDING
Supported by grants 2008ZX09312-008, 2011BAI08B02, 2012ZX09303, and 200902004 from the Ministry of Science and Technology, China; and the Chinese University of Hong Kong (Focused Investment Scheme B) and the Institute of Innovative Medicine, Chinese University of Hong Kong, China.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) Trial. Circulation 2005;111:2233–2240. [DOI] [PubMed] [Google Scholar]
- 2.Wong KS, Chen C, Fu J, et al. Clopidogrel Plus Aspirin Versus Aspirin Alone for Reducing Embolisation in Patients with Acute Symptomatic Cerebral or Carotid Artery Stenosis (CLAIR Study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–497. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 4.Feske SK. A little good. Circulation 2013;128:1598–1599. [DOI] [PubMed] [Google Scholar]
- 5.Hankey GJ. Dual antiplatelet therapy in acute transient ischemic attack and minor stroke. N Engl J Med 2013;369:82–83. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Johnston SC. Rationale and design of a randomized, double-blind trial comparing the effects of a 3-month clopidogrel-aspirin regimen versus aspirin alone for the treatment of high-risk patients with acute nondisabling cerebrovascular event. Am Heart J 2010;160:380–386. [DOI] [PubMed] [Google Scholar]
- 7.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO Investigators. N Engl J Med 1993;329:673–682. [DOI] [PubMed] [Google Scholar]
- 8.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MZ. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014;45:663–669. [DOI] [PubMed] [Google Scholar]
- 10.Wong KS, Li H, Chan YL, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke 2000;31:2641–2647. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi AI, Feldmann E, Gomez CR, et al. Intracranial atherosclerotic disease: an update. Ann Neurol 2009;66:730–738. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–1717. [DOI] [PubMed] [Google Scholar]
- 13.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong KS, Wang Y, Leng X, et al. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: an updated systematic review and meta-analysis. Circulation 2013;128:1656–1666. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM. Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007;6:961–969. [DOI] [PubMed] [Google Scholar]
- 16.Johnston SC, Easton JD, Farrant M, et al. Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) Trial: rationale and design. Int J Stroke 2013;8:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.