Abstract
Background:
Cerebrovascular disease is a cause of morbidity in HIV-infected populations. The relationship among HIV infection, brain arterial remodeling, and stroke is unclear.
Methods:
Large brain arteries (n = 1,878 segments) from 284 brain donors with and without HIV were analyzed to obtain media and wall thickness and lumen-to-wall ratio, and to determine the presence of atherosclerosis and dolichoectasia (arterial remodeling extremes). Neuropathologic assessment was used to characterize brain infarcts. Multilevel models were used to assess for associations between arterial characteristics and HIV. Associations between arterial characteristics and brain infarcts were examined in HIV+ individuals only.
Results:
Adjusting for vascular risk factors, HIV infection was associated with thicker arterial walls and smaller lumen-to-wall ratios. Cerebral atherosclerosis accounted for one-quarter of the brain infarcts in HIV+ cases, and was more common with aging, diabetes, a lower CD4 nadir, and a higher antemortem CD4 count. In contrast, a higher lumen-to-wall ratio was the only arterial predictor of unexplained infarcts in HIV+ cases. Dolichoectasia was more common in HIV+ cases with smoking and media thinning, and with protracted HIV infection and a detectable antemortem viral load.
Conclusions:
HIV infection may predispose to inward remodeling compared to uninfected controls. However, among HIV+ cases with protracted immunosuppression, outward remodeling is the defining arterial phenotype. Half of all brain infarcts in this sample were attributed to the extremes of brain arterial remodeling: atherosclerosis and dolichoectasia. Understanding the mechanisms influencing arterial remodeling will be important in controlling cerebrovascular disease in the HIV-infected population.
With the advent of effective combination antiretroviral therapy (cART), individuals with HIV live longer, but there remains an increased prevalence and incidence of stroke among HIV+ patients compared to the general population.1–3 Whether this simply reflects general increases in systemic arterial disease seen in aging with HIV or a more complex array of factors specific to the cerebral circulation is unclear.
A still unanswered question is whether HIV per se is independently associated with vasculopathic changes. There is evidence to consider HIV infection as proatherogenic.4 However, there also exist data supporting a nonatherosclerotic dolichoectatic arterial phenotype in individuals with HIV, characterized by dilation and tortuosity of cerebral and systemic large arteries.5,6 Atherosclerosis and dolichoectasia may represent extremes in the brain arterial remodeling continuum.7 Investigating the relationship between brain arterial remodeling and the presence of brain infarcts among HIV+ patients may help in targeting specific therapies to curb the rise of cerebrovascular disease in this population.
The goal of this analysis was to investigate whether brain arterial remodeling differs among HIV+ cases compared to age- and sex-matched uninfected controls, and to test the hypothesis that these markers of brain arterial remodeling are associated with the presence of brain infarcts in HIV+ cases.
METHODS
Arterial specimens were obtained from the Brain Arterial Remodeling Study collection, itself formed from 4 brain banks or tissue collections.8 For this analysis, patients with HIV (n = 142) were matched by age (±3 years) and sex to HIV-uninfected controls. The majority of patients with HIV (N = 137) came from the Manhattan HIV Brain Bank (MHBB, S. Morgello, PI). The MHBB has 2 sources of donors: the majority are enrolled while alive and followed prospectively, while a smaller number are obtained at the time of autopsy and clinical information obtained through review of the medical record. Cardiovascular disease (CVD) was defined by history of stroke (any), myocardial infarction, coronary artery disease, angina, or peripheral artery disease. Brain infarcts were defined by autopsy as a focal cavity corresponding to a vascular territory. The presumed etiology of brain infarct was obtained retrospectively from chart review and autopsy findings and attributed by consensus between a neuropathologist (senior author) and a vascular neurologist (first author; see e-Methods on the Neurology® Web site at Neurology.org).
For the histopathologic evaluation of brain large arteries, the circle of Willis or part of it was extracted from available formaldehyde-fixed brain tissues. Five-millimeter segments were cut from the proximal and distal portion of each available large brain artery and embedded in paraffin. Six-micron-thick sections were cut for hematoxylin & eosin and elastic Van Gieson stains. The variables of interest for this analysis were as follows: lumen diameter, media thickness, lumen-to-wall ratio (LWR, calculated as lumen diameter [numerator]: thickness of the arterial wall [denominator]), degree of stenosis, the presence of internal elastic lamina (IEL) fragmentation (or gaps), IEL duplication, arterial calcifications, atherosclerosis, and dolichoectasia. We considered LWR a continuous measure of arterial remodeling with a small LWR indicative of inward remodeling and a large LWR indicative of outward remodeling. We used atherosclerosis and dolichoectasia as nonoverlapping extremes of remodeling. We defined atherosclerosis as the presence of at least a clearly defined atheroma with a fibrotic cap (see e-Methods). Dolichoectasia was defined solely by an arterial size-adjusted LWR >2 SD for each sex obtained from this sample. Since sex is one of the major determinants of brain arterial diameters, we obtained the distribution of each arterial segment by sex to obtain the cutoffs for dolichoectasia.8 None of the arteries classified as dolichoectatic had concurrent evidence of atherosclerosis. We considered HIV vasculopathy a form of secondary dolichoectasia, given that the radiographic appearances of dolichoectasia in non-HIV cases and in those with HIV vasculopathy are indistinguishable, and because both conditions are indicative of extreme outward remodeling.6,7,9
Standard protocol approvals, registrations, and patient consents.
Institutional review board approval and patient consent were obtained by each of the cited brain collections at their respective institutions.
Statistical analysis.
Differences in the proportions of demographic and clinical characteristics among groups were compared with Student t test and χ2 as indicated. Multivariate logistic regression was performed to assess independent associations of demographic and clinical characteristics with HIV and to obtain odds of association and their 95% confidence intervals (CIs). To enable a fair comparison among arteries with different sizes and locations, we adjusted all analyses for arterial size, number of arteries per case, and the type of arteries included in each case.8 Additionally, media and wall thicknesses as well as LWR were normalized with log transformation. First we investigated whether brain large arteries from cases with HIV had different characteristics compared to the age- and sex-matched HIV-negative controls with 3 statistical models controlling progressively for demographics and vascular risk factors. Then we assessed whether any of these characteristics would be associated with the presence of cryptogenic brain infarcts in HIV+ cases. Finally, we assessed for associations among demographic, clinical, and pathologic variables with the 2 main arterial phenotypes studied in this study: atherosclerosis and dolichoectasia. We used multilevel mixed models with random effects for continuous variables and generalized linear models with a Poisson distribution and log link for categorical variables. Statistical significance was set at a p value of ≤0.05 and statistical interactions were considered significant at a p value of ≤0.10. The analysis was carried out with SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Sample characteristics.
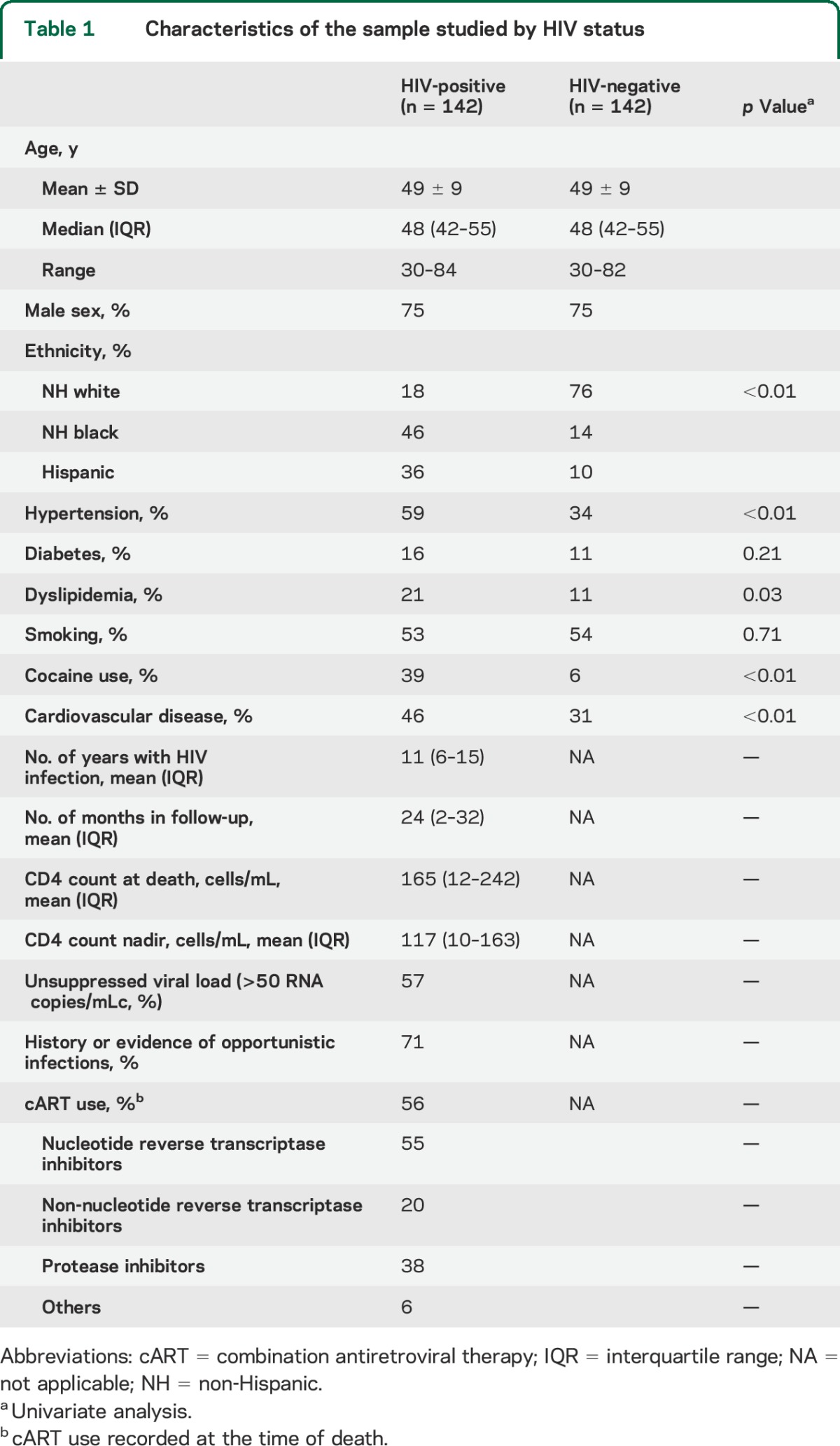
Sample characteristics are illustrated in table 1. HIV+ brain donors were more likely to be African American (odds ratio [OR] 3.2, 1.3–7.4), Hispanic (OR 7.0, 2.8–17.7), smokers (OR 4.7, 2.2–10.2), and to have used cocaine (OR 2.7, 1.1–6.6), after adjusting for covariates. CVD was more common in the HIV population; however, no significant association remained between HIV and CVD after adjusting for vascular risk factors (OR 1.2, 0.6–2.4).
Table 1.
Characteristics of the sample studied by HIV status
HIV as a predictor of brain arterial phenotypes.
Compared with HIV− cases, brain large arteries from HIV+ cases had a thicker media and thicker arterial walls with smaller LWR, suggesting a predisposition for inward remodeling. These associations became apparent only after adjustment for demographics and vascular risk factors. Arterial stenosis was also higher among cases with HIV, but it did not reach statistical significance. There was no difference in the rate of arterial calcification by HIV status (table 2).
Table 2.
Arterial characteristics associated with HIV infection

Brain infarcts among patients with HIV.
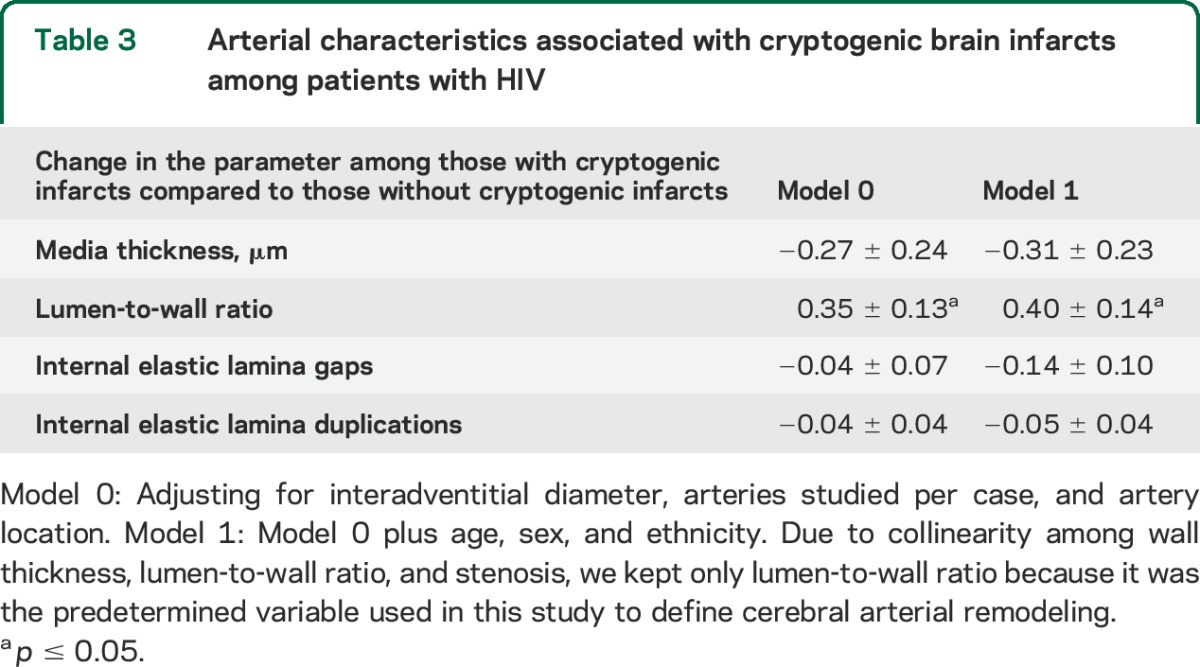
Thirty out of 142 cases with HIV (21%) had brain infarcts. Among these 30 cases, 7 had embolic sources (3 with bacterial endocarditis, 3 with dilated cardiomyopathy or intracavitary thrombus, and 1 with an aortic abscess after prostatic aortic valve replacement), 5 had brain opportunistic infections (2 cytomegalovirus, 2 cryptococcus, and 1 HIV leukoencephalopathy), 3 had disorders of coagulation (1 antiphospholipid syndrome, 2 disseminated intravascular coagulation due to cancer or sepsis), 7 had atherosclerosis in the brain arteries supplying the region of their infarcts (and thus attributed to intracranial atherosclerosis), and 8 were cryptogenic. The only arterial measure associated with cryptogenic brain infarcts was a higher LWR, suggestive of outward remodeling (figure). This reported association was significant even after controlling for demographic and vascular risk factors (table 3).
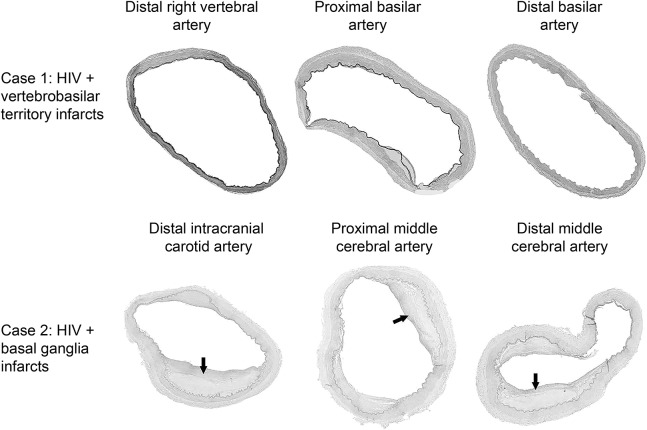
Figure. Examples of patients with HIV and brain infarcts with corresponding arterial pathology.
Patient 1 was a 43-year-old Hispanic man with HIV infection for 9 years treated with didanosine, abacavir, and nelfinavir at the time of death. His CD4 count nadir was 29 and at death his CD4 count was 118 and his viral load 354,870 copies/mLc. He had a history of hypertension and smoking. The brain autopsy demonstrated multiple small infarcts in the territory supplied by the vertebrobasilar system. The basilar artery and vertebral arteries shown here have a relatively thin wall with a dilated lumen. Patient 2 was a 64-year-old Hispanic woman with HIV infection for 15 years, treated with lamivudine/zidovudine and efavirenz, CD4 count nadir of 125 and at death of 537, with an undetectable viral load. She also had hypertension and smoking. Brain autopsy disclosed hypertensive retinopathy and circle of Willis atherosclerosis (arrows show thin-fibrous cap atheromas). Staining for opportunistic infections in brain parenchyma was unrevealing. The systemic autopsy disclosed left ventricular hypertrophy and systemic atherosclerosis.
Table 3.
Arterial characteristics associated with cryptogenic brain infarcts among patients with HIV
Predictors of brain large artery atherosclerosis and dolichoectasia among patients with HIV.
Intracranial large artery atherosclerosis among patients with HIV was associated with aging, diabetes, a higher CD4 count at death, and a low nadir CD4, whereas dolichoectasia was associated with smoking, a thinner media, and a protracted (increased duration of) HIV infection. A trend was also noted for protease inhibitor use and dolichoectasia (p = 0.059). All of these associations were independent of demographic, clinical, immunologic, and pharmacologic variables (table 4).
Table 4.
Cross-sectional associations of atherosclerosis and dolichoectasia among cases with HIV

We investigated statistical interactions post hoc. Intracranial atherosclerosis was more common among individuals who had both hypertension and diabetes (p = <0.001), or in individuals with diabetes (p = 0.04) or hypertension (p = 0.05) who used protease inhibitors. In contrast, dolichoectasia was more likely among individuals with a protracted HIV infection who also remained immunosuppressed during antemortem follow-up (p = 0.07) or who had detectable viral loads at death (p = 0.02).
DISCUSSION
In this cART-era autopsy sample, large brain arteries from HIV+ individuals had thicker arterial walls, thicker media, smaller LWR, and a trend for greater degrees of arterial stenosis than age- and sex-matched HIV-negative controls, independent of vascular risk factors. These findings suggest that in aggregate, in the cART era, some aspect of HIV infection promotes inward arterial remodeling when compared to uninfected individuals. The pathologic consequences of inward remodeling and of its most feared phenotype, atherosclerosis, are not minimal. In this sample, 1 out of 4 brain infarcts had no apparent cause other than large artery atherosclerosis, highlighting the importance of the noted association between HIV and markers of inward remodeling. In contrast, among HIV+ individuals with unexplained infarcts, an increased LWR (indicative of outward remodeling) was the only predictor of the presence of these infarcts. Consequently, half of the HIV+ individuals who had brain infarcts were associated with extreme phenotypes of arterial remodeling, i.e., atherosclerosis and dolichoectasia. The implications of these observations are that an increased risk of stroke in HIV may emerge from divergent intrinsic cerebrovascular biologies, and that focus should be placed on finding ways to distinguish variable mechanisms of disease in this population. A pertinent question arising is thus whether HIV per se plays a role in the development and natural history of atherosclerosis or dolichoectasia, independent of the risks conveyed by the typically highly morbid vascular profile documented in the HIV population.2,10
Our results provide some insight into this question. Brain artery atherosclerosis in HIV+ cases was more frequent among older individuals, in those with diabetes, diabetes, and hypertension, and in those who used protease inhibitors in the setting of hypertension or diabetes. This comes as no surprise, as diabetes and hypertension are well-accepted risk factors for large artery atherosclerosis, and protease inhibitors have been linked to myocardial infarction (usually attributed to atherosclerosis) in HIV.11,12 A novel finding in our study is that intracranial atherosclerosis was more likely among those with a lower nadir CD4 count and a higher CD4 count at the time of death. Other studies have reported similar association with extracranial atherosclerosis. For example, there is an association between history of an AIDS-defining condition or CD4 nadir <200 with higher aggregate atherosclerotic vascular disease or a faster progression of extracranial carotid atherosclerosis.4,13 Brain arterial (small and large) disease has been reported in up to 76% of HIV-infected patients in a large sample with stroke with median CD4 counts >400 cells/mm3 and CD4 nadir >200 cells/mm3, whereas in samples with more overall severe immunosuppression persistent at the time of stroke, the prevalence of brain arterial disease falls to 30%.3,14 The lower prevalence of small and large artery disease infarction in predominantly immunosuppressed samples is similar to the 25% reported in our sample, where the majority of patients had CD4 count <200 cells/mm3 as well.
A second novel finding reported here is that the only arterial measure associated with cryptogenic infarcts was an increased LWR. As we have documented previously, a higher LWR implies relative thinning of the wall or enlargement of the lumen that if extreme may cause dilative arteriopathy.15 Dilation is a cardinal feature of primary and secondary dolichoectasia and may lead to infarction through mechanical traction of penetrating arteries, in situ thrombosis due to stagnant flow, or intramural hemorrhage with obliteration of penetrating arteries.7,16 HIV vasculopathy is a form of secondary dolichoectasia, and, except for the clinical context,5,6,17 we know no reliable method to distinguish it from other forms of dolichoectasia. HIV vasculopathy is a diagnosis of exclusion and it accounts for roughly one-fifth of stroke cases in HIV, although its prevalence is probably higher in patients with severe immunosuppression.6 In this sample, dolichoectasia was more common among those who had HIV for longer periods of time and those who died with unsuppressed viral loads. A thicker media was inversely associated with the presence of dolichoectasia in this sample and thus confirms earlier observations that a thinner media may be a preclinical stage in the development of HIV vasculopathy.17 Smoking was independently associated with dolichoectasia in this sample. Since smoking is higher in the HIV population than in non-HIV controls, smoking may play an important role in the development of HIV vasculopathy.18 A role for arterial inflammation is plausible, as arterial specimens from patients with HIV vasculopathy frequently disclose inflammatory infiltrates.19–21
Although we lack temporal evidence of acute arterial disease (e.g., plaque rupture or in situ thrombosis in the setting of dolichoectasia) causing an acute brain infarct, the direct observation and thorough pathologic examination for alternative causes of infarcts increases the likelihood that the arterial phenotypes were linked causally to the brain infarcts. Furthermore, we did not make an effort to segregate brain infarcts with lacunar appearance from those attributed to large artery disease because we considered both pathologies as part of the spectrum of endothelial disease, as supported by research published by other groups.22 The strengths of this report include its large sample size, the systematic processing of arteries with good reliable methods, as well as the comprehensive antemortem characterization of HIV-positive individuals carried out by the MHBB. The results from this analysis should be interpreted with caution when compared to the general population with HIV in the United States and to that of other countries that might not share the same demographics. A further limitation includes the lack of biomarkers of remodeling that may be used to screen living individuals with HIV and that could shed light into the pathophysiology of atherosclerosis and dolichoectasia. Additionally, although this report encompasses one of the largest collections of brain arteries in donors with and without HIV, it is still possible that we were underpowered to confirm the statistical significance of some of the associations reported.
We found that large brain arteries from donors with HIV were more likely to have evidence of inward remodeling. Inward remodeling progression leads to arterial stenosis and atherosclerosis, which in this sample accounted for one-fourth of the brain infarcts in HIV+ cases. Intracranial large artery atherosclerosis was more common among those with low nadir CD4 count and higher CD4 counts at death, suggesting a role for the immune system in the development of atherosclerosis. Outward remodeling, expressed as a higher LWR, was the only arterial phenotype associated with cryptogenic infarcts. Dolichoectasia, an extreme manifestation of outward remodeling, was more common among patients with a protracted HIV infection and with high viral loads at death. Thinning of the media may be a prerequisite to further dilation of the vessels. Studying the mechanisms underlying these divergent arterial phenotypes may offer novel insights into brain arterial remodeling leading to stroke in the general population and in particular among those with HIV.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Deborah Mash (NIH N271201300028C) for contributing cases to this study and technical assistance.
GLOSSARY
- cART
combination antiretroviral therapy
- CI
confidence interval
- CVD
cardiovascular disease
- IEL
internal elastic lamina
- LWR
lumen-to-wall ratio
- MHBB
Manhattan HIV Brain Bank
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
Editorial, page 1098
AUTHOR CONTRIBUTIONS
Jose Gutierrez: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, and obtaining funding. James E. Goldman: drafting/revising the manuscript, analysis or interpretation of data, and accepts responsibility for conduct of research and final approval. Andrew J. Dwork: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, and contribution of vital reagents/tools/patients. Mitchell S.V. Elkind: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, and study supervision. Randolph S. Marshall: drafting/revising the manuscript and accepts responsibility for conduct of research and final approval. Susan Morgello: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, contribution of vital reagents/tools/patients, acquisition of data, and obtaining funding.
STUDY FUNDING
Supported by AHA 13CRP14800040 (PI Jose Gutierrez), NIH R25MH080663 and U24MH100931 (PI Susan Morgello), and NIH R01MH64168 (PI Andrew Dwork).
DISCLOSURE
J. Gutierrez, J. Goldman, and A. Dwork report no disclosures relevant to the manuscript. M. Elkind receives compensation for providing consultative services for Biogen IDEC, Biotelemetry/Cardionet, BMS-Pfizer Partnership, Boehringer-Ingelheim, Daiichi-Sankyo, and Janssen Pharmaceuticals; receives research support from diaDexus, Inc., and the NIH/NINDS; has given expert legal opinions on behalf of Merck/Organon (NuvaRing and stroke litigation); and serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. He receives royalties from UpToDate for chapters related to stroke. R. Marshall and S. Morgello report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mateen FJ, Post WS, Sacktor N, et al. Long-term predictive value of the Framingham Risk Score for Stroke in HIV-positive vs HIV-negative men. Neurology 2013;81:2094–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012;60:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses 2013;29:1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004;109:1603–1608. [DOI] [PubMed] [Google Scholar]
- 5.Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry 2007;78:1320–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez J, Ortiz G. HIV/AIDS patients with HIV vasculopathy and VZV vasculitis: a case series. Clin Neuroradiol 2011;21:145–151. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez J. Dolichoectasia and the risk of stroke and vascular disease: a critical appraisal. Curr Cardiol Rep 2014;16:525. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez J, Rosoklija G, Murray J, et al. A quantitative perspective to the study of brain arterial remodeling of donors with and without HIV in the Brain Arterial Remodeling Study (BARS). Front Physiol 2014;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez J, Carrasquillo J, Ortiz G, Wright CB. Vascular profile of dolichoectasia differs depending on the presenting symptoms. Edorium J Cardiol 2014;1:1–10. [Google Scholar]
- 10.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356:1723–1735. [DOI] [PubMed] [Google Scholar]
- 12.Rincon F, Sacco RL, Kranwinkel G, et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovasc Dis 2009;28:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel K, Wang J, Jacobson DL, et al. Aggregate risk of cardiovascular disease among adolescents perinatally infected with the human immunodeficiency virus. Circulation 2014;129:1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology 2007;68:1257–1261. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez J, Elkind MSV, Petito C, Chung DY, Dwork AJ, Marshall RS. The contribution of HIV infection to intracranial arterial remodeling: a pilot study. Neuropathology 2013;33:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez J, Sacco RL, Wright CB. Dolichoectasia: an evolving arterial disease. Nat Rev Neurol 2011;7:41–50. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez J, Glenn M, Isaacson RS, Marr AD, Mash D, Petito C. Thinning of the arterial media layer as a possible preclinical stage in HIV vasculopathy: a pilot study. Stroke 2012;43:1156–1158. [DOI] [PubMed] [Google Scholar]
- 18.Helleberg M, Afzal S, Kronborg G, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis 2013;56:727–734. [DOI] [PubMed] [Google Scholar]
- 19.Chetty R, Batitang S, Nair R. Large artery vasculopathy in HIV-positive patients: another vasculitic enigma. Hum Pathol 2000;31:374–379. [DOI] [PubMed] [Google Scholar]
- 20.Connor MD, Lammie GA, Bell JE, Warlow CP, Simmonds P, Brettle RD. Cerebral infarction in adult AIDS patients: observations from the Edinburgh HIV Autopsy Cohort. Stroke 2000;31:2117–2126. [DOI] [PubMed] [Google Scholar]
- 21.Nair R, Robbs JV, Naidoo NG, Woolgar J. Clinical profile of HIV-related aneurysms. Eur J Vasc Endovasc Surg 2000;20:235–240. [DOI] [PubMed] [Google Scholar]
- 22.Deplanque D, Lavallee PC, Labreuche J, et al. Cerebral and extracerebral vasoreactivity in symptomatic lacunar stroke patients: a case-control study. Int J Stroke 2013;8:413–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.