Mice lacking the transcription factor Nfatc1 develop skin tumors at a decreased rate in response to the carcinogen DMBA. Nfatc1 promotes the expression of DMBA-metabolizing enzymes and DNA damage in DMBA-treated keratinocytes.
Abstract
Immunosuppressive therapies using calcineurin inhibitors, such as cyclosporine A, are associated with a higher incidence of squamous cell carcinoma formation in mice and humans. Calcineurin is believed to suppress tumorigenesis in part through Nfatc1, a transcription factor expressed primarily in hair follicle bulge stem cells in mice. However, mice overexpressing a constitutively active Nfatc1 isoform in the skin epithelium developed increased spontaneous skin squamous cell carcinomas. Because follicular stem cells can contribute to skin tumorigenesis, whether the endogenous expression of Nfatc1 inhibits or enhances skin tumorigenesis is unclear. Here we show that loss of the endogenous expression of Nfatc1 suppresses the rate of DMBA/TPA-induced skin tumorigenesis. Inducible deletion of Nfatc1 in follicular stem cells before tumor initiation significantly reduces the rate of tumorigenesis and the contribution of follicular stem cells to skin tumors. We find that skin tumors from mice lacking Nfatc1 display reduced Hras codon 61 mutations. Furthermore, Nfatc1 enhances the expression of genes involved in DMBA metabolism and increases DMBA-induced DNA damage in keratinocytes. Together these data implicate Nfatc1 in the regulation of skin stem cell–initiated tumorigenesis via the regulation of DMBA metabolism.
INTRODUCTION
Stem cells reside within tissues to govern organ homeostasis and regeneration through the coordinated regulation of proliferation and differentiation. When these processes go awry, stem cells can contribute to diseases such as cancer. Indeed, tissue-resident stem cells can initiate tumorigenesis in the mammary gland, intestine, and skin (Barker et al., 2009; Lapouge et al., 2011; Visvader, 2011; White et al., 2011), and cancer stem cells can maintain tumor growth in the epidermis (Schober and Fuchs, 2011; Driessens et al., 2012; Boumahdi et al., 2014). However, the mechanisms controlling the contribution of stem cells to tumors remain largely unknown.
The skin provides an excellent model to explore stem cell function during tumorigenesis. Follicular stem cells are slow-cycling cells that reside within the bulge compartment, express the cell surface marker CD34, and become activated to regenerate the hair follicle during the native hair cycle (Cotsarelis et al., 1990; Blanpain et al., 2004; Morris et al., 2004; Tumbar et al., 2004). Bulge stem cells can contribute to cutaneous keratoacanthomas (Zito et al., 2014) and can also act as cells of origin in squamous cell carcinomas (SCCs; Lapouge et al., 2011; White et al., 2011; Li et al., 2013) during their activated state in a Pten-dependent manner (White et al., 2014). However, additional mechanisms that control the ability of stem cells to contribute to skin tumors are poorly understood.
We and others have shown that the transcription factor Nfatc1 is expressed within bulge stem cells in mice and that Nfatc1 and its upstream phosphatase calcineurin regulate bulge stem cell activity during native hair cycling (Horsley et al., 2008), aging (Keyes et al., 2013), and pregnancy (Goldstein et al., 2014). Of interest, patients receiving immunosuppressive treatment with calcineurin inhibitors, such as cyclosporine A and FK506, experience a significantly elevated risk of developing SCCs over the normal population (Euvrard et al., 2003).
Chemical carcinogenesis studies using 7,12-dimethylbenz[a]anthracene (DMBA), a tumor initiator that induces oncogenic Hras mutations, and 12-O-tetradecanoylphorbol 13-acetate (TPA), a phorbol ester that promotes cell proliferation (Abel et al., 2009), have enabled researchers to further characterize the role of calcineurin signaling in epidermal tumorigenesis. Mice lacking calcineurin B in the skin epithelium developed more tumors than controls when treated with DMBA/TPA, and Nfat proteins were implicated in the repression of tumor formation (Wu et al., 2010). However, mice overexpressing a constitutively active Nfatc1 isoform driven by the Hoxb7 promoter in the skin epithelium developed spontaneous skin SCCs (Tripathi et al., 2013). Thus it is unclear whether endogenous Nfatc1 in bulge stem cells inhibits or enhances skin tumorigenesis. To clarify the role of Nfatc1 in skin tumor formation, we subjected mice lacking Nfatc1 in the epidermis (Keratin14-Cre; Nfatc1fl/fl: Nfatc1, conditional knockout [cKO]; Figure 1A; Horsley et al., 2008) to chemical carcinogenesis with DMBA/TPA (Abel et al., 2009). Our comprehensive study suggests that Nfatc1 increases skin tumor initiation by regulating DMBA metabolism and enhancing stem cell contribution to tumorigenesis.
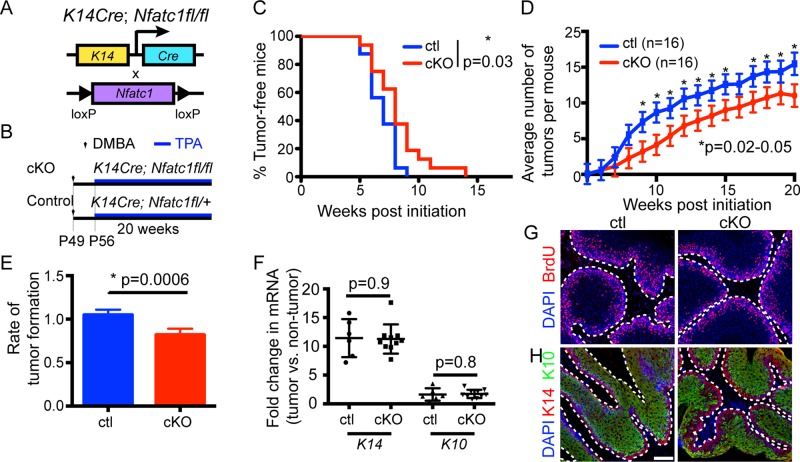
FIGURE 1:
Epidermal Nfatc1 deletion decreases DMBA/TPA tumorigenesis. (A) Schematic of K14-specific Nfatc1 deletion. (B) DMBA/TPA tumorigenesis regime. (C) Percentage of tumor-free cKO/control mice during DMBA/TPA tumorigenesis (16 mice/genotype). *p = 0.03; log-rank test. (D) Average tumor number in cKO/control mice. Data: mean ± SEM. The profiles (p = 0.03) and several time points were significantly different (*); mixed-effect model. (E) Tumor formation rate between cKO/control mice is significantly different, p = 0.0006; mixed-effect model; time: continuous variable. (F) Real-time PCR for K14 and K10 in cKO/control tumors. Data: mean ± SEM (3 mice/genotype). (G, H) Immunostaining for (G) BrdU (red) and (H) K14 (red) and K10 (green) in cross sections of cKO/control tumors 8–10 wk post-DMBA. DAPI, blue. Scale bar, 50 μm.
RESULTS
Reduced skin papilloma formation in the absence of epidermal Nfatc1
To determine whether endogenous expression of Nfatc1 influences skin tumor susceptibility, we examined the response of Nfatc1 cKO mice and heterozygous littermates to DMBA/TPA carcinogenesis (Figure 1B). Treating 7-wk-old mice in the telogen stage of the hair cycle with a single dose of DMBA followed by a biweekly dose of TPA for 20 wk (Abel et al., 2009) resulted in papilloma formation in control mice after 6 wk (Figure 1C). However, the majority of Nfatc1 cKO mice treated with DMBA/TPA developed tumors after 8–10 wk (Figure 1C). Analysis of the number of tumors in control and Nfatc1 cKO mice during a 20-wk time course using mixed-effect models revealed that Nfatc1 cKO mice developed fewer tumors at multiple time points after week 8 and that the profiles for tumor formation between the control and cKO mice were significantly different (Figure 1D).
Because tumor formation increased for both control and Nfatc1 cKO mice over time, we used a mixed-effect model with higher statistical power by maintaining time as a continuous variable to determine whether the rate of tumor formation or tumor number per week was altered in Nfatc1 cKO mice. After week 5, Nfatc1 cKO mice developed 20% fewer tumors per week than control mice (Figure 1E). Thus the rate of tumor formation was significantly reduced in Nfatc1 cKO mice compared with control mice. Characterization of papillomas from control and Nfatc1 cKO mice 8–10 wk after DMBA treatment indicated similarities in tumor size (unpublished data), proliferation (Figure 1G), and K14 and K10 mRNA and protein expression (Figure 1, F and H).
Nfatc1 enhances the rate of skin tumor initiation but not promotion
To determine whether Nfatc1 affects skin tumorigenesis before or after DMBA initiation (Zoumpourlis et al., 2003), we crossed K19CreERT mice (Means et al., 2008) with Nfatc1fl/fl mice to generate Nfatc1 inducible knockout (Nfatc1 iKO mice; Figure 2A). We confirmed that tamoxifen treatment reduced Nfatc1 expression within hair follicle bulge cells in Nfatc1 iKO mice relative to vehicle-treated controls (Figure 2B). To test whether Nfatc1 regulates tumor initiation, we treated Nfatc1 iKO mice with tamoxifen to induce Cre recombinase activity and subsequent Nfatc1 deletion before DMBA treatment (ODT; Figure 2C). In contrast, to determine whether Nfatc1 controls tumor promotion, we treated Nfatc1 iKO mice with tamoxifen after DMBA treatment (DOT; Figure 2C).
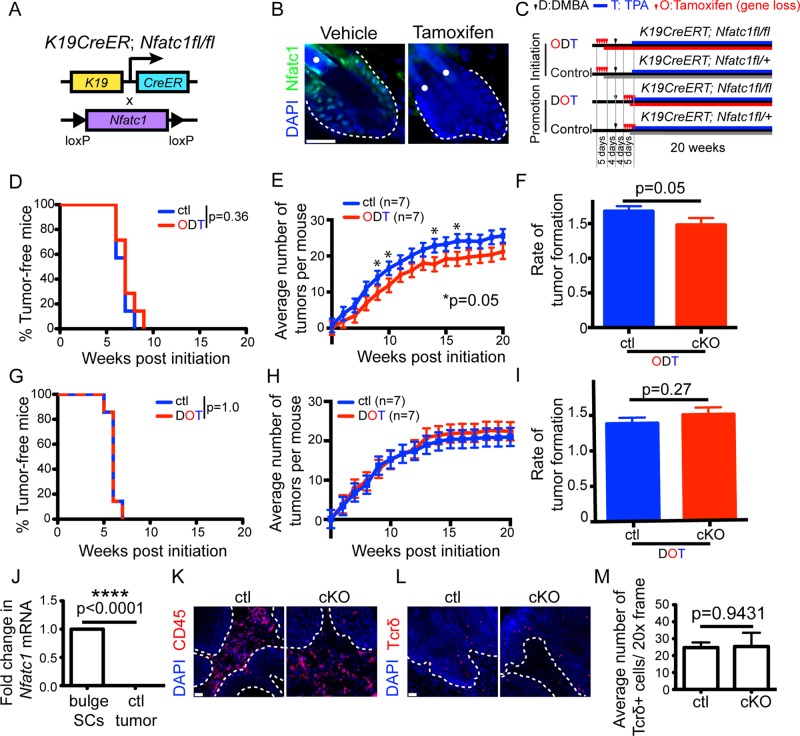
FIGURE 2:
Nfatc1 deletion decreases the rate of tumor initiation but not tumor promotion. (A) Schematic of inducible K19-specific Nfatc1 deletion. (B) Nfatc1 immunostaining (green) in iKO mice 5 d after tamoxifen/vehicle. (C) DMBA/TPA initiation (ODT) and promotion (DOT) regimes. (D, G) Percentage of tumor-free iKO/control mice in (D) ODT or (G) DOT regime (n = 16 mice/genotype). (E, H) Average tumor number during (E) ODT or (H) DOT regime. Data: mean ± SEM (seven mice/genotype). Several time points were significantly different (*); mixed-effect model. (F, I) Tumor formation rate during (F) ODT regime is significantly different but not during (I) DOT regime (mixed-effect model; time: continuous variable). (J) Real-time PCR of Nfatc1 in tumor cells relative to FACS-sorted bulge cells. Data: mean ± SD (six mice/group). Immunostaining for (K) CD45 (red) and (L) Tcrδ (red) in cKO/control tumors 8–10 wk post-DMBA. (M) Quantification of Tcrδ+ cells in cKO/control tumors. Data: mean ± SEM (three mice/genotype). DAPI, blue; dotted line, epithelial–stromal barrier; white dots, hair shaft autofluorescence. Scale bar, 25 μm (B), 50 μm (K, L).
When Nfatc1 was deleted before DMBA treatment (ODT; Figure 2C), Nfatc1 iKO mice and control mice developed tumors between 6 and 8 wk (Figure 2D). However, Nfatc1 iKO mice developed fewer papillomas than littermate controls at several time points during TPA treatment (Figure 2E). In addition, the rate of tumor formation was significantly reduced in Nfatc1 iKO mice when Nfatc1 was deleted before DMBA treatment (Figure 2F). Reduced Cre activity in the inducible K19CreERT mouse model may explain why the reduction in skin tumorigenesis is not as significantly reduced as with the K14Cre model. However, when Nfatc1 was deleted after DMBA treatment but before TPA treatment (DOT; Figure 2C), Nfatc1 iKO mice developed a similar number of papillomas at the same rate as littermate control mice (Figure 2, G–I). Collectively these data suggest that Nfatc1 regulates the rate of tumor initiation and does not affect tumor promotion.
Consistent with the inability of Nfatc1 to affect tumor promotion, Nfatc1 mRNA was not detected within papillomas of control mice as compared with its high enrichment in bulge stem cells isolated by fluorescence activated cell sorting (FACS; Figure 2J). These results are similar to previous studies demonstrating that Nfatc1 mRNA and protein levels are highly down-regulated in skin cancer stem cells relative to hair follicle stem cells (Schober and Fuchs, 2011). Because immune cells have been implicated as regulators of skin tumor formation (de Visser et al., 2006; Ostrand-Rosenberg, 2008; Demehri et al., 2009; Zamarron and Chen, 2011), we investigated whether epidermal deletion of Nfatc1 modulated the presence of immune cells in skin tumors. Immunofluorescence staining for the pan–immune cell marker CD45 revealed the presence of hematopoietic cells in tumors from both Nfatc1 cKO and control mice (Figure 2K). We also examined the presence of dendritic epidermal T-cells (DETCs), a subpopulation of T-cells that resides within the skin and expresses the γδ T-cell receptor (Tcr; Stingl et al., 1987). Immunofluorescence staining and quantification of Tcrδ+ cells in papillomas from Nfatc1 cKO mice and littermate controls revealed similar numbers of DETCs within the tumor (Figure 2, L and M). These data suggest that Nfatc1 regulates tumor formation at the earliest stages of development rather than by affecting tumor promotion via alterations in the tumor immune cell stroma.
Nfatc1 enhances bulge cell contribution to tumors in vivo
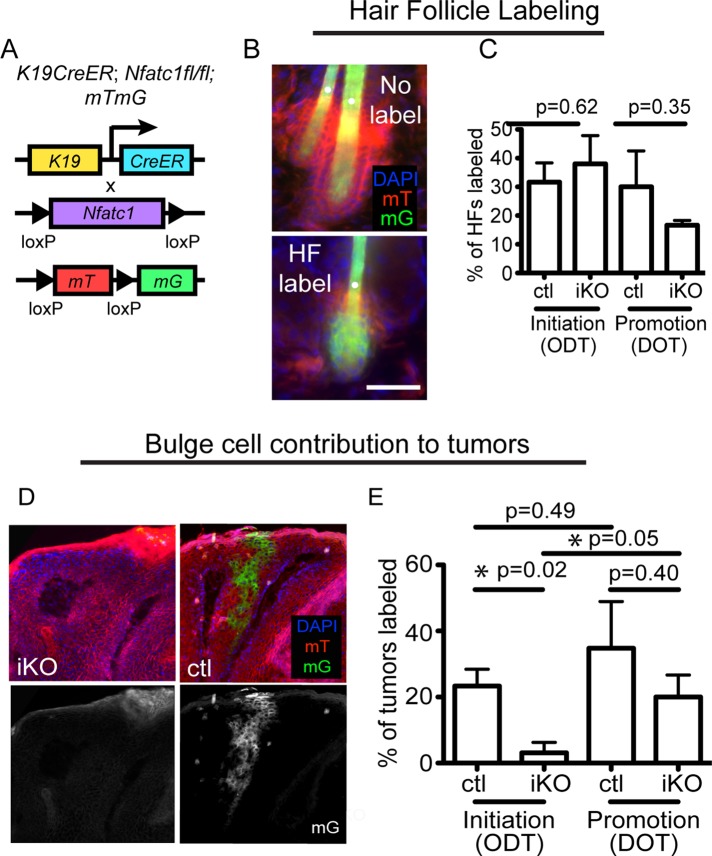
We next analyzed whether Nfatc1 could regulate follicular stem cell contribution to tumors. To this end, we used a genetic lineage tracing approach to trace bulge stem cells and their progeny during tumorigenesis in control and Nfatc1 iKO mice. Specifically, we crossed Nfatc1 iKO mice to mice ubiquitously expressing a fluorescent membrane Tomato/membrane enhanced green fluorescent protein GFP (GFP; mTmG) reporter construct (Muzumdar et al., 2007; Figure 3A). In the absence of Cre activity, all cells express mT. However, treatment with tamoxifen induces K19CreERT activity, resulting in a heritable loss of mT expression and the induction of mG expression in K19-expressing bulge stem cells and their progeny. These mice were subjected to both the initiation and promotion tumorigenesis regimes (Figure 2C). After tamoxifen treatment, a similar percentage of hair follicles in both genotypes contained mG+ cells (Figure 3, B and C). Furthermore, we observed the contribution of K19-expressing bulge cells to skin tumors after 8–12 wk of the carcinogenesis regime (Figure 3D), and quantification of skin tumor labeling revealed a decrease in bulge cell contribution to tumors in Nfatc1 iKO mice during the initiation regime (Figure 3E). However, Nfatc1 deletion after tumor initiation (DOT) did not affect bulge stem cell contribution to tumors (Figure 3E). Together these findings indicate that Nfatc1 expression in hair follicle stem cells enhances their ability to contribute to skin papillomas during tumor initiation.
FIGURE 3:
Nfatc1 deletion decreases stem cell contribution to papillomas during tumor initiation. (A) Schematic of inducible K19-specific Nfatc1 deletion and mTmG labeling. (B) Representative images of K19CreERT;mTmG follicles after tamoxifen. Images show unlabeled (only mT+ cells) or labeled (containing mG+ cells) follicles. (C) Percentage of mG+ hair follicles 8–12 wk post-DMBA (initiation, ODT; promotion, DOT). Data are mean ± SEM for 50–100 hair follicles from four individual mice/group. (D) Representative sagittal section of a tumor containing mG+ cells derived from K19-expressing bulge cells. (E) Percentage of tumors containing mG+ cells in iKO mice and littermate controls from sagittally sectioned tumors during ODT and DOT regimes. Data are mean ± SEM of three or four individual mice/genotype for 8–15 tumors from each mouse. Tumors were harvested 8–12 wk post-DMBA. DAPI, blue. White dots denote hair shaft autofluorescence. Scale bar, 25 μm.
Loss of Nfatc1 alters Hras codon mutations in response to DMBA
The initiating events for mouse skin tumors induced by DMBA largely involve mutation of the Hras gene at codon 61 (Balmain et al., 1984). These mutations are caused by metabolites generated by cytochrome P-450 enzymes such as Cyp1a1 and Cyp1b1 and microsomal epoxide hydrolase (Epxh1) after engagement of the aryl hydrocarbon receptor (Ahr) and the aryl hydrocarbon receptor nuclear translocator (Arnt) within keratinocytes and epidermal Langerhans cells (Modi et al., 2012).
To determine whether the reduced rate of tumor initiation in Nfatc1 cKO mice results from alterations in DMBA metabolism, we analyzed Langerhans cells, which metabolize DMBA and enhance chemical carcinogenesis (Modi et al., 2012). To this end, we immunostained epidermal sheets from Nfatc1 cKO mice (Figure 1A) with antibodies against major histocompatibility complex (MHC) class II, which marks Langerhans cells within the skin epithelium. No changes in the presence of MHC class II+ Langerhans cells were observed between Nfatc1 cKO and control mice (Figure 4A).
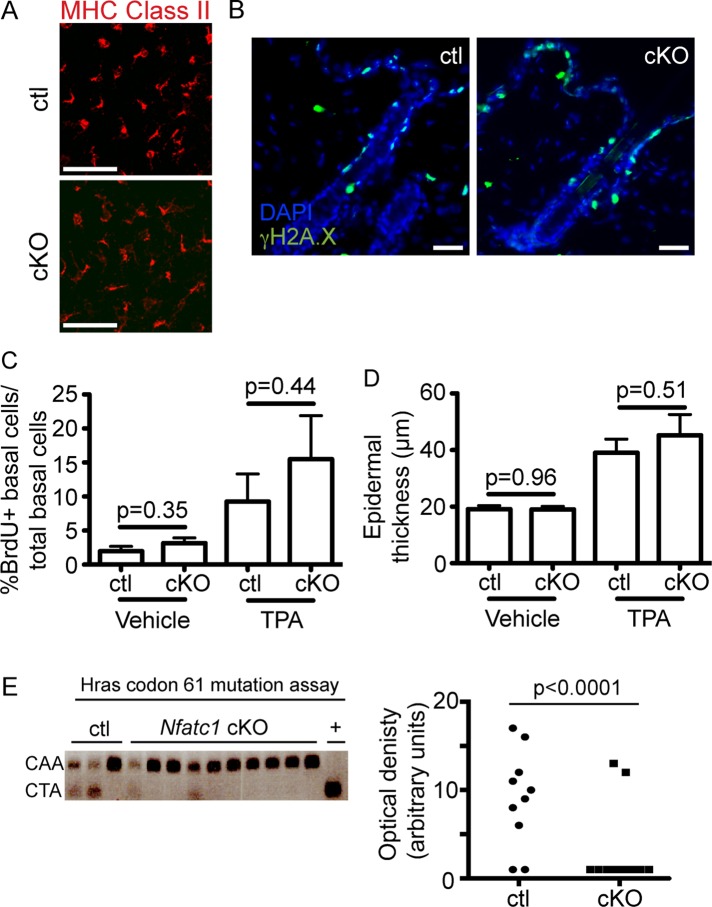
FIGURE 4:
Epidermal Nfatc1 deletion reduces DMBA-induced Hras codon mutations. (A) MHC class II immunostaining (red) from cKO/control epidermal sheets. (B) γH2A.X immunostaining (green) in cKO/control mice 24 h post-DMBA/vehicle. (C) Percentage of BrdU+ epidermal cells normalized to total basal cells 24 post-TPA/vehicle. Data: mean ± SEM (three or four mice/group; >4000 cells quantified/ mouse). (D) Epidermal thickness 24 h post-TPA/vehicle. Data: mean ± SEM (four mice/group). (E) Hras codon 61 mutations from cKO/control tumors 8–10 wk post-DMBA. +, CarC DNA. Optical density of CTA mutant bands. Chi-squared test. DAPI, blue. Scale bar, 50 μm.
DNA damage occurs in keratinocytes after DMBA treatment (Hennings et al., 1993). To analyze the response of keratinocytes to DMBA in Nfatc1 cKO mice (Figure 1A), we treated mice with a single topical dose of carcinogen and analyzed DNA damage after 24 h by immunostaining for γH2A.X, a histone variant induced by DNA damage. No global differences in the presence of γH2A.X+ cells were observed between control and Nfatc1 cKO mice after either vehicle or DMBA treatment (Figure 4B). To determine whether a difference in TPA responsiveness could account for the decreased tumor formation observed in Nfatc1 cKO mice, we pulsed mice with 5-bromo-2′-deoxyuridine (BrdU) to mark proliferating cells after one application of TPA or a vehicle control. Quantification of BrdU+ cells in the epidermis revealed equivalent levels of proliferation between Nfatc1 cKO mice and littermate controls 24 h after either vehicle or TPA treatment (Figure 4C). Similarly, no significant differences in epidermal thickness were observed between Nfatc1 cKO mice and littermate controls under vehicle or TPA-treated conditions (Figure 4D).
To further examine the response of Nfatc1 cKO mice to DMBA-induced tumorigenesis, we analyzed tumors from control and Nfatc1 cKO mice for the presence of DMBA-induced mutations in Hras codon 61 from CAA to CTA, which creates a new Xba1 restriction enzyme site (Sachs et al., 2012). Whereas the mutant Hras codon was detected in a majority of tumors in control mice, few tumors from Nfatc1 cKO mice displayed detectable Hras mutations (Figure 4E). These data indicate that DMBA-induced skin tumors from Nfatc1 cKO mice exhibit fewer Hras codon 61 mutations than littermate controls.
Nfatc1 regulates expression of DMBA-metabolizing enzymes
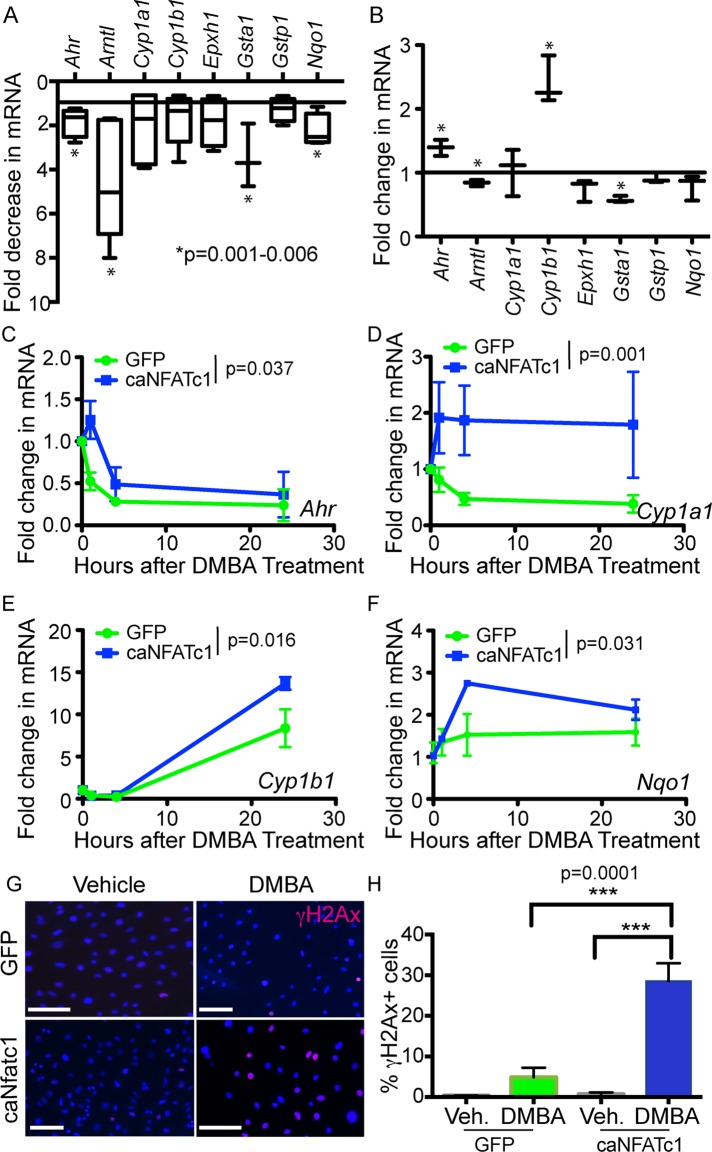
Because DMBA metabolism is responsible for the induction of mutations in Hras during chemically induced tumorigenesis, we next analyzed expression of genes involved in DMBA metabolism by real-time PCR. We found that expression of Ahr, Arntl, Gsta1, and Nqo1 is significantly down-regulated in bulge cells from Nfatc1 cKO mice relative to littermate controls (Figure 5A). To probe whether Nfatc1 could drive expression of these DMBA-metabolizing enzymes, we infected mouse keratinocytes with retrovirus expressing either a constitutively active form of NFATc1 (caNFATc1; Horsley et al., 2008) or an empty vector GFP control (Figure 5B). From these analyses, we observed a significant increase in Ahr and Cyp1b1 expression and a decrease in Arntl and Gsta1 expression (Figure 5B). We next investigated whether caNFATc1 influenced expression of these DMBA-metabolizing enzymes 1, 4, and 24 h after DMBA treatment. Four of these genes (Ahr, Cyp1a1, Cyp1b1, and Nqo1) were significantly more highly expressed in caNFATc1-expressing keratinocytes after DMBA treatment relative to controls. caNFATc1 expression also increased the percentage of DMBA-treated keratinocytes with γH2Ax nuclear foci, indicating that Nfatc1 expression promotes DNA damage after DMBA treatment (Figure 5, G and H). Taken together, these data suggest that Nfatc1 controls the expression of genes involved in DMBA metabolism, leading to enhanced DNA damage after DMBA exposure.
FIGURE 5:
Nfatc1 regulates expression of DMBA-metabolizing enzymes. (A) Real-time PCR analysis of mRNA expression for DMBA-metabolizing enzymes in FACS-purified sorted cKO bulge cells compared with control bulge cells. Data are mean ± SD (three or four mice/genotype). *p < 0.05; one-way ANOVA. (B) Real-time PCR for DMBA-metabolizing enzymes in caNFATc1 compared with control-infected keratinocytes. *p < 0.05; one-way ANOVA. (C–F) Real-time PCR for (C) Ahr, (D) Cyp1a1, (E) Cyp1b1, and (F) Nqo1 mRNA in caNFATc1- and control-infected keratinocytes at 0, 1, 4, and 24 h. The p value reflects two-way ANOVA for the effect of Nfatc1 expression. (G) γH2A.X immunostaining (red) in GFP- and caNFATc1-infected keratinocytes 24 h post-DMBA/vehicle. DAPI, blue. Scale bar, 50 μm. (H) Quantification of γH2A.X+ GFP- and caNFATc1-infected keratinocytes after 24 h in vehicle or DMBA. Data: mean ± SEM (three or four replicates for each treatment in two independent experiments; >179–1435 cells analyzed). One-way ANOVA.
DISCUSSION
Here we identify a role for Nfatc1 during the initiation of chemically induced epidermal tumorigenesis. The tumor-enhancing roles for Nfatc1 in the skin are distinct from the role of calcineurin, the upstream phosphatase that regulates Nfat’s nuclear localization, which inhibits skin tumorigenesis (Wu et al., 2010). Patients treated with calcineurin inhibitors have an increased disposition for developing SCCs (Euvrard et al., 2003), and mouse and human cell studies have revealed that genetic and pharmacological inhibition of calcineurin/Nfat signaling increases chemically induced skin tumor onset (Wu et al., 2010). Using the same chemical tumorigenesis paradigm as Wu et al. (2010), we find that mice lacking Nfatc1 in the epidermis do not display enhanced tumor formation but instead have reduced tumor formation, suggesting that calcineurin may inhibit skin tumorigenesis through its regulation of additional Nfat isoforms or other proteins, such as Atf3 or p53.
Our data and those of others (Li et al., 2013) are consistent with cells from the bulge and outside the bulge contributing to DMBA-induced tumors. Several types of keratinocytes are likely origins of SCCs, including those outside of the bulge (Lapouge et al., 2011; White et al., 2011). Although inefficient Cre activity could yield heterogeneity in our lineage tracing data, loss of Nfatc1 before tumor initiation decreases the contribution of bulge stem cells and their progeny to skin tumors. The minor fraction of keratinocytes that express Nfatc1 in the bulge and contribute to tumors in this model may explain the moderate reduction in tumorigenesis in Nfatc1 cKO mice.
Recent data reveal that follicular stem cell activation is required for stem cell–induced tumor initiation (White et al., 2014). Nfatc1 is a major driver of follicular stem cell quiescence (Horsley et al., 2008; Keyes et al., 2013), and the reduced rate of tumorigenesis after Nfatc1 deletion seems contrary to its role in stem cell activity. Because we treated both Nfatc1 cKO mice and littermate controls with DMBA during telogen and promoted tumorigenesis with TPA, which enhances proliferation throughout the skin epithelium (Figure 4C) and induces hair follicle stem cell activity (Stenn and Paus, 2001), our study did not elucidate whether Nfatc1’s role in stem cell activation influences tumor formation. White et al. (2014) used an inducible genetic mouse model to express an oncogenic Kras allele in hair follicle stem cells to dissect the requirement of stem cell activity during skin tumorigenesis. Whether Nfatc1 alters SCC formation in other tumor induction paradigms, including a genetically activated Ras mouse model, will be an interesting area of future study.
Our findings are consistent with previous work demonstrating that Nfatc1 enhances tumorigenesis in multiple cell types (Mancini and Toker, 2009; Muller and Rao, 2010). In fibroblasts, constitutive Nfatc1 activation induces cellular transformation through disruption of contact-mediated cell growth (Neal and Clipstone, 2003). Nfatc1 can also promote cancer cell invasion in breast and lung cancer cell lines (Oikawa et al., 2013) and promote angiogenesis in tumor-associated vasculature (Ryeom et al., 2008). Furthermore, Nfat activation has been associated with lymphomas (Marafioti et al., 2005; Pham et al., 2005), leukemias (Medyouf et al., 2007), and pancreatic cancers (Buchholz et al., 2006; Koenig et al., 2010).
Our data also resonate with a recent study demonstrating that constitutive overexpression of a nuclear form of Nfatc1 is sufficient to drive the formation of SCC-like tumors in the skin (Tripathi et al., 2013). Although Nfatc1 was implicated in the creation of a proinflammatory microenvironment in the skin through regulation of cytokine gene expression (Tripathi et al., 2013), our studies suggest that endogenous Nfatc1 promotes bulge cell contribution to tumors in a role independent of inflammation. We do not detect overt alterations in skin inflammatory response (Horsley et al., 2008) or cytokine gene expression during homeostasis after Nfatc1 inhibition (Goldstein et al., 2014) or inflammation during tumorigenesis in Nfatc1 cKO mice.
In our study, we discovered a link between Nfatc1 and DMBA-induced DNA damage within follicular stem cells. Polyaromatic hydrocarbons such as DMBA are highly prevalent in industrial pollution and are detoxified into adducts that cause DNA damage by several enzymes that are induced in part by the transcription factor Ahr. Indeed, mice treated with extracts of airborne particles from industrial pollution form SCCs in an Ahr-dependent manner (Matsumoto et al., 2007). We show that Nfatc1 enhances the expression of Ahr and its downstream target Cyp1a in DMBA-treated keratinocytes and leads to an elevated DNA damage response in DMBA-treated keratinocytes. The role of Nfatc1 in regulating DNA damage is likely balanced by additional mechanisms in bulge cells to promote DNA repair, such as elevated expression of Bcl-2 (Sotiropoulou et al., 2010) and stabilization of p53 (Petersson et al., 2015). Of interest, Nfatc1 can directly regulate the expression of the cytochrome P450 superfamily member CYP2E1 (Wang et al., 2010), which metabolizes ethanol and other small polar molecules, suggesting that Nfatc1 may be involved broadly in metabolism of xenobiotics. Defining whether Nfatc1 directly or indirectly regulates the expression of P450 family members to affect skin tumorigenesis may reveal novel mechanisms involved in skin tumor initiation.
MATERIALS AND METHODS
Animal use
All animals were housed and handled according to the institutional guidelines of Yale University. Nfatc1fl/fl (Aliprantis et al., 2008), Keratin14Cre (Vasioukhin et al., 1999), Keratin19CreERT (Means et al., 2008), and mTmG (Muzumdar et al., 2007) mice have been described previously. For K14Cre/Nfatc1 and K19CreERT/Nfatc1 tumorigenesis studies, mice were maintained in the outbred CD-1 background, and age- and sex-matched Cre+; Nfatc1fl/+ littermates were used as controls. For the K19creERT/Nfatc1/mTmG lineage tracing experiments, mice were in a mixed CD-1, 129×1/SvJ background. Activation of CreERT was performed by topical administration of tamoxifen (10 mg/ml in ethanol; Sigma-Aldrich, St. Louis, MO) for 5 d. For BrdU experiments, mice were injected intraperitoneally with 50 mg/kg BrdU (Sigma-Aldrich) for 4 h before being killed.
Chemical carcinogenesis
To induce skin tumors, both control and Nfatc1 cKO mice in the telogen phase of the hair cycle (Horsley et al. 2008) were shaved on the back and administered one topical dose of DMBA (20 μg in 100 μl acetone; Sigma-Aldrich). Mice received topical treatments of TPA (5 μg in 100 μl ethanol; Sigma-Aldrich) twice weekly for 20 wk, and tumors were counted weekly. Histological analyses of tumors from Nfatc1 cKO and control mice were performed by a veterinary pathologist and revealed the presence of skin papillomas. One control mouse developed an SCC, which metastasized to the lung and inguinal lymph nodes.
Histology and immunofluorescence
For epidermal thickness analyses and immunofluorescence, tissues were embedded in OCT compound (Tissue-Tek; Sakura Finetek, Torrance, CA), frozen, sectioned, fixed in 3.7% formaldehyde, and stained with hematoxylin and eosin. When applicable, the M.O.M. kit (Vector Labs, Burlingame, CA) was used to prevent nonspecific binding of mouse secondary antibodies. The following primary antibodies were used: BrdU (rat, Bu 1/75 [ICR1], 1:300; Abcam, Cambridge, MA); CD45 (rabbit, 30-F11, 1:250; eBioscience, San Diego, CA); γδTcr (hamster, GL3, 1:200; BD Biosciences, San Jose, CA); γH2A.X (mouse, JBW301, 1:100; Millipore, Billerica, MA); keratin 14 (chicken, 1:500; kind gift of Julie Segre, National Institutes of Health); keratin 10 (rabbit, 1:500; kind gift of Julie Segre), and Nfatc1 (mouse, 7A6, 1:25; Santa Cruz Biotechnology, Santa Cruz, CA). For BrdU staining, tissue sections were incubated in 2 N HCl (37°C, 30 min) and 0.1 M sodium borate, pH 8.5 (room temperature, 10 min), before primary antibody incubation. The following secondary antibodies were used: anti-chicken, anti-rabbit, anti-mouse, anti-rat, and anti-hamster conjugated to rhodamine red X or Alexa 488 (1:250; Jackson ImmunoResearch, West Grove, PA). Sections were mounted in Prolong Gold anti-fade reagent with 4′,6′-diamidino-2-phenylindole (DAPI; Life Technologies, Grand Island, NY). Imaging was performed on a Zeiss Imager M.1 fluorescence microscope (Zeiss, Oberkochen, Germany), and images were acquired with an AxioCam MR3 camera and quantified with ImageJ (National Institutes of Health, Bethesda, MD).
Quantification of Langerhans cells in epidermal sheets
Mouse ears were collected, split into dorsal and ventral sides, and floated epidermal side up on a Ca2+/Mg2+-free phosphate-buffered saline (PBS) solution containing 20 mM EDTA for 2 h at 37°C. Epidermal sheets were gently peeled from the dermis, washed in PBS containing Ca2+/Mg2+, and fixed in cold acetone for 20 min at −20°C. Sheets were washed in PBS, incubated for 1 h at 37°C in 2% (wt/vol) bovine serum albumin (BSA) in PBS, and stained overnight at 4°C with MHC class II (1-A/1-E) primary antibody (rat, M5/114.15.2, 1:200; eBioscience, San Diego, CA). After being washed in PBS, sheets were stained overnight at 4°C with a rhodamine red X–conjugated anti-Rat secondary antibody (1:250; Jackson ImmunoResearch) in 1% (wt/vol) BSA in PBS. After washing in PBS, epidermal sheets were mounted on slides with Prolong Gold anti-fade reagent (Life Technologies) and visualized by confocal microscopy.
Analysis of DMBA responsiveness in vivo and Hras codon mutations
To analyze the response of DMBA-metabolizing enzymes to DMBA treatment in control and Nfatc1 iKO mice, we treated back skin with vehicle and DMBA and isolated mRNA after 24 h. Analysis of mutations in Hras1 was performed as described previously (Sachs et al., 2012). Briefly, genomic DNA was isolated from skin tumors 8–10 wk after DMBA treatment using phenol-chloroform-isoamyl alcohol (Life Technologies). Codon 61 of Hras1 was amplified by nested PCR using Hras primer pairs 1 and 2 (Supplemental Table S1). Amplification with Hras primer pair 1 generated a 267–base pair product. Subsequent nested PCR amplification with Hras primer pair 2 generated a 178–base pair product. A 5-μg amount of DNA was digested at 37°C overnight with the restriction enzyme Xba1 and resolved on a 2% agarose gel. Genomic DNA from CarC cells (provided by M. Girardi, Yale University), which contains mutations at both Hras alleles, was used as a positive control.
FACS, RNA isolation, cDNA synthesis, and real-time PCR
For bulge stem cell isolation, subcutaneous fat was gently removed from skins with a scalpel. Skins were floated on 0.25% trypsin-EDTA overnight at 4°C and for 1 h at 37°C. Trypsin was inactivated with medium containing chelexed fetal bovine serum (FBS), and epidermal cells were gently removed from the skins with a scalpel. Cells were filtered and resuspended as single-cell suspensions in staining buffer (SB) containing PBS/4% chelexed FBS. Cells were incubated with the following antibodies for 30 min on ice in the dark: CD34-biotin (1:50; eBioscience) and Cd49f-PE (1:75; BD Biosciences). Cells were washed, resuspended in SB, and incubated with streptavidin-APC (1:200; BD Biosciences) for 30 min on ice in the dark. Cells were washed and resuspended at 1 × 106 cells/ml. Propidium iodide, 0.5 g/ml (Sigma-Aldrich), was added to the cell suspension immediately before sorting. Cells were sorted on a FACS Aria III equipped with FACS DiVA software (BD Biosciences).
For RNA isolation from FACS-sorted cells, 150,000 bulge cells were sorted into 600 μl of TRIzol LS (Life Technologies). For tumor RNA isolation, papillomas and nontumor skins from Nfatc1 cKO mice and littermate controls were homogenized in TRIzol (Life Technologies). Total RNAs were extracted according to manufacturer’s protocol and purified using the RNeasy kit (Qiagen, Venlo, Netherlands).
To generate cDNA, equal amounts of RNA were reverse transcribed using oligo(dT) primers (Superscript III First-Strand Synthesis Kit; Life Technologies). Real-time PCR was performed on a Light Cycler 480 II using SYBR GREEN reagents (Roche, Indianapolis, IN). Primers used are listed in Supplemental Table S1. Relative quantification was calculated with the ΔΔCt method using β-actin as a reference gene.
Cell culture
MSCV-GFP (control) and constitutively active NFATc1 (caNFATc1) retroviral vectors have been described previously (Neal and Clipstone, 2003; Horsley et al., 2008). Briefly, retroviral vectors were transfected with Xtremegene HD (Roche) into Phoenix cells to generate retroviral supernatants. Supernatants were filtered at 0.45 μm, and infections of primary mouse keratinocytes were performed by adding retroviral supernatant, chelexed FBS, and 4 μg/ml Polybrene and centrifuging at 800 × g for 30 min at 32°C. After centrifugation, retroviral supernatant was removed, and keratinocytes were refed with growth medium. Cells were grown for 48 h before collection in TRIzol (Invitrogen) for RNA isolation. For in vitro DMBA treatments, MSCV-GFP– or caNFATc1–infected cells were treated with 0.15 μg/ml DMBA for indicated time points up to 24 h.
Statistical analysis
For tumor frequency, statistical analyses were performed using a log-rank test. Analysis of differences in Hras codon mutations between control and cKO mice was performed using the chi-square test. Statistical analyses between two groups were performed using a Student’s t test, between multiple groups using a one-way analysis of variance (ANOVA), or between groups over time with a two-way ANOVA. To assess the differences in tumor number over time between control and cKO mice, we used a mixed-effect model with genotype and week as (discrete) fixed effects and a random intercept for each mouse. This model, conceptually similar to a repeated-measures ANOVA, allows us to estimate the tumor number difference at each week taking into account the within-mouse correlation and can account for missing values. In addition, the weekly rate of tumor formation after week 5 was evaluated using the equation Nit = αo + β1t + β2t(GKO) + ui + εit, where Nit is the number of tumors per week for mouse i, αo is the intercept, ui is a random term for each mouse, β1 represents the rate for the control mice, t is the time after week 5, β2 is the difference in tumor number per week between groups, GkO = 1 for KO mice and 0 for control mice, and εit is the error term. The mixed-model approach using R and all other analyses were performed using GraphPad Prism version for Macintosh (GraphPad, La Jolla, CA). For all analyses, p ≤ 0.05 was accepted for statistical significance.
Supplementary Material
Acknowledgments
We thank Z. Zhou and K. Nelson (Yale Cell Sorting Facility) for FACS sorting and G. Rivera, A. Graham, and A. Chun for technical assistance. M. Girardi graciously provided DNA from CarC cells. Horsley lab members provided valuable discussions and critical reading of the manuscript. R.Z. is funded by the Lo Graduate Fellowship in Excellence in Stem Cell Research. J.G. was funded by National Institutes of Health Training Grant 5T32HG 3198-7. V.H. was a Pew Scholar in Biomedical Research and is funded by the National Institutes of Health (AR060295) and the Connecticut Department of Public Health (12SCBYALE01).
Abbreviations used:
- BrdU
5-bromo-2′-deoxyuridine
- cKO
conditional knockout
- DETC
dendritic epidermal T-cell
- DMBA
7,12-dimethylbenz[a]anthracene
- FACS
fluorescence-activated cell sorting
- iKO
inducible knockout
- MHC
major histocompatibility complex
- mTmG
membrane Tomato/membrane enhanced green fluorescent protein
- SCC
squamous cell carcinoma
- Tcr
T-cell receptor
- TPA
12-O-tetradecanoylphorbol 13-acetate.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E15-05-0282) on August 26, 2015.
REFERENCES
- Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR, Glimcher LH. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118:3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmain A, Ramsden M, Bowden GT, Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984;307:658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, Gress TM, Ellenrieder V. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25:3714–3724. doi: 10.1038/sj.emboj.7601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009;16:55–66. doi: 10.1016/j.ccr.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Fletcher S, Roth E, Wu C, Chun A, Horsley V. Calcineurin/Nfatc1 signaling links skin stem cell quiescence to hormonal signaling during pregnancy and lactation. Genes Dev. 2014;28:983–994. doi: 10.1101/gad.236554.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H, Glick AB, Greenhalgh DA, Morgan DL, Strickland JE, Tennenbaum T, Yuspa SH. Critical aspects of initiation, promotion, and progression in multistage epidermal carcinogenesis. Proc Soc Exp Biol Med Soc. 1993;202:1–8. doi: 10.3181/00379727-202-43511a. [DOI] [PubMed] [Google Scholar]
- Horsley V, Aliprantis A, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes BE, Segal JP, Heller E, Lien WH, Chang CY, Guo X, Oristian DS, Zheng D, Fuchs E. Nfatc1 orchestrates aging in hair follicle stem cells. Proc Natl Acad Sci USA. 2013;110:E4950–E4959. doi: 10.1073/pnas.1320301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig A, Linhart T, Schlengemann K, Reutlinger K, Wegele J, Adler G, Singh G, Hofmann L, Kunsch S, Büch T. NFAT-induced histone acetylation relay switch promotes c-Myc-dependent growth in pancreatic cancer cells. Gastroenterology. 2010;138:1189–1199. doi: 10.1053/j.gastro.2009.10.045. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou P, Blanpain C. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci USA. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Park H, Trempus CS, Gordon D, Liu Y, Cotsarelis G, Morris RJ. A keratin 15 containing stem cell population from the hair follicle contributes to squamous papilloma development in the mouse. Mol Carcinog. 2013;52:751–759. doi: 10.1002/mc.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M, Toker A. NFAT proteins: emerging roles in cancer progression. Nat Rev Cancer. 2009;9:810–820. doi: 10.1038/nrc2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafioti T, Pozzobon M, Hansmann ML, Ventura R, Pileri SA, Roberton H, Gesk S, Gaulard P, Barth TF, Du MQ, et al. The NFATc1 transcription factor is widely expressed in white cells and translocates from the cytoplasm to the nucleus in a subset of human lymphomas. Br J Haematol. 2005;128:333–342. doi: 10.1111/j.1365-2141.2004.05313.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Ide F, Kishi R, Akutagawa T, Sakai S, Nakamura M, Ishikawa T, Fujii-Kuriyama Y, Nakatsuru Y. Aryl hydrocarbon receptor plays a significant role in mediating airborne particulate-induced carcinogenesis in mice. Environ Sci Technol. 2007;41:3775–3780. doi: 10.1021/es062793g. [DOI] [PubMed] [Google Scholar]
- Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H, Alcalde H, Berthier C, Guillemin MC, dos Santos NR, Janin A, Decaudin D, de The H, Ghysdael J. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007;13:736–741. doi: 10.1038/nm1588. [DOI] [PubMed] [Google Scholar]
- Modi BG, Neustadter J, Binda E, Lewis J, Filler RB, Roberts SJ, Kwong BY, Reddy S, Overton JD, Galan A, et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science. 2012;335:104–108. doi: 10.1126/science.1211600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Neal JW, Clipstone NA. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J Biol Chem. 2003;278:17246–17254. doi: 10.1074/jbc.M300528200. [DOI] [PubMed] [Google Scholar]
- Oikawa T, Nakamura A, Onishi N, Yamada T, Matsuo K, Saya H. Acquired expression of NFATc1 downregulates E-cadherin and promotes cancer cell invasion. Cancer Res. 2013;73:5100–5109. doi: 10.1158/0008-5472.CAN-13-0274. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson M, Reuter K, Brylka H, Kraus A, Schettina P, Niemann C. Interfering with stem cell-specific gatekeeper functions controls tumour initiation and malignant progression of skin tumours. Nat Commun. 2015;6:5874. doi: 10.1038/ncomms6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LV, Tamayo AT, Yoshimura LC, Lin-Lee YC, Ford RJ. Constitutive NF-kappaB and NFAT activation in aggressive B-cell lymphomas synergistically activates the CD154 gene and maintains lymphoma cell survival. Blood. 2005;106:3940–3947. doi: 10.1182/blood-2005-03-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom S, Baek KH, Rioth MJ, Lynch RC, Zaslavsky A, Birsner A, Yoon SS, McKeon F. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell. 2008;13:420–431. doi: 10.1016/j.ccr.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Sachs N, Secades P, van Hulst L, Kreft M, Song JY, Sonnenberg A. Loss of integrin alpha3 prevents skin tumor formation by promoting epidermal turnover and depletion of slow-cycling cells. Proc Natl Acad Sci USA. 2012;109:21468–21473. doi: 10.1073/pnas.1204614110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci USA. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulou PA, Candi A, Mascré G, DeClercq S, Youssef KK, Lapouge G, Dahl E, Semeraro C, Denecker G, Marine JC, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572–582. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
- Stenn KE, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Stingl G, Koning F, Yamada H, Yokoyama WM, Tschachler E, Bluestone JA, Steiner G, Samelson LE, Lew AM, Coligan JE, et al. Thy-1+ dendritic epidermal cells express T3 antigen and the T-cell receptor gamma chain. Proc Natl Acad Sci USA. 1987;84:4586–4590. doi: 10.1073/pnas.84.13.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Wang Y, Coussens M, Manda KR, Casey AM, Lin C, Poyo E, Pfeifer JD, Basappa N, Bates CM, et al. Activation of NFAT signaling establishes a tumorigenic microenvironment through cell autonomous and non-cell autonomous mechanisms. Oncogene. 2013;33:1840–1849. doi: 10.1038/onc.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Wang J, Hu Y, Nekvindova J, Ingelman-Sundberg M, Neve EP. IL-4-mediated transcriptional regulation of human CYP2E1 by two independent signaling pathways. Biochem Pharmacol. 2010;80:1592–1600. doi: 10.1016/j.bcp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- White AC, Khuu JK, Dang CY, Hu J, Tran KV, Liu A, Gomez S, Zhang Z, Yi R, Scumpia P, et al. Stem cell quiescence acts as a tumour suppressor in squamous tumours. Nat Cell Biol. 2014;16:99–107. doi: 10.1038/ncb2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC, Tran K, Khuu J, Dang C, Cui Y, Binder SW, Lowry WE. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci USA. 2011;108:7425–7430. doi: 10.1073/pnas.1012670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, Hofbauer GF, Dotto GP. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–372. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito G, Saotome I, Liu Z, Ferro EG, Sun TY, Nguyen DX, Bilguvar K, Ko CJ, Greco V. Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk. Nat Commun. 2014;5:3543. doi: 10.1038/ncomms4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoumpourlis V, Solakidi S, Papathoma A, Papaevangeliou D. Alterations in signal transduction pathways implicated in tumour progression during multistage mouse skin carcinogenesis. Carcinogenesis. 2003;24:1159–1165. doi: 10.1093/carcin/bgg067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.